Evaluating the Ability of the Biomarkers to Identify the Possibility of Diagnosing Sepsis in the Enrolled Patients
Received: 07-Jul-2023 / Manuscript No. DPO-23-105255 / Editor assigned: 12-Jul-2023 / PreQC No. DPO-23-105255(PQ) / Reviewed: 26-Jul-2023 / QC No. DPO-23-105255 / Revised: 03-Aug-2023 / Manuscript No. DPO-23-105255 / Accepted Date: 03-Aug-2023 / Published Date: 10-Aug-2023 DOI: 10.4172/2476-2024.8.S13.005 QI No. / DPO-23-105255
Abstract
Background: Severe trauma could induce sepsis due to the loss of control of the infection, which may eventually lead to death. Accurate and timely diagnosis of sepsis with severe trauma remains challenging both for clinician and laboratory. Combinations of markers, as opposed to single ones, may improve diagnosis. We therefore compared the diagnostic characteristics of routinely used biomarkers of sepsis alone and in combination, trying to define a biomarker panel to predict sepsis in severe patients.
Methods: This prospective observational study included patients with severe trauma (ISS 16 or more) in the EICU at a university hospital. Blood samples were collected at 8 a.m. every day after admission to the EICU, until the day included patients were transferred out of EICU. Plasma levels of PCT, CRP, IL-6 and SAA were measured using commercial ELISA kits. Receiver operating characteristic (ROC) curves were used to evaluate the ability of the biomarkers to identify the possibility of sepsis in the enrolled patients. Logistic regression models were used to identify independent risk factors for sepsis.
Results: A total of 100 patients were eligible for analysis. Of these, 52 were diagnosed with sepsis. CRP yielded the highest discriminative value with an area under the ROC curve (AUC) of 0.82 (82% Confidence Interval (CI), 0.73–0.91; P<0.001), followed by PCT (AUC 0.77 (0.68–0.86); P<0.001). Whereas, in multiple logistic regression, SAA, CRP, and PCT were found to be independent predictors of sepsis. Bioscore which was composed of SAA, CRP, and PCT, with AUC=0.89(95%CI, 0.82-0.95), cut-off=0.28, sensitivity=0.77, specificity=0.9, P<0.001, was shown to be far superior to that of each individual biomarker taken individually.
Conclusion: Compared with single markers, the biomarker panel of PCT, CRP, and SAA was more predictive of sepsis in severe polytrauma patients.
Keywords: c-reactive protein; Procalcitonin; Serum amyloid A; Sepsis; Severe trauma
Abbreviations
CRP: C-Reactive Protein; PCT: Procalcitonin; SAA: Serum Amyloid A; IL-6: Interleukin-6; SIRS: Systemic Inflammatory Response Syndrome; EICU: Emergency Intensive Care Unit; ISS: Injury Severity Score; SOFA: Sepsis-Related Organ Failure Assessment; APACHE: Acute Physiology and Chronic Health Evaluation; GCS: Glasgow Score; MV: Mechanical Ventilation; EDTA: Ethylenediaminetetraacetic Acid; ROC: Receiver Operating Characteristic; AUC: Areas Under the Receiver Operating Characteristic Curves
Introduction
Polytrauma means an anatomical injury of Abbreviated Injury Scale (AIS) ≥ 3 in at least two body regions with the presence of SIRS on at least one day during the first 72 hours [1]. These patients are at risk of higher morbidity and mortality than the summation of expected morbidity and mortality of their individual injuries. Severe traumas induce a systemic inflammatory response that may be followed by an anti-inflammatory response, which contributes to a state of transient immunosuppression [2-5]. A number of factors like poor blood perfusion, wound infection and stress response will lead to a series of pathophysiological processes such as ischemia and hypoxia, infection and sepsis, septic shock or multiple organ dysfunction syndrome, which eventually lead to death. Sepsis may induce fatal organ failures due to the loss of control of the infection, thereby leading to septic shock. There are 31,500,000 cases of sepsis every year worldwide, with 5,300,000 death and 17% mortality rate, and it costs 170 billion dollars annually to treat the sepsis patients [6,7]. Therefore, it has become one of the most vital issues to lower the occurrence and mortality of sepsis in the field of critical medicine.
Despite the progress in the management of primary injury and supportive care in polytrauma patients, the incidence and mortality rate of post-traumatic sepsis have not been reduced to an acceptable level. If the incidence and outcome of post-traumatic sepsis can be predicted early, and the intervention measures can be implemented early for the high-risk injured patients, the incidence and mortality rate can be effectively decreased. Therefore, early intervention to prevent subsequent or worsening clinical deterioration is a key to the successful treatment of patients with potentially severe sepsis [8,9]. However, it is often difficult to determine which of the post-traumatic patients with signs of infection on initial evaluation have, or will develop, more severe illness. Therefore, the development of new biomarkers is desirable. However, to our knowledge, to date there is no single accepted biomarker or combination of biomarkers for use in patients with suspected sepsis.
Many potential biomarkers have been investigated, but only CRP and PCT are currently used on a routine basis [10-12]. Concentration of IL-6 is in relation with the severity of injury, and SAA and IL-6 are also of potential interest [13]. Because sepsis is comprised of an array of signaling proteins from various cascades, we hypothesized that use of a multiple marker approach would improve clinical utility compared with the use of a single marker. That means, the search for a single magic bullet marker might ultimately be fruitless, but a combination of markers could improve diagnosis, prognosis and treatment efficacy, and thus surviva [10]. Here, we performed a prospective study aimed at evaluating the diagnostic accuracy of PCT, CRP, IL-6 and SAA alone or in combination for differential diagnosis of post-traumatic sepsis, to possibly define a panel of biomarkers that would assess risk of sepsis in critically ill post-traumatic patients at ICU admission.
Materials and Methods
Study population
This prospective observational study was carried out over a 16-month period (August 2021 to November 2022) in the EICU of the Third Hospital of Hebei Medical University (a 2000-bed university, and also a trauma emergency center at provincial level), China. Inclusion criteria were EICU patients aged 18 or older, and an ISS 16 or more. Clinical exclusion criteria were age of less than 18 years, ISS of less than 16, pregnancy, chronic corticosteroid or Immunosuppressant therapy, do-not-resuscitate status and cardiac arrest [14,15].
Approval of the institutional review board and informed consent were obtained from our hospital before inclusion. Informed consent was obtained directly from each patient/legal representative before enrollment.
Data collection
All polytrauma patients admitted were followed up prospectively until the day they were tranferred out of EICU or died. During admission, clinical and therapeutic data were collected. Clinical data collection comprised demographics (age, gender), ISS, SOFA score [16]. The APACHE II score, comorbid conditions (e.g.,hypertension, diabetes, vascular diseases, and other diseases including arrhythmia, cirrhosis, rheumatoid arthritis, diseases of the thyroid gland or end-stage renal diseases), social factors (history of smoking or alcohol use), vital signs (GCS, body temperature, pulse rate, respiratory rate, blood pressure, oxygen saturation), infection characteristics (sources and microorganisms identified) [17]. Therapeutic data were also collected on admission to the EICU, including the use of MV, the use of vasopressors, the length of ICU stay and mortality rate were also recorded. The latest diagnostic criteria for sepsis 3.0 were infection+SOFA ≥ 2 [18].
Sample collection and biomarker assays
EDTA anticoagulated blood samples were collected at 8 a.m. every day after admission to the EICU (that is, at day 0, 1, 2…until the day included patients were transferred out of EICU). Samplings were acquired through central venous or arterial catheter, as the severity of the trauma required such equipments. Blood samples were stored immediately at 4ºC and then centrifuged (3000 g × 10 min) within 2 h after collection. Plasma levels of PCT, CRP, IL-6 and SAA were measured using commercial ELISA kits, according to the manufacturers’ recommendations (Dry fluorescence immunoassay analyzer QD-S300, Nanjing Vazyme MedTech Co., Ltd, Nanjing, China). Interassay and intraassay coefficients of variation were lower than 15%. The detection limits were 0.046 ng/ml, 3 mg/L, 7 pg/ml and 10 mg/L, respectively.
Statistical analyses
All statistical analyses were performed using R4.0 and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA). Continuous variables are described by x ± s or median (interquartile range). Normal measurement data were analyzed by t-test, and non-normal measurement data were analyzed by nonparametric test. The count data were described by frequency and analyzed by chi-square test. ROC curves were used to evaluate the ability of the biomarkers to identify the possibility of sepsis in the enrolled patients. Logistic regression models were used to identify independent risk factors for sepsis. P<0.05 was considered statistically significant.
Results
Characteristics of the inception cohort patients
Among the 100 patients enrolled in this study, 52 (52%) were diagnosed with sepsis. Clinical and demographic characteristics, comorbidity and prognosis are summarized in Table 1. At admission, the proportion of male, age, ISS, SOFA score and APACHE II score were higher in sepsis group than in control group, and the total score of GCS was lower in sepsis group than in control group (P<0.05). Also, the lengths of ICU stay and mortality rate were significantly higher in sepsis group than in control group (P<0.05).
| Characteristic | Patients without Sepsis (n=48) | Patients with Sepsis (n=52) | P |
|---|---|---|---|
| Sex, n (%) | - | - | 0.969 |
| Male | 38 | 41 | - |
| Female | 10 | 11 | - |
| Age, yr* | 51.00(29.75) | 65.50(20.75) | 0.001 |
| ISS | 23.85 ± 7.33 | 37.15 ± 8.28 | <0.001 |
| SOFA Score | 10.50(18.00) | 17.00(28.75) | 0.001 |
| APACHE2 Score | 12.50(15.75) | 27.00(23.75) | <0.001 |
| Comorbiditya | - | - | - |
| Hypertension | 6 | 20 | 0.003 |
| Diabetes | 3 | 11 | 0.032 |
| Vascular diseases diseases | 4 | 7 | 0.413 |
| Other diseasesb | 9 | 5 | 0.188 |
| History of smoking | 22 | 21 | 0.582 |
| Alcohol abuse | 23 | 20 | 0.34 |
| GCS | 9.00(9.00) | 3.00(1.00) | <0.001 |
| Body temperature, ºC | 36.80(1.08) | 36.60(0.67) | 0.103 |
| Pulse, b/m | 78.00(26.00) | 89.00(25.00) | 0.078 |
| Respiratory rate, /m | 17.00(3.75) | 15.50(6.00) | 0.43 |
| Blood pressure, mmHg | 106.27 ± 19.01 | 109.21 ± 19.09 | 0.442 |
| SPO2 | 97.50(6.50) | 96.00(5.00) | 0.617 |
| Duration of Mechanical ventilation, n (%) | 9.00(9.75) | 11.50(11.75) | 0.078 |
| Vasopressors, n (%) | 22 | 33 | 0.077 |
| Length of ICU saty, d | 11.50(10.75) | 21(20.25) | 0.002 |
| Mortality rate, n(%) | 9 | 23 | 0.006 |
Note: Data are expressed as n (%), unless otherwise indicated. aSeveral patients had more than one comorbidity (for example, some had both hypertension and cardiovascular diseases). bArhythmia, cirrhosis, rheumatoid arthritis, diseases of the thyroid gland or end-stage renal diseases. ISS, Injury Severity Scoring. SOFA, Sepsis-related Organ Failure Assessment. APACHE, Acute Physiology And Chronic Health Evaluation. GCS, glasgow Score.
Table 1: Baseline characteristics of the inception cohort.
At admission and during the first seven days in the hospital, blood cultures, urine cultures, sputum cultures, swabs cultures and cerebrospinal fluid cultures were conducted in all enrolled patients. All 52 patients in sepsis group were classified as having infection, and a clinically relevant pathogen was isolated from the sepsis patients. The expert panel classified the infections and found that all of the infections were caused by bacteria. The primary sites of infection and microorganisms isolated are summarized in Table 2.
| Sites of infection (n, %) | Pathogens isolated |
|---|---|
| Respiratory system (18,35%) | Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Enterobacter cloacae, Stenotrophomonas maltophilia, Staphylococcus aureus, Enterobacter cloacae |
| Blood stream (12,23%) | Staphylococcus cohnii subsp. ureae, Staphylococcus hominis subsp. Hominis, Acinetobacter baumannii, Staphylococcus warneri, Staphylococcus lugdunensis |
| Urinary tract (6,12%) | Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae |
| Cerebral nervous system (8,15%) | Staphylococcus aureus, Staphylococcus haemolyticus, Staphylococcus pidermidis, Micrococcus luteus, Pseudomonas maltophilia, Escherichia coli, Staphylococcus hominis subsp. Homini, Sphingomonas paucimobilis |
| Skin and soft tissues (8,15%) | Staphylococcus warneri, Klebsiella pneumoniae, Acinetobacter baumannii, Enterococcus faecium (group D) |
Table 2: Sites of infection and pathogens isolated.
Patients’ blood samples were collected at 8 a.m. every day after admission to the EICU, and finally five samples of each patient were chosen for analysis. For the sepsis group, the five time points of the samples were the day of sepsis, 24 h before or after sepsis, 48 h before and after sepsis; for the non-sepsis group, blood samples of five days from the day of admission to the EICU were analyzed. The levels of the four biomarkers are shown in Table 3. SAA (on sepsis, and at 24 hours and 48 hours after sepsis), CRP (at 48 hours and 24 hours before sepsis, on sepsis, and at 24 hours and 48 hours after sepsis), PCT (at 48 hours and 24 hours before sepsis, on sepsis, and at 24 h and 48 h after sepsis), IL-6 (on sepsis, and at 24 h after sepsis) were significantly higher in patients with sepsis compared with non-sepsis (P<0.05).
| Biomarkers | Time points | Control group(N=48) | Sepsis group(N=52) | Statistics | P value |
|---|---|---|---|---|---|
| SAA | pre48 h | 269.02 ± 87.10 | 280.76 ± 84.67 | t=-0.683 | 0.496 |
| pre24 h | 321.84 ± 146.81 | 329.23 ± 126.46 | t=-0.270 | 0.788 | |
| sepsis | 284.42(95.17) | 371.69(82.76) | Z=-4.237 | <0.001 | |
| post24 | 267.57 ± 92.58 | 338.18 ± 125.89 | t=-3.173 | 0.002 | |
| post48 | 254.51 ± 101.32 | 318.00 ± 121.27 | t=-2.828 | 0.006 | |
| CRP | pre48 h | 170.17 ± 80.04 | 219.45 ± 101.99 | t=-2.672 | 0.009 |
| pre24 h | 212.85(160.09) | 260.43(172.63) | Z=-2.270 | 0.023 | |
| sepsis | 191.40(119.09) | 363.22(230.92) | Z=-5.450 | <0.001 | |
| post24 | 165.72(161.31) | 320.64(202.13) | Z=-3.919 | <0.001 | |
| post48 | 98.14(195.61) | 273.04(186.10) | Z=-3.401 | 0.001 | |
| PCT | pre48 h | 3.57(5.34) | 6.39(5.88) | Z=-3.108 | 0.002 |
| pre24 h | 2.95(3.49) | 9.17(20.49) | Z=-3.446 | 0.001 | |
| sepsis | 3.43(5.72) | 10.47(22.41) | Z=-4.678 | <0.001 | |
| post24 | 1.88(3.25) | 9.90(18.92) | Z=-3.874 | <0.001 | |
| post48 | 1.58(4.89) | 8.68(16.65) | Z=-3.946 | <0.001 | |
| IL-6 | pre48 h | 60.08(126.39) | 36.22(148.54) | Z=-1.656 | 0.098 |
| pre24 h | 109.31(164.37) | 144.00(184.28) | Z=-1.338 | 0.181 | |
| sepsis | 71.81(117.13) | 236.51(315.10) | Z=-4.326 | <0.001 | |
| post24 | 78.68(78.95) | 121.66(143.87) | Z=-2.829 | 0.005 | |
| post48 | 89.02(78.82) | 120.18(89.01) | Z=-1.911 | 0.056 |
Note: The levels of the four biomarkers after admission to the EICU at five points; SAA-Serum Amyloid A; CRP=C-Reactive Protein; PCT= Procalcitonin; IL-6=Interleukin-6.
Table 3: Levels of the four biomarkers compared between sepsis group and control group.
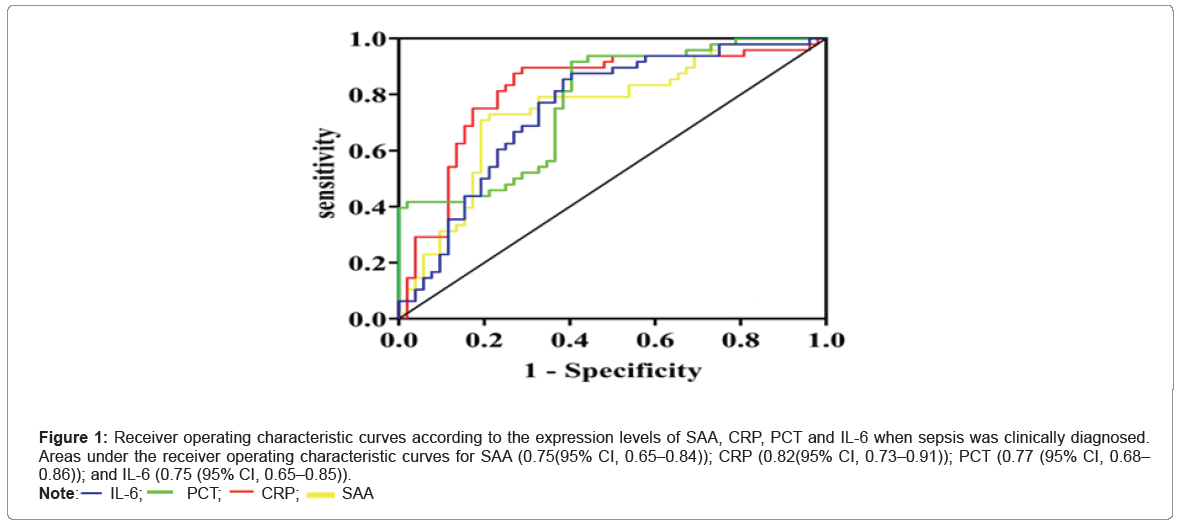
As shown in Figure 1, CRP yielded the highest discriminative value with an Area Under the ROC Curve (AUC) of 0.82 (82% CI, 0.73–0.91; P<0.001), followed by PCT (AUC 0.77 (0.68–0.86); P<0.001). Table 4 summarizes the performances of each of these biomarkers in diagnosing sepsis. CRP proved to be the optimal individual marker in terms of specificity (90%) and sensitivity (71%).
| Biomarker | Cut-off value | Sensitivity (%)* | Specificity (%) + | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| SAA | 319.7 | 0.79 | 0.73 | 0.75(0.65-0.84) | <0.001 |
| CRP | 270.84 | 0.71 | 0.9 | 0.82(0.73-0.91) | <0.001 |
| PCT | 7.27 | 0.6 | 0.92 | 0.77(0.68-0.86) | <0.001 |
| IL-6 | 194.06 | 0.6 | 0.88 | 0.75(0.65-0.85) | <0.001 |
Abbreviations: AUC-Areas Under the Receiver Operating Characteristic Curves; CI-Confidence Interval.
Table 4: Clinical performance of biomarkers in diagnosing sepsis.
Figure 1: Receiver operating characteristic curves according to the expression levels of SAA, CRP, PCT and IL-6 when sepsis was clinically diagnosed. Areas under the receiver operating characteristic curves for SAA (0.75(95% CI, 0.65–0.84)); CRP (0.82(95% CI, 0.73–0.91)); PCT (0.77 (95% CI, 0.68–0.86)); and IL-6 (0.75 (95% CI, 0.65–0.85)). 
In multiple logistic regressions, SAA, CRP, and PCT were found to be independent predictors of sepsis (Table 5).
| Variable | Coefficient | Standard error | Wald value | Odds radio | 95% CI | P value |
|---|---|---|---|---|---|---|
| SAA | 0.007 | 0.003 | 6.948 | 1.008 | 1.002-1.013 | 0.008 |
| CRP | 0.004 | 0.002 | 4.156 | 1.004 | 1.000-1.008 | 0.041 |
| PCT | 0.102 | 0.04 | 6.401 | 1.108 | 1.023-1.199 | 0.011 |
| IL6 | 0.002 | 0.002 | 1.39 | 1.002 | 0.999-1.006 | 0.238 |
Table 5: Multiple logistic-regression analysis of biomarkers in diagnosing sepsis.
Combination of PCT, CRP, and SAA index in a bioscore
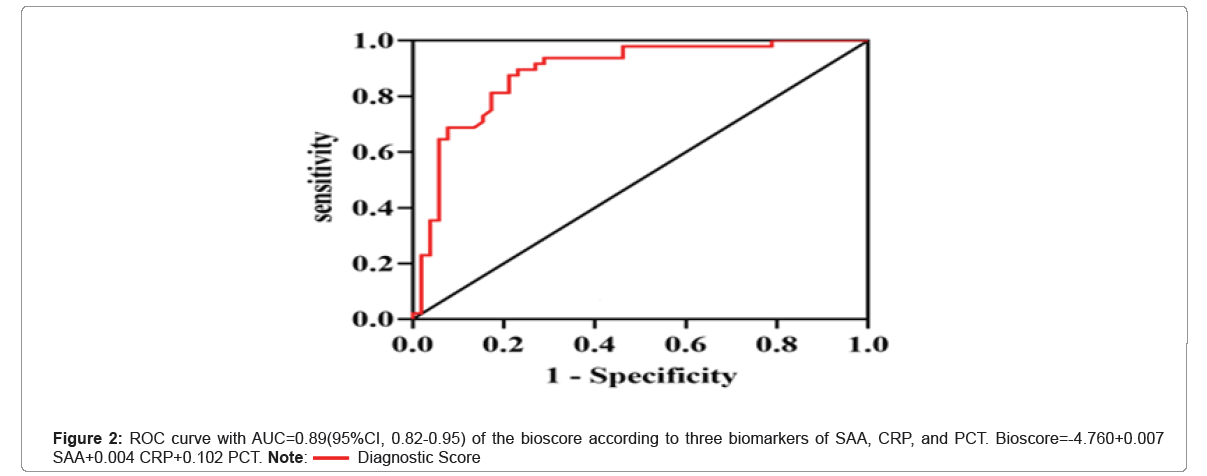
Since SAA, CRP, and PCT were found to be independent predictors of sepsis from the result of multiple logistic regressions, we need to determine whether the combination of these three biomarkers into a single bioscore could improve the diagnostic performance. We used a sepsis bioscore Nathan I. Shapiro, Stephen Trzeciak, Judd E. Hollander, et al., A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 2009; 37: 96-104] to report the multimarker assessment.
This score was calculated using standard methods by utilizing the derived equation from the multivariate regression model:
Raw Score=β0(intercept)+β1(marker 1 quartile)+β2(marker 2 quartile)...βn(marker n quartile)
For the multimarker assessment for sepsis, bioscore=-4.760+0.007 SAA+0.004 CRP+0.102 PCT was used to calculate that bioscore for each patient. When the bioscore was entered into the multiple logistic regression model in Figure 2, its performance with AUC=0.89(95%CI, 0.82-0.95), cut-off=0.28, sensitivity=0.77, specificity=0.9, P<0.001, was shown to be far superior to that of each individual biomarker taken individually.
Identification of independent risk factors for sepsis
We used logistic regression models to identify independent risk factors for sepsis by analyzing patients' clinical variables and bioscore. Clinical variables included sex, age, ISS, SOFA score, APACHE2 score, history of smoking, history of drinking alcohol, GCS score, vital signs of body temperature, pulse, respiratory rate, blood pressure, oxygen saturation of pulse, and usage of vasopressors. Bioscore was composed of the three biomarkers, SAA, CRP, and PCT, as mentioned in Figure 2.
Univariate logistic regression analysis revealed that age (OR=1.045, 95% CI:1.018-1.074, P=0.001), ISS (OR=1.248, 95% CI:1.146-1.360, P<0.001), SOFA score (OR=1.047, 95% CI:1.015-1.080, P=0.004), APACHE2 score (OR=1.077, 95%CI:1.035-1.121, P<0.001), GCS score (OR=0.636, 95% CI:0.524-0.772, P<0.001), and bioscore (OR=3.194, 95% CI:1.940-5.258, P<0.001) were associated with sepsis, as shown in Table 6.
| Variate | B | Standard error | Wald value | Odds radio | 95%CI | P value |
|---|---|---|---|---|---|---|
| Sex | 0.019 | 0.492 | 0.002 | 1.02 | 0.389-2.672 | 0.969 |
| Age | 0.044 | 0.014 | 10.356 | 1.045 | 1.018-1.074 | 0.001 |
| ISS | 0.222 | 0.044 | 25.904 | 1.248 | 1.146-1.360 | <0.001 |
| SOFA score | 0.046 | 0.016 | 8.442 | 1.047 | 1.015-1.080 | 0.004 |
| APACHE2 score | 0.074 | 0.02 | 13.366 | 1.077 | 1.035-1.121 | <0.001 |
| History of smoking | -0.222 | 0.405 | 0.302 | 0.801 | 0.362-1.770 | 0.583 |
| History of drinking alcohol | -0.387 | 0.406 | 0.907 | 0.679 | 0.307-1.505 | 0.341 |
| GCS score | -0.453 | 0.099 | 20.935 | 0.636 | 0.524-0.772 | <0.001 |
| Temperature | -0.629 | 0.37 | 2.897 | 0.533 | 0.258-1.100 | 0.089 |
| Pulse | 0.02 | 0.012 | 2.662 | 1.02 | 0.996-1.044 | 0.103 |
| Respiratory rate | -0.029 | 0.05 | 0.327 | 0.972 | 0.881-1.072 | 0.567 |
| Blood pressure | 0.008 | 0.011 | 0.599 | 1.008 | 0.987-1.030 | 0.439 |
| Oxygen saturation of pulse | 0.028 | 0.033 | 0.7 | 1.028 | 0.964-1.097 | 0.403 |
| Vasopressors | 0.719 | 0.408 | 3.099 | 2.053 | 0.922-4.571 | 0.078 |
| Bioscore | 1.161 | 0.254 | 20.827 | 3.194 | 1.940-5.258 | <0.001 |
Table 6: Univariate logistic regression analysis of factors for sepsis.
Then, all the significant univariates were taken into multiple logistic analysis, and proved that only ISS (OR=1.265, 95% CI:1.077-1.487, P=0.004), SOFA score (OR=1.184, 95% CI:1.005-1.394, P=0.043), and bioscore (OR=3.067, 95% CI: 1.187-7.925, P=0.021) were the independent risk factors, as shown in Table 7.
| Variate | B | Standard error | Wald value | Odds radio | 95%CI | P value |
|---|---|---|---|---|---|---|
| Age | 0.009 | 0.039 | 0.059 | 1.009 | 0.936-1.089 | 0.808 |
| ISS | 0.235 | 0.082 | 8.174 | 1.265 | 1.077-1.487 | 0.004 |
| SOFA score | 0.169 | 0.084 | 4.083 | 1.184 | 1.005-1.394 | 0.043 |
| APACHE2 score | 0.169 | 0.094 | 3.218 | 1.184 | 0.984-1.424 | 0.073 |
| GCS score | -0.242 | 0.205 | 1.4 | 0.785 | 0.526-1.172 | 0.237 |
| Bioscore | 1.121 | 0.484 | 5.353 | 3.067 | 1.187-7.925 | 0.021 |
Table 7: Multiple logistic regression analysis of risk factors for sepsis.
Discussion
In this study, we assembled a cohort of patients with severe trauma and studied several common biomarkers, including PCT, CRP, IL-6 and SAA, with the overall goal of creating a panel that would allow discrimination of ICU patients who are at increased risk of sepsis. Of all the clinical data, ISS and SOFA score were found to be independent risk factors for post-traumatic sepsis. Of the biomarkers, we showed here that PCT, CRP and SAA were useful in the diagnosis of sepsis. Subsequently, we combined these markers into a simple score, called “bioscore”, which turned out to be associated with an impressive diagnostic value for having or not having sepsis. The results of this study have several potential implications.
In this study, we assembled a cohort of patients with severe trauma and studied several common biomarkers, including PCT, CRP, IL-6 and SAA, with the overall goal of creating a panel that would allow discrimination of ICU patients who are at increased risk of sepsis. Of all the clinical data, ISS and SOFA score were found to be independent risk factors for post-traumatic sepsis. Of the biomarkers, we showed here that PCT, CRP and SAA were useful in the diagnosis of sepsis. Subsequently, we combined these markers into a simple score, called “bioscore”, which turned out to be associated with an impressive diagnostic value for having or not having sepsis. The results of this study have several potential implications. differences in the occurrence rate of post-traumatic sepsis is thought to be the differences in the injury severity in the patients in each study. A prospective study including 183 trauma patients reported that ISS was relevant as the risk factor for sepsis [23]. Another retrospective study reviewed 422 trauma patients with ISS ≥ 15 and found that ISS was an associated factor for sepsis-3 [24].
Second, this study showed SOFA score, instead of APACHE2 score, was the independent risk factor for post-traumatic sepsis. We may possibly find answers from the evolution history of the concept of sepsis. In 2016, the European Society of Intensive Care Medicine and the Society of Critical Care Medicine (SCCM) created a task force that proposed Sepsis-3, a new definition for sepsis [25]. The new definition excluded the establishment of SIRS criteria to define sepsis and made it more nonspecific as any life-threatening organ dysfunction caused by the dysregulated host response to infection. The task force claimed that SOFA had a better predictive validity for sepsis than SIRS criteria. It had better prognostic accuracy and the ability to predict in-hospital mortality.
Third, four biomarkers of SAA, CRP, PCT, and IL-6 showed different results. All of them were significantly higher in patients with sepsis than in those without sepsis at several specific time points as described previously, but only SAA, CRP, and PCT were found to be independently predictive of sepsis except IL-6 as shown in this study. To date, CRP and PCT are quite widely used and studied biomarkers during sepsis in recent studies. Although PCT is considered superior to CRP in a number of studies, it is not a definitive test for diagnosing sepsis because PCT levels can also be increased in other conditions [26-28]. And the combination of these two biomarkers may improve their ability to exclude sepsis [29]. SAA has been proposed as a potential biomarker for sepsis due to its rapid and robust induction in the early stages of the disease. Several studies have investigated the utility of SAA in predicting sepsis, either alone or in combination with other biomarkers. Many animal studies found that SAA concentration was higher in septic groups and can be used as a marker to rule out sepsis and nonsurvival [30,31]. Yuan et al., concluded that the level of SAA in sepsis is most useful in combination with other markers such as CRP and PCT as well as determining their correlation and SAA could be promising and meaningful in the diagnosis of neonatal sepsis [32]. Another study showed that SAA had overall better diagnostic accuracy in predicting neonatal late-onset sepsis compared to CRP and specificity [33]. IL-6 is considered as useful in septic patients but increase of its level is not specifically linked to infectious conditions [34]. Another study showed that IL-6 could be used as both diagnostic and prognostic biomarkers for sepsis and septic shock diagnosed in accordance with the Sepsis-3 definitions, and IL-6 was superior to PCT in both diagnostic and prognostic value for sepsis and septic shock [35]. Above all, numerous studies have produced a variety of results for these four biomarkers. However, to our knowledge, there is no study testing the diagnostic and prognostic values of the bioscore composed of the four biomarkers. The “bioscore” concept is considered biologically plausible as it incorporates biomarkers involved in key components of pathophysiology of sepsis. The multimarker bioscore offers a distinct mechanistic advantage over single-marker approaches [36-38]. In this study, the bioscore of these routinely available biomarkers was shown to be far superior to that of each individual biomarker taken individually in the prediction of sepsis, which was found to be associated with an impressive diagnostic accuracy and be useful in clinical practice in rapidly assigning the patients to having or not having sepsis.
This study has several limitations. First, the patients enrolled in the group were in post-traumatic period and the final results were post-traumatic sepsis or non-sepsis, which may restrict the pathophysiology of sepsis. Second, although we collected blood samples dynamically after admission, time-points in the final study were still five fixed cross-sectional points, and this did not precisely reflect the dynamic process of sepsis evolution. Third, although these four indicators of PCT, CRP, IL-6 and SAA are common and easy to measure in clinical practice, whether they are the optimal combination still needs further study.
Conclusion
This prospective study illustrates the high value of bioscore combining PCT, CRP and SAA, which shows a significant predictive value for sepsis in severe post-traumatic patients. Further investigations are warranted to validate these findings and to assess whether the bioscore may be successfully used in ICUs and on which screening criteria. And if possible, we may measure these biomarkers using a Poit-of-Care-Test (POCT) device to rapidly assay biomarkers and to produce the sepsis bioscore as its output to predict the possibility of sepsis. This approach offers a practical opportunity to improve bedside testing method of ICU patients with suspected sepsis in a clinically useful manner. This study, furthermore, provides robust thresholds for all readily obtained parameters as a strong basis for further multicenter studies.
Declarations
Acknowledgements
Not applicable.
Author contributions
ML designed the study, planned the analysis and drafted the manuscript. Y-JQ and X-LZ gave suggestions for the study design, and analyzed data. C-HZ, R-JC and D-ZH helped collect samples and were involved in collection of data. S-MD gave suggestions for the study design and revised the manuscript. All authors read and approved the final manuscript.
Funding
This project was funded by the Scientific Research Fund Project of Hebei Provincial Department of Health (20211688).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Third Hospital of Hebei Medical University approval number: W2021-089-1; January 21, 2021]. Written informed consent was obtained directly from each patient/legal representative before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- Butcher N, Balogh ZJ (2009) The definition of polytrauma: the need for international consensus. Injury 40: S12-22.
- Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101: 36-47.
- Khalid N, Patel PD, Alghareeb R, Hussain A, Maheshwari MV (2022) The effect of sepsis on myocardial function: A review of pathophysiology, diagnostic criteria, and treatment. Cureus 14: e26178.
- Bone RC (1996) Immunologic dissonance: a continuing evolution in our understanding of the Systemic Inflammatory Response Syndrome (SIRS) and the Multiple Organ Dysfunction Syndrome (MODS). Ann Intern Med 125: 680-687.
- Ward NS, Casserly B, Ayala A (2008) The Compensatory Anti-Inflammatory Response Syndrome (CARS) in critically ill patients. Clin Chest Med 29: 617-625.
- Liu VX, Morehouse JW, Marelich GP, Soule J, Russell T, et al. (2016) Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values. Am J Respir Crit Care Med 193: 1264-1270.
- Zilberman-Itskovich S, Elukbi Y, Sibony RW, Shapiro M, Yovel DZ, et al. (2022) The epidemiology of multidrug-resistant sepsis among chronic hemodialysis patients. Antibiotics (Basel), 11: 1255.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, et al. (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34: 1589-1596.
- Park SK, Shin SR, Hur M, Kim WH, Oh EA, et al. (2017) The effect of early goal-directed therapy for treatment of severe sepsis or septic shock: A systemic review and meta-analysis. J Crit Care 38: 115-122.
- Carrigan SD, Scott G, Tabrizian M (2004) Toward resolving the challenges of sepsis diagnosis. Clin Chem 50: 1301-1314.
- Meisner M (2005) Biomarkers of sepsis: clinically useful? Curr Opin Crit Care 11:473-480.
- Marshall JC, Vincent JL, Fink MP, Cook DJ, Rubenfeld G, et al. (2003) Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25-26, 2000. Crit Care Med 31: 1560-1567.
- Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M (2011) Analytic review: Interleukin-6 in surgery, trauma, and critical care: part I: Basic science. J Intensive Care Med 26: 3-12.
- Grandic L, Olic I, Pogorelic Z, Mrklic I, Perko Z (2017) The value of injury severity score and abbreviated injury scale in the management of traumatic injuries of parenchymal abdominal organs. Acta Clin Croat 56: 453-459.
- MacKenzie EJ, Shapiro S, Eastham JN (1985) The abbreviated injury scale and injury severity score. levels of inter- and intrarater reliability. Med Care 23: 823-835.
- Vincent JL, Moreno R, Takala J, Willatts S, Mendonca AD, et al. (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med 22: 707-710.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE, et al. (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818-829.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801-810.
- Kisat M, Villegas CV, Onguti S, Zafar SN, Latif A, et al. (2013) Predictors of sepsis in moderately severely injured patients: an analysis of the National Trauma Data Bank. Surg Infect (Larchmt) 14: 62-68.
- Muckart DJ, Bhagwanjee S (1997) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference definitions of the systemic inflammatory response syndrome and allied disorders in relation to critically injured patients. Crit Care Med 25: 1789-1795.
- Osborn TM, Tracy JK, Dunne JR, Pasquale M, Napolitano LM (2004) Epidemiology of sepsis in patients with traumatic injury. Crit Care Med 32: 2234-2240.
- Wafaisade A, Lefering R, Bouillon B, Sakka SG, Thamm OC, et al. (2011) Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med 39: 621-628.
- Park JH, Choi SH, Yoon YH, Park SJ, Kim JY, et al. (2016) Risk factors for sepsis in Korean trauma patients. Eur J Trauma Emerg Surg 42: 453-458.
- Chung S, Choi D, Cho J, Huh Y, Moon J, et al. (2018) Timing and associated factors for Sepsis-3 in severe trauma patients: A 3-Year single trauma center experience. Acute Crit Care 33: 130-134.
- Chakraborty RK, Burns B (2023) Systemic inflammatory response syndrome, in StatPearls: Treasure Island (FL).
- Shilpakar R, Paudel BD, Neupane P, Shah A, Acharya B, et al. (2019) Procalcitonin and C-Reactive protein as markers of bacteremia in patients with febrile neutropenia who receive chemotherapy for acute leukemia: A prospective study from nepal. J Glob Oncol 5: 1-6.
- Shokripour M, Omidifar N, Salami K, Moghadami M, Samizadeh B, et al. (2020) Diagnostic accuracy of immunologic biomarkers for accurate diagnosis of bloodstream infection in patients with malignancy: Procalcitonin in comparison with c-reactive protein. Can J Infect Dis Med Microbiol 2020: 8362109.
- Park JH, Kim DH, Jang HY, Kim MJ, Jung SH, et al. (2014) Clinical relevance of procalcitonin and C-reactive protein as infection markers in renal impairment: a cross-sectional study. Crit Care 18: 640.
- Han JH, Nachamkin I, Coffin SE, Gerber JS, Fuchs B, et al. (2015) Use of a combination biomarker algorithm to identify medical intensive care unit patients with suspected sepsis at very low likelihood of bacterial infection. Antimicrob Agents Chemother 59: 6494-6500.
- Barr B, Nieman NM (2022) Serum amyloid A as an aid in diagnosing sepsis in equine neonates. Equine Vet J 54:922-926.
- Hoeberg E, Sånge A, Saegerman C, Bohlin A, Nostell K, et al. Serum amyloid A as a marker to detect sepsis and predict outcome in hospitalized neonatal foals. J Vet Intern Med, 2022. 36: 2245-2253.
- Yuan H, Huang J, Lv B, Yan W, Hu G, et al. (2013) Diagnosis value of the serum amyloid A test in neonatal sepsis: a meta-analysis. Biomed Res Int 2013: 520294.
- Ucar B, Yildiz B, Aksit MA, Yarar C, Colak O, et al. (2008) Serum amyloid A, procalcitonin, tumor necrosis factor-alpha, and interleukin-1beta levels in neonatal late-onset sepsis. Mediators Inflamm 2008: 737141.
- Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, et al. (2001) Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med, 2001. 164: 396-402.
- Song J, Park DW, Moon S, Cho HJ, Park JH, et al. (2019) Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis 19: 968.
- Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, et al. (2009) A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med 37: 96-104.
- Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, et al. (2012) A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine 57: 332-336.
- Gibot S, Béné MC, Noel R, Massin F, Guy J, et al. (2012) Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med 186:65-71.
Citation: Li M, Yan-Jun Q, Xin-Liang Z, Chun-Hua Z, Rui-Juan C, et al. (2023) Evaluating the Ability of the Biomarkers to Identify the Possibility of Diagnosing Sepsis in the Enrolled Patients. Diagnos Pathol Open S13:005. DOI: 10.4172/2476-2024.8.S13.005
Copyright: © 2023 Li M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 869
- [From(publication date): 0-2023 - Dec 03, 2024]
- Breakdown by view type
- HTML page views: 800
- PDF downloads: 69