Research Article Open Access
Evaluating Quality Control of Radiotracer 18F-FDG: Experience in the United Hospital, Bangladesh
| Samiya Mahjabin1*, Tarana Mahzabin2, Zahid Hasan Mahmood1and MA Wahab3 | |
| 1Department of Electrical and Electronic Engineering, University of Dhaka, Dhaka-1200, Bangladesh | |
| 2Community Development Centre,Chittagong, Bangladesh | |
| 3United Hospital, Dhaka-1212, Bangladesh | |
| Corresponding Author : | Samiya Mahjabin Department of Electrical and Electronic Engineering University of Dhaka, Dhaka-1200, Bangladesh Tel: +88-01922827026 Email: smmahjabin@gmail.com |
| Received April 29, 2015; Accepted June 24, 2015; Published June 26, 2015 | |
| Citation: Mahjabin S, Mahzabin T, Mahmood ZH,Wahab MA (2015) Evaluating Quality Control of Radiotracer 18F-FDG: Experience in the United Hospital, Bangladesh. OMICS J Radiol 4:191. doi: 10.4172/2167-7964.1000191 | |
| Copyright: ©2015 Mahjabin S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
In the present research the quality of 18F-FDG (fluorodeoxyglucose) is maintained to reduce hazards of the patients and produce good quality of image that helps interpret the image with proper diagnosis. The quality of produced 18F-FDG in the United Hospital, Bangladesh is checked in the quality control laboratory and compared with the guideline criteria of the International Atomic Energy Agency. This 18F-FDG has been injected into 2500 patients and found no immediate or afterward hazards to any patients till now. Radionuclide purity, radiochemical purity, chemical purity, pH are assessed to have the quality assurance of 18F-FDG studied. Radionuclide purity is validated by half-life measurement and gamma ray detection. The calculated average half-life (110.26 min) and halflife 18F (109.7 min) are found in good agreement. The peak from TLC result sheet gives only the peak of unhydrolyzed FDG which implies more than 99% 18F-FDG is evident in this experiment. Chromatographic spectrum of residual solvents like Acetonitrile and Ethanol are analyzed to access the chemical purity with respect to permissible concentration. The pH of 18F-FDG is found within 4.5 to 6.5.
|
Abstract
In the present research the quality of 18F-FDG (fluorodeoxyglucose) is maintained to reduce hazards of the patients and produce good quality of image that helps interpret the image with proper diagnosis. The quality of produced 18F-FDG in the United Hospital, Bangladesh is checked in the quality control laboratory and compared with the guideline criteria of the International Atomic Energy Agency. This 18F-FDG has been injected into 2500 patients and found no immediate or afterward hazards to any patients till now. Radionuclide purity, radiochemical purity, chemical purity, pH are assessed to have the quality assurance of 18F-FDG studied. Radionuclide purity is validated by half-life measurement and gamma ray detection. The calculated average half-life (110.26 min) and half-life 18F (109.7 min) are found in good agreement. The peak from TLC result sheet gives only the peak of unhydrolyzed FDG which implies more than 99% 18F-FDG is evident in this experiment. Chromatographic spectrum of residual solvents like Acetonitrile and Ethanol are analyzed to access the chemical purity with respect to permissible concentration. The pH of 18F-FDG is found within 4.5 to 6.5.
Keywords
18F-FDG; PET/CT; Procedure of quality control
Introduction
Highly sensitive molecular imaging method positron emission tomography (PET) with 18F-FDG (fluorodeoxyglucose) specifies the exact location of active malignant sites for diagnosis, staging, planning and subsequent follow ups [1]. It has greater sensitivity and specificity for disease detection compared with CT and MR imaging [2].Also it gives better temporal and spatial resolution than SPECT [3]. Moreover, it improves delineation of target volume at both the primary and metastatic lesions, minimizes unnecessary irradiation of normal tissues and reduces the risk of local recurrence [1]. During therapy, it is used to obtain an early readout of tumor metabolic response and get reliable long term prognostic information only 3 month after the completion of therapy [4].
In Bangladesh, United Hospital has introduced for the first time the facility to produce 18F-FDG [5]. This hospital has indoor and outdoor facilities with a capacity of 450 beds, functioning 250 beds at present under 26 departments having almost all subspecialty branches. Nuclear Medicine department is equipped with 9.6 MeV MINItrace cyclotron (GE Healthcare, USA) [6] facility with standard quality control laboratory where FDG quality control is done regularly in each batch from 23rd May in 2011 .This Medical cyclotron has facility to produce 18F, 11C, 13NH3 but at present only 18F is produced with capacity of 2Ci per run with 2 run per day. The quality of this produced 18F-FDG must be ensured for getting good quality of image & trying to lessen hazard of the patients as per GMP (Good Manufacturing Practice). This technical report describes how radio nuclide purity, radio chemical purity, chemical purity, pH are checked in the United Hospital quality control laboratory and compared it with standard method. Methodology
Radionuclide purity
Radionuclide sample is not 100% pure and contains some contaminants which arise from the production process or the decay of the primary radioisotope. Radionuclide impurities increase the radiation dose received by the patient and degrade the quality of any imaging procedure performed. The presence of 18F radionuclide in sample is determined by the radionuclide purity analysis.
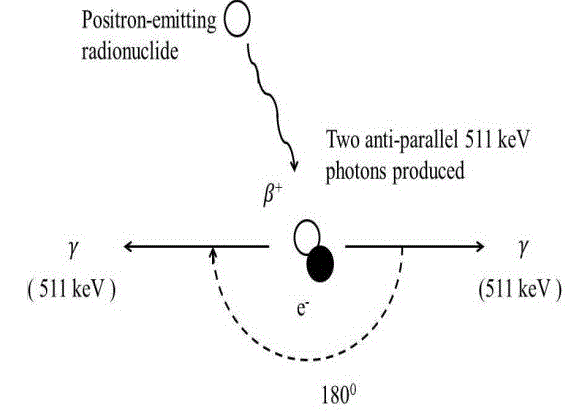
In British pharmacopeia, the presence of 18F radionuclide can be identified by half-life measurement and the gamma spectrum detection. Though first one is more reliable method, the second one reveals the percentage purity [7]. In quality control laboratory, CRC®-25PET Dose Calibrator (Capintec, USA) [8] is used for half-life measurement and Osprey™ multi-channel analyzer (MCA) with Thallium-doped sodium iodide NaI (Tl) scintillation detector (Canberra, USA) [9] is used to detect gamma spectrum. A tiny amount, <1 µl of 18F-FDG solution with dead time <15% is put in the test tube inside the Scintillation detector. Then the interaction of the emitted positron of 18F with the atoms in the crystals of the Scintillation detector emits 511 keV gamma photons as shown in figure 1. These Gamma photons are directed to the photomultiplier which converts gamma photons into electrons by photoelectric effect. After that, multiplications of electrons occur in the series of dynode. Then preamplifier produces voltage pulse from these electrons. The Multichannel Analyzer (MCA) reshapes this voltage pulse and converts this electrical signal into a digital signal [10]. The signal sent by MCA is stored, displayed & analyzed by operation computer. Dose Calibrator is used to check the radionuclide identity of 18F-FDG. A glass vial containing (<10 µl) 18F-FDG is placed in the Argon gas filled chamber of Dose Calibrator which consists of anode and cathode those are connected to an external power source. Radioactive decay of 18F-FDG produces high energy photon. Ion pairs are produced due to the interaction between high energy photons and Argon gas atoms. These ion pairs migrate towards the anode and cathode and produce current. This current is proportional to the radioactivity of 18F. When isotope selector button is pressed, the result of the division of current and activity conversion factor of 18F is found which represents the radio activity of 18F [11]. After that, we take an average value of the observed half-life of Dose calibrator which is as same as the half-life of 18F. Radiochemical Purity
In radiopharmacy, biodistribution of the radiopharmaceutical is determined by radiochemical purity. Radiochemical impurity obscures the diagnostic image obtained and renders the investigation meaningless due to its different patterns of biodistribution.
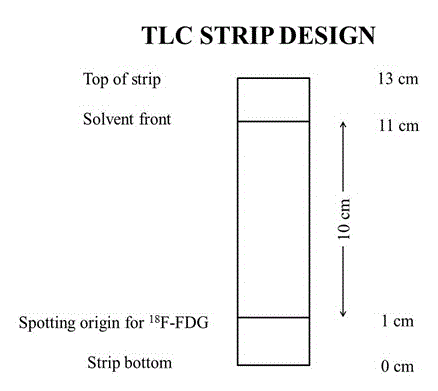
In British pharmacopeia, both thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) are accurate and reliable method to determine the radiochemical purity and radiochemical identity of 18F-FDG. Though TLC is easier, it takes longer time [7]. To check the radiochemical purity of 18F-FDG by TLC method, Peak Simple Chromatography Data System (SRI Instrument, USA) [12] is used in United Hospital quality control lab. In TLC, according to the monographs, most usually <1 μl of 18F-FDG is applied with the aid of micropipette on the TLC strip. When the solution dries, the plate is put into 95% Acetonitrile solution. The solvent migrates up the plate (usually 10 cm from the spot) as shown in figure 2. The strip is removed when the solvent reaches the front. The dried TLC strip is placed into the chromatographic ruler (platform) between two folia strips and insert the ruler into the lead shield of the detector. A slit-collimated NaI(TI) scintillation radiation detector is used to quantify the regional distribution of radioactivity on the strip. The radioactivity distribution between the start and the solvent front points is recorded and plotted as radioactive peaks by this detector. The potential radiochemical impurities such as free 18F-FDG and incompletely hydrolyzed intermediate are easily separated from the authentic 18F-FDG using silica gel thin-layer chromatography. Chemical purity
In 18F-FDG, the mannose triflate precursor is the main chemical contaminant and organic solvent but it is not analyzed in this laboratory because of its non-toxic characteristics. Acetonitrile & Ethanol are also possible chemical contaminants and to ensure the chemical purity of 18F-FDG the amount of them has been checked. 430-Gas Chromatograph ( Bruker, USA) [13] is used to check the chemical purity of 18F-FDG.
A very small amount approximately 1µl of 18F-FDG sample is injected into a hot injector port (which is covered by a rubber septum) of the GC. The temperature of the injector is higher than the components boiling points. Inside the hot injector chamber, the solvent components of 18F-FDG evaporate into the gas phase. The gaseous components of the 18F-FDG are pushed by carrier gas helium on to the GC capillary column. Sample passing rate through the column is directly proportional to the temperature of the column. The separation of the organic compounds take place between the carriers gas (the mobile phase) and the high boiling liquid (the stationary phase) within the GC capillary column. Each compound passes through a detector just before exits the GC instrument. The mixture components reach the detector at varying times due to differences in the partitioning between mobile and stationary phases. The signal is sent by this detector to the recorder through amplifier .The result with all peaks is sent into the system computer which stores, displays, and analyzes the data by using the Galaxie™ Chromatography Data Software System(Bruker, USA) [13]. pH measurement
For testing the pH value of 18F-FDG, British pharmacopeia does not specify any method [7]. pH value of 18F-FDG can be measured by using pH meter or pH paper with pH reference standards. Narrow band pH paper which is verified by standard pH buffers displays a colour change for each 0.5 pH unit and gives an approximate value of pH. However, pH meter gives specific value [14-19]. In United Hospital quality control laboratory (UHL), properly calibrated SevenMulti pH meter (Mettler- Toledo, Switzerland) and Merck pH paper both are used to check the pH. The physiological range for pH of 18F-FDG is 4.5-8.5 as per European Pharmacopoeia acceptance criteria and it is consistent from batch to batch.
Results
In United hospital quality control laboratory, the radionuclide purity, radiochemical purity, chemical purity and pH of 18F-FDG had been checked.
Radionuclide Purity
OsprayTM MCA with NaI (TI) scintillation detector [19-22] was used to check the radionuclide purity by gamma spectrum detection. The horizontal position of the peak as shown in figure 3 was determined by the gamma ray energy and the area of the peak was determined by the intensity of the gamma ray and the efficiency of the detector. 511 keV peak of gamma spectrum produced by 18F was detected in this detector. This single peak represented that 18F-FDG solution contained >99% 18F. No other significant amount of radionuclide impurity was present here.
CRC®-25PET Dose Calibrator was used to check the radionuclide identity of 18F-FDG by half-life measurement because gamma spectrum detection was not enough to confirm the presence of 18F. As per instruction from the manual supplied by the company the parameters tabulated below were measured [6] (Table 1). From the observed output of Dose Calibrator as shown in table 2, it was found that the radioactivity decreased gradually when time increased. At the initial the radioactivity of 18 F was 16.34 mCi and after 10 min it was 15.44 mCi. The average value of the observed half-life (110.26min) was nearly same as the half-life (109.7 min) of 18F which was shown in table 3. Radio chemical Purity
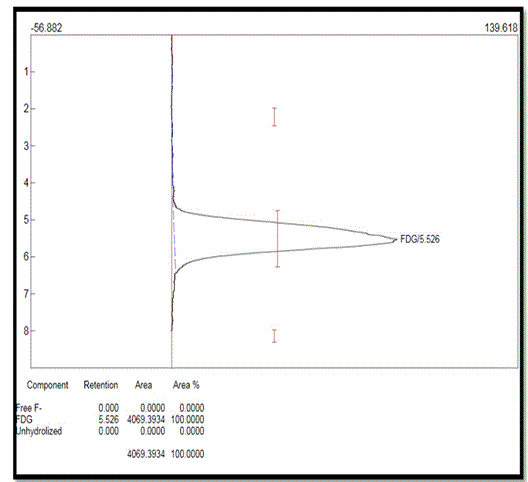
In quality control laboratory, TLC method was used to check the radio chemical purity of 18F-FDG. The retention factors of the free 18F-, 18F-FDG, and unhydrolysed 18F-FDG were about 2.0 to 2.3, 5.2 to 5.7 and 8.0 to 8.2 respectively. Retention factor (Rf) is the distance traveled by the individual components from the starting point to the solvent front. TLC result is varied according to different brands of TLC plates and operation conditions.
In figure 4, no peak was found in between 2 to 2.3 and 8 to 8.2, so the free 18F- and unhydrolysed 18F-FDG concentration were not detectable here. It was approximately found < 0.9% whereas peak in between 5.2 to 5.7 implied the presence of 18F-FDG. From the above illustration, it was found that more than 99% 18F-FDG was present in this sample. Chemical purity
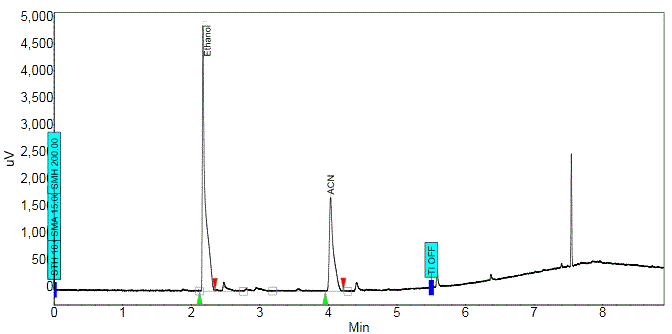
The chemical purity of 18F-FDG was checked by Gas Chromatography method in quality control laboratory. The amount of analyte, e.g., Ethanol and Acetonitrile (ACN), present in a sample was determined by the area under the peak of chromatogram.
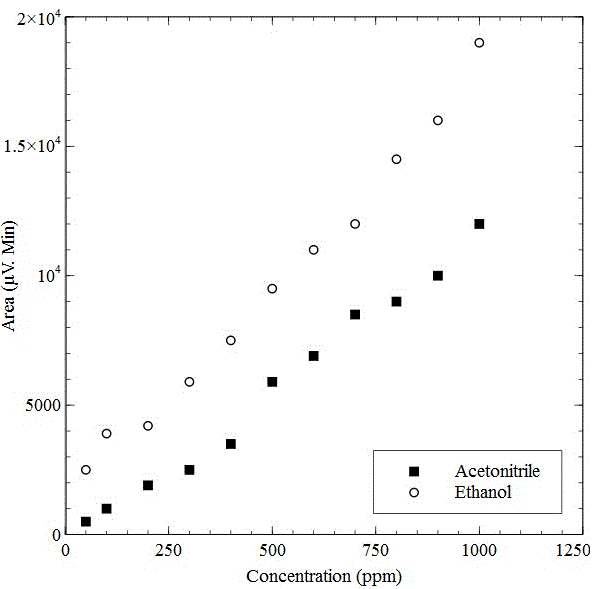
% component of the analyte had been worked out by the following formula Area = (height) x (width at ½ height) %Component 1 = [(area under peak 1)/ (total area)] x 100% From chromatogram (Figure 5), the area of residual Acetonitrile and Ethanol were found (Table 4). The calibration curve (Figure 6) of residual solvents, Acetonitrile and Ethanol, showed linear relation of area and concentration. According to the graph, when the area of residual Acetonitrile was 3500 µV.Min, the concentration was 400ppm. From the observed results of chromatographic spectrum (Table 4), the area of Acetonitrile was found 118 µV.Min which correspond very low residual concentration (<70 ppm). Same was seen in case of Ethanol (EtOH), the area of EtOH was found 235.8 µV.Min from the chromatographic spectrum (Table 4), which also correspond very low residual concentration (<50 ppm). As per European Pharmacopoeia acceptance criteria, residual solvents concentrations in 18F-FDG were given in table 5. From the above discussion, it was found that the observed residual concentration of Acetonitrile and Ethanol are within the acceptance limit. pH
Digital pH meter and narrow-band pH paper were used to measure the pH of 18F-FDG. The range of pH was found within 4.5 to 6.5.
Discussion
In radionuclide purity analysis, the outcome shows 511 keV peak that implies only presence of 18F radionuclide. In radiochemical purity analysis, only 18F-FDG is detected but the free 18F- and unhydrolysed 18F-FDG concentration are not detectable. To check the chemical purity of 18F-FDG by gas chromatogram only the amount of Ethanol and Acetonitrile has been checked. 18F-FDG contains very small amount of Kryptofix, NaOH and Mannose triflate. Due to their negligible volume and insignificant effect in human body their amount has not been checked. Bacterial Endotoxin test take more time to get its result whereas the half-life of 18F is 109.7min. For this reason, it is difficult to wait for the Bacterial Endoxin result after it is injected into the patient’s body. Also the presence of allergenic substance in 18F-FDG has not been encountered, as it is very rare case. The total production of 18F-FDG is in a sterile vial automatic sealed procedure and thus the chance of contamination is very low and still random samples weekly sending to pathological laboratory for culture and sensitivity. After, finishing the 18F-FDG quality analysis, radiopharmaceutical 18F-FDG is sent to the PET-CT injection room for patient scanning.
Conclusion
This quality control investigation found that the sample contains more than 99% 18F-FDG without any other significant impurities, if remains, it would be within acceptable limit. Thus the sample is suitable to inject into the patient’s body. No immediate or delayed side effect has been reported even after a week of injecting 18F-FDG to any patient. Epidemiological studies with clinical patients could be performed to assess the quality control of radiotracer in a greater extent.
Acknowledgement
We thank Dr. Pradip Deb, from the Department of Medical Sciences at the RMIT University, for his important comments and suggestions.
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 16529
- [From(publication date):
June-2015 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 11800
- PDF downloads : 4729
