Evaluating Endosulfan Removal in Horizontal Subsurface Flow Constructed Wetlands
Received: 03-Oct-2018 / Accepted Date: 20-Nov-2018 / Published Date: 23-Nov-2018 DOI: 10.4172/2155-6199.1000454
Keywords: Horizontal subsurface flow constructed wetland; Endosulfan removal; Inflow concentrations; Retention time; Molasses; Effective microorganisms
Introduction
Wastewater treatment in constructed wetlands (CWs) offers a sustainable, eco-friendly and economical technology than others conventional wastewater treatment system [1]. They provide physical, chemical and biological processes of removal of pollutants (Adeniran et al.). It includes processes of sedimentation, hydrolysis, adsorption, microbial degradation and direct uptake by plants. Horizontal subsurface flow CWs (HSSFCWs) are increasingly popular, in particular due to their reduced surface requirements. They are also revealed to provide different redox conditions in which pollutants get into contact with a network of aerobic, anoxic and anaerobic zones during their passage through the HSSFCWs [1]. Furthermore, they are reported to offer a high purification efficiency of organic matter including numerous pesticides [2-4]. However, studies are limited in assessing the feasibility of using HSSFCWs for removal of most of the priority pollutants given by the European Water Framework Directive at field conditions.
Endosulfan is one of the selected chlorinated pollutants prioritized by the European Water Framework Directive due to its persistent nature and many adverse effects in the environment. It has been widely used in agriculture to control pests of a wide range of crops, i.e., cereals, tea, coffee, cotton, rice, sorghum, cashew, soy, fruits and vegetables [5]. As a consequence, endosulfan is one of the most commonly detected pesticides in surface waters emanating from agricultural activities in Ethiopia [6-12]. Thus, it is of great concern, particularly because of its environmentally persistency and semivolatile nature [13] and severe neurotoxicity [14]. Thus, an effective management on its application sites is of critical importance.
Technical grade endosulfan is commercially available as a mixture, typically containing >95% of two diastereoisomers, known as α- and β- endosulfan in ratios from 2:1 to 7:3 in dependence of the technical mixture [15]. The two isomers are subject to degradation resulting in endosulfan sulfate via oxidation or endosulfan diol via hydrolysis in aquatic systems [13]. Like other persistent organic pollutants in treated effluents, levels of endosulfan isomers are usually higher than their allowed threshold [16]. The magnitude of its removal processes assumed to be influenced by different factors including pesticide inflow concentrations, retention time and availability of nutrients or activities of microbial community.
Biostimulation via nutrient supply leads to stimulating indigenous microorganisms [17] whereas bioaugmentation adds effective catabolic microbes into an indigenous microbial community [18]. Both are known to improve removal of organic pollutants in various types of CWs [18,19]. Over the last decade, many investigators have been dedicated for testing the effect of biostimulation and bioaugmentation strategies to clean various types of wastewater in different treatment systems [18,20,21]. For example, sucrose biostimulated in lab-scale CWs related with a higher removal of endosulfan isomers [19]. Molasses as biostimulants is also reported to enhance pollutants removal through the processes of bioremediation [22]. Sugar cane molasses primarily provide a carbon and energy source for microbes as well as trace elements that are required for the production of microbial enzymes involved in the mineralization of pollutants. However, sugar cane molasses as a relatively cheap biostimulant for the removal of endosulfan in CWs have not been studied.
The uses of effective microorganisms (EM) as bioaugments for better reduction of contaminants from wastewaters assumed to offer a feasible treatment options [23]. The EM product is based on the original formula developed by Higa and Parr [24] and is known as EM1. EM1 is consisted of actinomycetes, phototrophic bacteria, lactic acid bacteria and yeast tested for efficient bioremediation of various kinds of contaminants [23,25]. It is a combination of selected microorganisms about 80 species capable of producing multiple benefits for pollutants removal [25]. Certain studies show a high potential of EM for enhancing organic pollutants removal in different wastewater treatment systems [26,27]. However, there are limited studies showing bioremediation of endosulfan via bioaugmentation with effective microbial consortia such as EM1 [25]. Thus, the major aim of the present study was to determine endosulfan removal efficiencies in low-priced and easy-to-maintain HSSFCWs at various endosulfan inflow concentrations, retention times, sugar cane molasses supplementation and EM bioaugmentation at field conditions.
Materials and Methods
Description of the treatment system
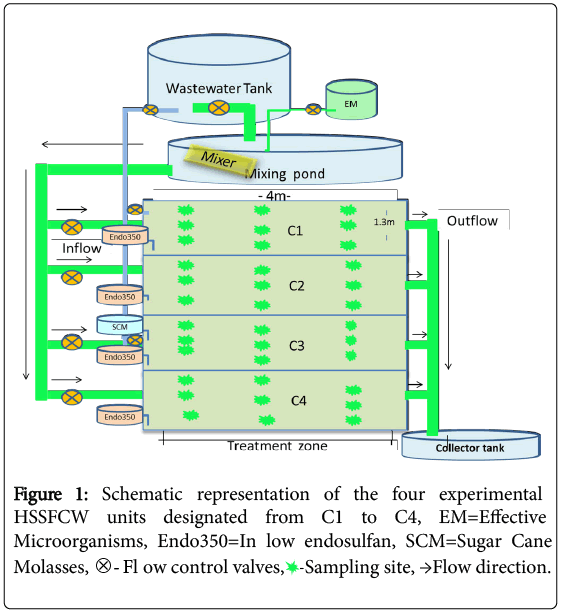
A pilot-scale experimental constructed wetland (CW) was established in a privately owned flower farm nearby Debrzeit Town, some 55 km south-east of the capital Addis Ababa, Ethiopia. The CW consisted of four replicated HSSFCW units designated as C1, C2, C3 and C4. Figure 1 represents a schematic sketch of the HSSFCW units used for this particular experiment. The surface area of each CW was 5.2 m2 (length: 4.0 m and width: 1.3 m) filled with gravels of a particle size of 2-4 mm for a depth of 0.5 m. The gravel forms the wetland media for plant anchorage, microbial biofilm formation and provides an extensive surface area for particle sedimentation. Each of the CW unit was planted with 39 Typha latifola sprout (locally adapted plants with a good performance for mitigation of agrochemical contaminated wastewater emanating from a flower farm as has been shown in a previous experiment (unpublished data). The experiment has been conducted from December 2016 to May 2017, for six months.
Experimental procedures
Endosulfan containing wastewater was prepared by mixing technical-grade endosulfan (Ensosulfan 350 EC) with the wastewater collected from the drainage of a privately owned flower farm. The wastewater was apparently stable in physicochemical compositions with the following average inflow wastewater parameters of pH: 7.78 ± 0.2, temperature: 23.1 ± 1.7°C, electrical conductivity: 0.91 ± 0.02 mS/cm, BOD5: 82.6 ± 0.9 mg/L, COD: 148.5 ± 1.5 mg/L, NH4+: 4.5 ± 0.7 mg/L, NO3- : 11.3 ± 0.9 mg/L, TP: 8.6 ± 5.5 mg/L and SO42: 44.6 ± 0.5 mg/L. The endosulfan was mixed with the wastewater in different ratios in order to get the final endosulfan inflow concentrations of 0.5, 1, 5, and 10 μg/L in the synthetic wastewater.
The effects of both initial endosulfan concentrations and retention time on endosulfan removal performance of the CW units were evaluated using four different endosulfan concentrations and retention times. The prepared four endosulfan concentrations (0.5, 1, 5, and 10 μg/L) were analogous to the range of the residual endosulfan concentrations detected in surface waters of the study area [12]. The 0.5, 1, 5, and 10 μg/L endosulfan-wastewater mixtures were allowed to enter the treatment zones of C1, C2, C3 and C4 respectively that were operating in continuous mode (Figure 1). The effects of four hydraulic retention times (4, 6, 8, and 10 days) on removal efficiency of endosulfan isomers was also evaluated for each of the four endosulfan concentrations (0.5, 1, 5, 10 μg/L). The retention time for the treatment system was adjusted by a flow controlled valve and electromagnetic flow meter that was supplied by Sensotronic System, Ahmedabad, Gujarat, India.
Furthermore, influences of addition of sugar cane molasses as biostimulate (MS) and EM as mixed bioaugment on the removal of endosulfan isomers were evaluated. A controlled experiment in C1 without MS and EM was conducted for comparison. MS were added from the supplementation tank to the wastewater containing 10 μg/L endosulfan in a ratio of molasses: wastewater 1:40 in C3, which was the lowest amount that was reported to provide effective degradation of organic compounds in Lamichhane et al. [22]. Activated EM was purchased from Wajaji private EM supplies in Debrezeit, Ethiopia. Whereas molasses was purchased from Wonji sugar factory, Ethiopia and mainly contains sucrose as a carbon and energy source for microbes as well as trace elements that are required for the production of microbial enzymes. EM was prepared by mixing 1 volume of EM to 1 volume of molasses and 18 volumes of chlorine-free water following the manufacturer’s protocol [24]. EM, molasses and water were well mixed in a clean airtight plastic container and incubated at room temperature for around 15 days by an intermittent release of the fermentation gas produced. The pH of the product shifted from initially 6.8 to 3.7 indicated the completion of the activation process. Microbial cell density was monitored by viable cell counts, which approximately yielded 5.8 × 108 CFU/ml. Finally, the prepared EM solution was added to C4 CW unit in a proportion of 1: 40 (V:V) EM solution to wastewater containing 10 μg/L endosulfan.
Sampling processes
Inflow and outflow water samples from each CW units (C1, C2, C3 and C4) were collected for analysis of the levels of physicochemical parameters and endosulfan isomers. Wetland sediments, water and plant tissues samples were also collected from the treatment zones of each CW unit to examine the fate of endosulfan in the late phase of the experiment. The samples were collected at a distance of 1, 2, and 3 m intervals (in longitudinal direction, 4 m total CW length) and at a distance of 0.3, 0.6, and 0.9 m (crosswise direction, 1.3 m width CW) (Figure 1). Water samples were collected from these 9 selected sites of the treatment zone. Similarly, sediments (fine gravels and accumulated organic matter) were collected at a depth of 25-35 cm from the plant roots of treatment zones of each CW unit which was apparently dominated by roots of the wetland plant. The plant aerial tissue of plants with similar growth conditions was also collected from the 9 selected spots of each CW units. Thereafter, the 9 sub-samples were mixed and homogenized in order to generate one composite representative samples. The samples were all run in triplicate (n=3) on interval of three days of sampling. The collected water, plant and sediment samples were kept in a cooler and immediately transported to the lab for analysis.
Endosulfan extraction and determination
The endosulfan in the water samples (1000 ml) was extracted according to the EPA Method 3510C using 60 ml of methylene chloride. The extraction procedure was repeated 6 times and concentrated to 1 ml using a Kuderna-Danish Apparatus. Sediments and chopped plant tissue samples (15 grams) were extracted according to the AOAC Official Method 2007.01 using 15 ml of 1% Acetic Acid (HOAc) in Acetonitrile (MeCN), 6 grams of Anhydrous Magnesium Sulfate, and 1.5 grams of Anhydrous Sodium Acetate via shaking for 1 minute in 50 ml centrifuge tubes and centrifugation at 4000 rpm for 1 minute. A portion of the MeCN extract (8 ml of the upper layer) was transferred into a 50 ml centrifuge tube and mixed with 400 mg of Primary Secondary Amine (PSA), 400 mg of C-18, 400 mg Graphitized Carbon Black (GCB), and 1.2 g of Magnesium Sulfate were added and then shaken for 1 minute and centrifuged at 4000 rpm for 1 minute. About 1 ml of the upper layer was transferred to a GC auto-sampler vial for analysis by Gas Chromatography/Tandem Mass Spectrometry (GC/MS). Endosulfan isomers were determined by injecting 1 μL of the sample at 250°C using an Agilent 7890 Gas Chromatograph coupled with an Agilent 7000C Triple Quadrupole Mass Spectrometer equipped with an Agilent J and W DB-1701P column (30 m × 0.25 mm × 0.25 μm). Hydrogen was used as a carrier gas and nitrogen (1.5 ml/min) as a collision gas.
Monitoring bacterial density
The microbial load of water and sediment samples was estimated using total viable plate count technique procedure as described previously [28,29]. Samples were prepared to appropriate dilutions; (sediment samples to 10-6-10-12 and water samples to 10-1 to 10-9 in normal saline solutions (0.85%), from which 0.1 ml of each was inoculated on nutrient agar plates. The cultures were incubated for 48 hours at 37°C. Upon appearance of bacterial colonies, plates with identifiable and countable colonies were selected and counted. All results obtained were expressed on a log basis per gram sediment or ml of sample water.
Data analysis
To assess treatment performance of each HSSFCW, endosulfan removal efficiencies were calculated on the basis of endosulfan concentrations in the inflow and outflow. The removal percentage was determined as shown below: where R% is removal percentage, C is the endosulfan concentration in mg/L, subscript i and o represent inflow and outflow samples, respectively.
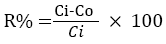
Graphical analyses were performed using Microsoft Origin, Minitab and Past software. Statistical analysis of the data was performed by using the Past software (version 2.08). Data of means with standard deviation were used in graph presentation. One-way analysis of variance (ANOVA) was used for statistically difference between means of observation. Differences in performance were considered to be statistically significant if P ≤ 0.05.
Results and Discussion
Effects of endosulfan concentrations and retention time
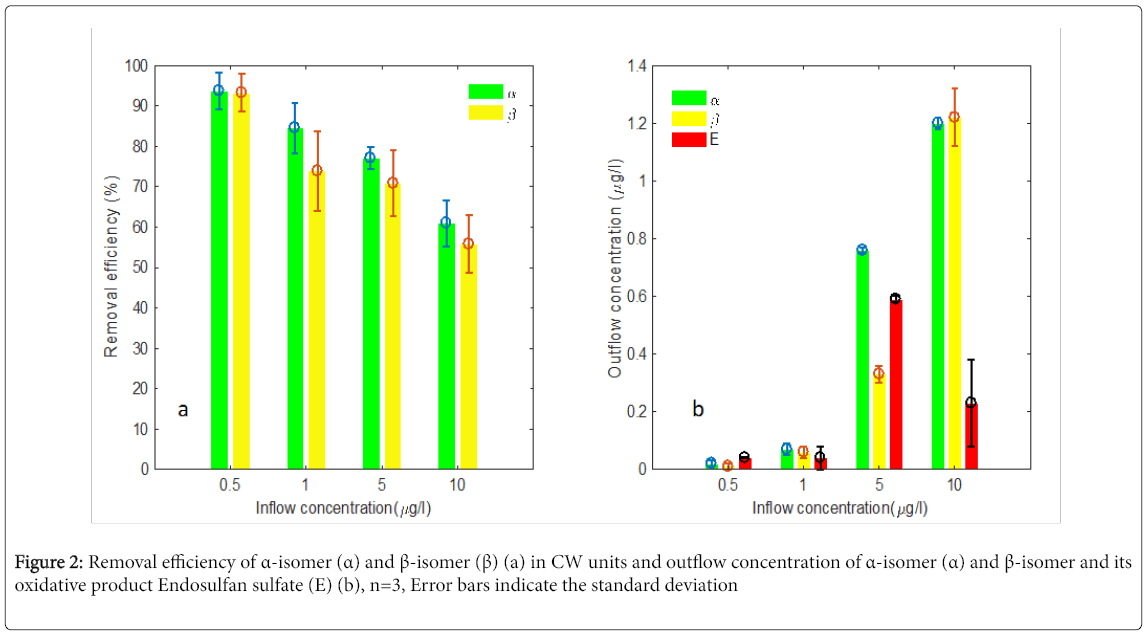
Endosulfan removal potential of Typha planted CW units with a retention time of 4 days was evaluated using 4 different concentrations (0.5, 1, 5 and 10 μg/L) of endosulfan. In average more than 90.0% of each α- and β-isomers of endosulfan was degraded when the initial endosulfan inflow concentration was 0.5 μg/L (Figure 2a). The removal efficiency was 61.0% and 55.8% for the α- and β-isomers respectively when the initial endosulfan inflow concentration was increased to 10 μg/L. The removal performance showed declining trend as feed concentration increase (Figure 2) and removal efficiency was statistically higher (P<0.05) at inflow concentration of 0.5 μg/L compared to the inflow of 1, 5 and 10 μg/L. The decrease of the removal efficiency as increase of inflow concentrations could suggest remarkable effect of inflow concentration on remediation processes in treatment zone of the CW units. In Zhao et al. [19] endosulfan removal efficiency >85% on 4 days of retention time was obtained from feed of 100 μg/L in laboratory-scale batch-operated vertical-flow CWs. The low removal of endosulfan from low concentration in our study could be the difference in treatment configuration as ours is a continuous operated horizontal CWs at field conditions.
The presence of gravel, plant roots, biofilm and organic matter complexes at anoxic conditions are reported to allow the HSSFCW to eliminate endosulfan by the hydrolysis path way [4,30]. However, detection of endosulfan sulfate in the outflow samples along with the reduction of the α- and β-isomers of endosulfan in this study implicated degradation of endosulfan by oxidation in the treatment zones of the CW units (Figures 2a and 2b). Oxygenation in the gravel beds by plant roots and diffusion of oxygen from atmosphere to inflow water may consider facilitating the observed oxidative degradation of endusulfan. Since we could not determine the endosulfan intermediates produced via hydrolysis, it remains uncertain whether oxidation represented the sole elimination pathway in this study. Though endosulfan sulfate was reported to be more stable in aqueous system [31], the relatively low amount of endosulfan sulfate detected along with a remarkable removal of the α- and β-isomers and the opposite at lower removal of endosulfan (Figures 2a and 2b) does not necessarily support a low biodegradability of endosulfan sulfate in our study.
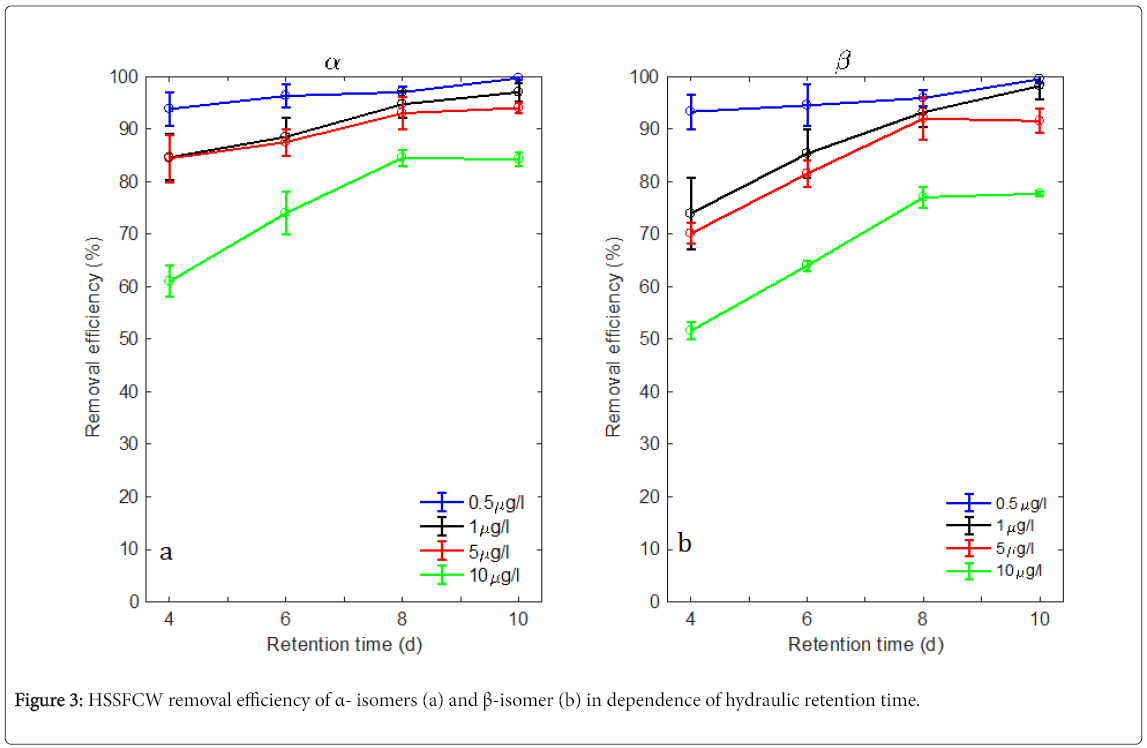
Figures 3a and 3b reveal the effects of various retention times, i.e., 4, 6, 8, and 10 days on endosulfan removal efficiency in the HSSFCW units. Extending the retention time from 4 days to 10 days, the removal of α-isomer of endosulfan increased by 5.9, 12.4, 9.6, and 23.2% for endosulfan inflow concentrations of 0.5, 1, 5, and 10 μg/L respectively (Figure 3a). A similar pattern was observed for the endosulfan β- isomer removal, which increased by 5.6, 24.3, 21.4, and 26.1%, respectively (Figure 3b). In general, an increasing in retention time resulted in an increasing trend in removal efficiencies for both α- and β-isomers. The statistical analysis also showed a significantly higher removal of both isomers at a retention time of 10 days compared to 4 days, regardless of the tested inflow concentrations. However, as the increase of endosulfan inflow concentration, the effect of retention time became more pronounced (Figures 3a and 3b). Similar trends were found in Rose et al. and Zhao et al. [19], which both showed enhanced endosulfan removal efficiencies as a result of extending in retention time in their experimental CWs. Hydraulic retention time affects the time water stays in the CWs and thus flow depth, substrate porosity and potential contact of microbial community with the pesticide. Thus, extending retention may lead to prolonged contact of endosulfan degrading wetland microbial consortia with the pesticide and may accounted for improved removal of endosulfan and its degradation product. Our results generally show that a retention time of 10 days in our HSSFCW system resulted in enhanced microbial degradation of endosulfan isomers, whereby the degradation efficiency greatly decreased with the increase in the pesticide’s incoming concentration.
Effects of bio-stimulation and bio-augmentation
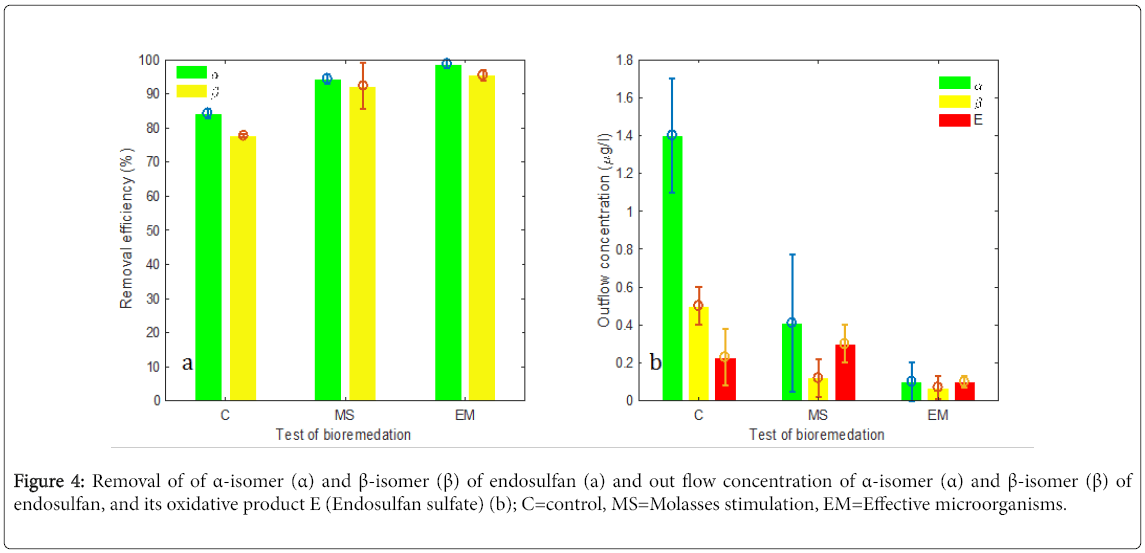
In this study, MS and EM as mixed microbial inoculums were evaluated for their bioremediation potential of endosulfan. Removal of endosulfan improved to 94.3+1.3% and 92.3+6.7% for α-and β-isomers respectively under supplementation of molasses from inflow endosulfan of 10 μg/L and a retention time of 10 days (Figure 4a). It is higher by 10.1% and 14.6% for α-and β-isomers respectively compared to removal efficiency without addition of MS (Figure 4a). This finding supports previous findings indicating that addition of an auxiliary carbon source to a treatment system significantly increases microbial biodegradation efficiency of organic pollutants [19,32-34]. MS suppose to stimulate wetland microbial consortia for increasingly consuming the organic contaminants directly as a carbon source or indirectly via co-metabolism [22,35-38]. Likewise, MS assumed to enhance endosulfan bioremediation in our study by increasing bacterial density (Figure 5) and stimulating their activities through provision of carbon and certain trace elements.
The use of EM bioaugmentation further improved the removal efficiency of endosulfan by >5.0% compared to MS at same operational conditions (Figure 4a). The increase in removal efficiency in the CW unit was also accompanied with the greatly reduced outflow concentrations of both α-and β-isomers as well as endosulfan sulfate (Figure 4b) suggesting EM-bioaugmentation could positively impact on removal efficiency of endosulfan the CW units. A similar effect of bioaugmention was observed in Zhao et al. [19], which improved removal by 5.9 and 7.6% for α- and β-endosulfan respectively compared to the corresponding control. However, differences of CWs design, endosulfan concentration and bioaugments may account for a higher removal of α- and β-endosulfan by more than 15.0% and 19.0% compared to the corresponding control in our study.
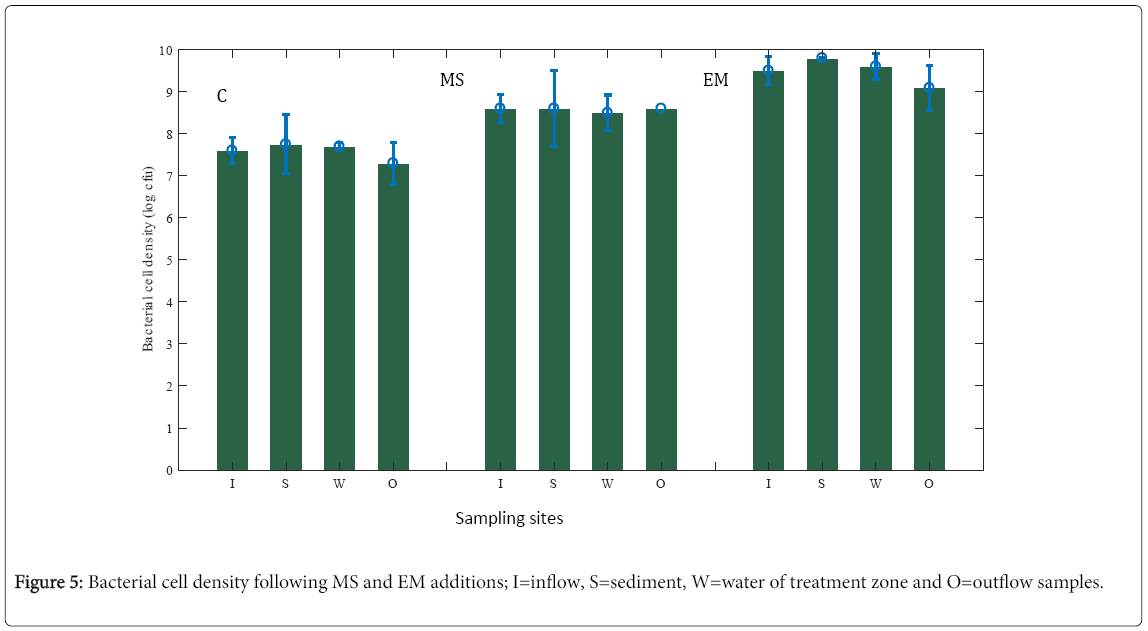
In this study, the removal efficiency of endosulfan was also paralleled with wetland bacterial density as determined by viable cell counts (Figure 5). The higher removal efficiency of endosulfan linked with the higher bacterial density following EM-bioaugmentation (Figure 5). On the other hand, the lower endosulfan removal efficiency correspond to the lower bacterial density in non-MS and EMbioaugemented CW units (Figure 5) suggest wetland microbial density as important aspect in transformation of endosulfan. Due to their dynamic and inducible complex enzymatic systems which enable the efficient degradation of xenobiotics, wetland microbial consortia along with the EM-bioaugment assumed to play a key role in endosulfan removal. The bacterial cell density in our CW units was similar to the result reported in Lananan et al. [25] and ranged between 5.24 × 106 and 9.14 × 109 CFU/mL. In this study, the observed higher bacterial density (but insignificant number of fungi) in the EM-bioagmentation may not only result in a higher degradation of α- and β-isomers, but also of their oxidation product endosulfan sulfate. Thus, it verifies the potential importance of EM-bioaugment for successful bioremediation of residual endosulfan in wastewater emanating from farm when using in continuous operating HSSFCW system at field conditions.
In general, previous studies indicate that both biostimulation and bioaugmentation improve the removal of various organic pollutants by 0–12% as compared to the respective control treatments [19,39-41]. Similarly, MS and EM bioagmentation greatly improved the endosulfan removal efficiency by >10 and 15% respectively in this study which may be due to difference of treatment conditions. In our study, the average levels endosulfan isomers in outflow were also reduced to a levels close to the permitted discharge limit of 0.1 μg/L (Figure 4b), through the application of EM- bioagmentation. Thus, our result verifies the potential importance of sugar cane molasses and EM-bioaugment for successful bioremediation of residual endosulfan in wastewater emanating from agricultural activities when using in continuous operating HSSFCW system at field conditions.
Mass distribution of endosulfan in the CW units
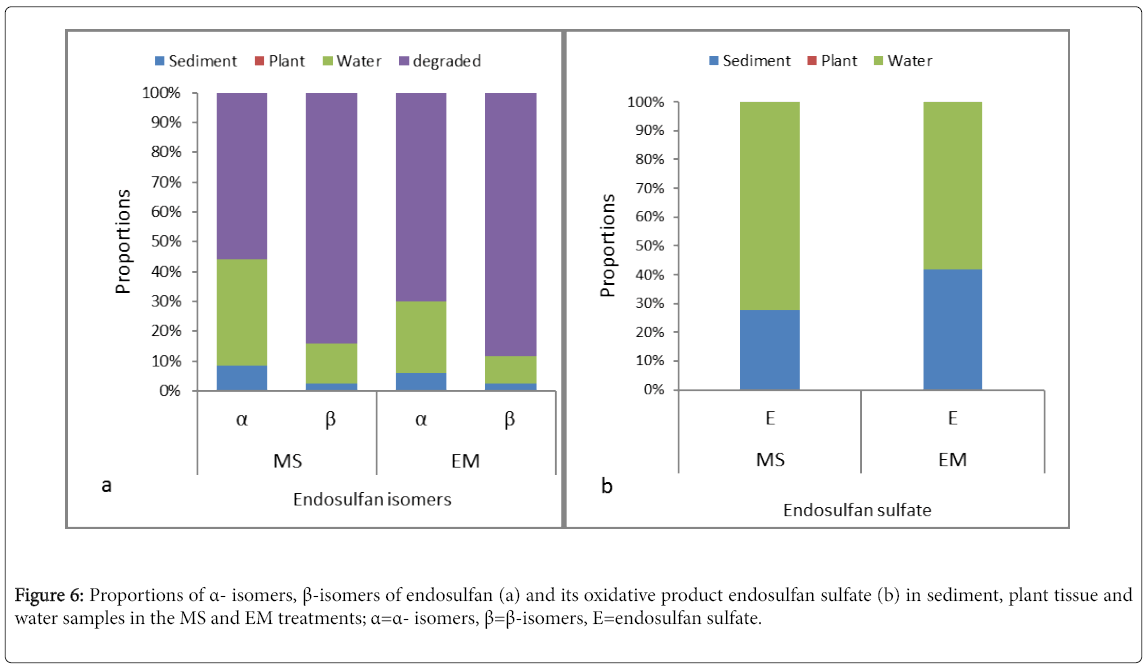
To determine the fate of endosulfan isomers in the CW units, a mass proportion analysis was conducted by using water, plant and sediment samples from the treatment zones of C3 and C4, which showed relatively better endosulfan remova Endosulfan is reported commonly to be absorbed by plants, accumulate by plant tissues and is released via evapotranspiration [19,42]. However, none of the endosulfan isomers were detected in plant samples in the present study and thus, the effect of phytoremediation was not clear. On the other hand, different proportions of endosulfan isomers were detected from sediments and water samples of the CW units (Figure 6a). In average, reduced percentages of incoming α- and β-endosulfan (<8.5% and <2.5% in sediment and <36% and <13.5% in water samples, respectively) were found in the treatment zone of the CW units. The low detection of pollutants such as endosulfan in the CW units’ effluent accompanied with low concentrations in the gravel bed of the CW units suggested that biodegradation represents the major elimination pathways for the pollutants [4]. Furthermore, the detection of endosulfan sulfate (Figure 6b) paralleled with low proportions of the α- and β-isomers of endosulfan could support biotransformation of endosulfan in the treatment zone of the HSSFCW units in this study.
Conclusions
This study investigated removal of endosulfan isomers from wastewater in low-priced HSSFCWs. Thereby, the removal efficiency of endosulfan isomers was found to be influenced by inflow endosulfan concentration. Extending retention times also led to a greatly improved removal of endosulfan isomers, regardless of the inflow concentrations. Furthermore, our results confirmed the positive effects of MS biostimulation and EM bioaugmentation for bioremediation of endosulfan isomers. The use of EM as bioaugmente lead to the removal of endosulfan isomers close to the discharging limit of 0.1 μg/L from 10 μg/L inflow endosulfan at a retention time of 10 days. The improved removal efficiency was linked with increased bacterial densities in the treatment zone of the HSSFCW units, particularly after EM addition. The reduction of endosulfan α- and β-isomers with detection of endosulfan and without any accumulation of plant tissues and a low substrate sorption suggests that biotransformation represents the main removal pathway for endosulfan in the treatment zone of the CW units. In general, our results revealed a high potential of a continuous operating HSSFCWs for bioremediation of residual endosulfan emanating from agricultural activities, in particular when used in combination with MS and EM-bioaugmentation.
Acknowledgements
The author would like to acknowledge the financial support provided by the Ethiopian Horticulture Producers and Exporters Association (EHPEA), Addis Ababa, Ethiopia.
References
- Vymazal J, Brˇezinová T (2015) The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ Int 75: 11-20.
- Agudeloa RM, Gustavo PG, Aguirrec NJ, Moratód J, Jaramilloa ML (2010) Simultaneous removal of chlorpyrifos and dissolved organic carbon using horizontal sub-surface flow pilot wetlands. Ecol Engin 36: 1401-1408.
- Borges AC, Calijuri MC, Matos AT, Maria Eliana Lope ME, Ribeiro de Queiroz R (2009) Horizontal subsurface flow constructed wetlands for mitigation of ametryn-contaminated water. Wat SA 35: 1816-7950.
- Matamoros V, Puigagut J, Grace J, Bayona JM (2007) Behavior of selected priority organic pollutants in horizontal subsurface flow constructed wetlands: A preliminary screening. Chemosphere 69: 1374-1380.
- Mengistie BT, Mol APJ, Oosterveer P (2016) Pesticide use practices in the central rift valley of Ethiopia. Environ Dev Sustain 19: 301-324.
- Hanssen VM, Nigatu AW, Zeleke ZK, Moen BE, Bråtveit M (2015) High prevalence of respiratory and dermal symptoms among Ethiopian flower farm workers. Arch Environ Occup Health 70: 204-213.
- Hiluf GK, Ayalew A (2015) Assessment of pesticide use, practice and environmental effects on the small holder farmers in the north shoa zone of Amhara National Regional State of Ethiopia. J Res Agri Environ Sci 2: 16-24.
- Nigatu AW, Bråtveit M, Moen BE (2016) Self-reported acute pesticide intoxications in Ethiopia. BMC Public Health 16: 1-8.
- Shemsu (2016) Assessments of pesticide use and practice in Bule Hora Districts of Ethiopia. Haya: Saudi J Life Sci 1: 103-108.
- Teklu BM, Adriaanse PI, Ter Horst MMS, Deneer JW, Van den Brink PJ (2015) Surface water risk assessment of pesticides in Ethiopia. Sci Total Environ 508: 566-574.
- Teklu BM, Hailu A, Wiegant DA, Scholten BS, Van den Brink PJ (2016) Impacts of nutrients and pesticides from small and large scale agriculture on the water quality of Lake Ziway, Ethiopia. Environ Sci Pollut Res Int 25: 13207-13216.
- Teklu BM, Adriaanse PI, Van den Brink PJ (2016) Monitoring and risk assessment of pesticides in irrigation systems in Debra Zeit, Ethiopia. Chemosphere 161: 280-291.
- Weber J, Halsall CJ, Muir D, Teixeira C, Small J, et al. (2010) Endosulfan, a global pesticide: A review of its fate in the environment and occurrence in the Arctic. Sci Total Environ 408: 2966-2984.
- Silva MH, Beauvais SL (2010) Human health risk assessment of endosulfan: toxicology and hazard identification. Regul Toxicol Pharmacol 56: 4-17.
- Herrmann M (2002) Preliminary risk profile of endosulfan. Berlin Germany: Umweltbundesamt.
- Gregoire C, Elsaesser D, Huguenot D, Lange J, Lebeau T, et al. (2009) Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems. Environ Chem Lett 7: 205-231.
- Garcia-Blanco S, Venosa AD, Suidan MT, Lee K, Cobanli S, et al. (2007) Biostimulation for the treatment of an oil-contaminated coastal salt marsh. Biodegradation 18: 1-15.
- Nzila A, Razzak SA, Zhu J (2016) Bioaugmentation: An Emerging Strategy of Industrial Wastewater Treatment for Reuse and Discharge. Int J Environ Res Public Health 13: 1-20.
- Zhao C, Xie H, Mu Y, Xu X, Zhang J, et al. (2014) Bioremediation of endosulfan in laboratory-scale constructed wetlands: effect of bioaugmentation and biostimulation. Environ Sci Pollut Res 21: 12827-12835.
- Fahrenfeld N, Zoeckler J, Widdowson MA, Pruden A (2013) Effect of biostimulants on 2,4,6-trinitrotoluene (TNT) degradation and bacterial community composition in contaminated aquifer sediment enrichments. Biodegradation 24: 179-190.
- Romero-Aguilar M, Tovar-Sánchez E, Sánchez-Salinas E, Mussali-Galante P, Sánchez-Meza JC, et al. (2014) Penicillium sp. as an organism that degrades endosulfan and reduces its genotoxic effects. SpringerPlus 3: 536.
- Lamichhane KM (2012) Molasses enhanced phyto and bioremediation treatability study of explosives contaminated Hawaiian soils. J Hazard Mate 243: 334-339.
- Monica S, Karthik L, Mythili S, Athiavelu A (2011) Formulation of Effective Microbial Consortia and its Application or Sewage Treatment. J Microbial Biochem Technol 3: 051-055.
- Higa T, Parr JF (1994) Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment. International Nature Farming Research Center Atami, Japan.
- Lananan F, Hamid SHA, Din WNS, Ali N, Khatoon H, et al. (2014) Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int Biodeter Biodegr 95: 127-134.
- Mgana SM, Mbuligwe SE, Kassenga GR, Mohamed SM (2013) Treatment of tropical domestic wastewater by USASB reactor inoculated with effective microorganisms consortium - cod and suspended solids removal. Proceedings of the 13th International Conference of Environmental Science and Technology, Athens, Greece.
- Rashed EM, Massoud M (2015) The effect of effective microorganisms (EM) on EBPR in modified contact stabilization system. J HBRC 11: 384-392.
- APHA (2012) Standard Methods for the Examination of Water and Wastewater. 22nd edn.
- Calheiros CSC, Duque AF, Moura A, Henriques IS, Correia A, et al. (2009) Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour Technol 100: 3228-3235.
- Braeckevelt M, Rokadia H, Imfeld G, Stelzer N, Paschke H, et al. (2007) Assessment of in situ biodegradation of monochlorobenzene in contaminated groundwater treated in a constructed wetland. Environ Pollut 148: 428-437.
- Kong L, Zhu S, Zhu L, Xie H, Su K, et al. (2013) Biodegradation of organochlorine pesticide endosulfan by bacterial strain Alcaligenes faecalis JBW4. J Environ Sci 25: 2257-2264.
- Goswami S, Singh DK (2009) Biodegradation of α and β endosulfan in brothmedium and soil microcosm by bacterial strain Bordetella sp. B9. Biodegradation 20: 199-207.
- Kumar M, Philip L (2006) Bioremediation of endosulfan contaminated soil and water–Optimization of operating conditions in laboratory scale reactors. J Hazard Mate 36: 354-364.
- Meng P, Pei H, Hu W, Shao Y, Li Z (2014) How to increase microbial degradation in constructed wetlands: influencing factors and improvement measures. Bioresour Technol 157: 316-326.
- Abraham J, Silambarasan S (2014) Biomineralization and formulation of endosulfan degrading bacterial and fungal consortiums. Pesti Biochem Physiol 116: 24-31.
- Kumar K, Devi SS, Krishnamurthi K, Kanade GS, Chakrabarti T (2007) Environment and isolation of endosulfan degrading and detoxifying bacteria. Chemotherapy 68: 479-488.
- Semrany S, Favier L, Djelal H, Taha S, Amrane A (2012) Bioaugmentation: possible solution in the treatment of Bio-Refractory Organic Compounds (Bio-ROCs). J Biochem Eng 69: 75-86.
- Singh NS, Singh DK (2011) Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium. Biodegradation 22: 845-857.
- Calvin NN, Onyedika NC, EmmanueL GC, Nkiruka IB (2017) Biodegradation of endosulfan by mixed bacteria culture strains of Pseudomonas aeruginosa and Staphylococcus aureus. Sci Worl J 12: 9-12.
- Taccari M, Milanovic V, Comitini F, Casucci C, Ciani M (2012) Effects of biostimulation and bioaugmentation on diesel removal and bacterial community. Int Biodeter Biodegr 66: 39-46.
- Wu Z, Dong H, Zou L, Lu D, Liu Z (2011) Enriched microbial community in bioaugmentation of petroleum-contaminated soil in the presence of wheat straw. Appl Biochem Biotechnol 164: 1071-1082.
- Hwang JI, Lee SE, Kim JE (2015) Plant uptake and distribution of endosulfan and its sulfate metabolite persisted in soil. PLoS ONE 10: e0141728.
Citation: Meaza M, Grossart HP, Ashagrie H, Assefa F (2018) Evaluating Endosulfan Removal in Horizontal Subsurface Flow Constructed Wetlands. J Bioremediat Biodegrad 9:454. DOI: 10.4172/2155-6199.1000454
Copyright: © 2018 Meaza M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3780
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2961
- PDF downloads: 819

 - Fl ow control valves,
- Fl ow control valves, 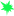 -Sampling site, →Flow direction.
-Sampling site, →Flow direction.