Etiology Model for Elevated Histamine Levels Driving High Reactogenicity Vaccines (including COVID-19) Associated Menstrual Adverse Events
Received: 19-Apr-2022 / Manuscript No. JIDT-22-55132 / Editor assigned: 21-Apr-2022 / PreQC No. JIDT-22-55132 (PQ) / Reviewed: 05-May-2022 / QC No. JIDT-22-61286 / Revised: 10-May-2022 / Manuscript No. JIDT-22-61286 (R) / Published Date: 17-May-2022
Abstract
Introduction: Some women are experiencing menstrual changes, including altered menstrual duration, volume (heavier bleeding), increased dysmenorrhea, and worsened Premenstrual Syndrome (PMS) following Coronavirus disease 2019 (COVID-19) spike vaccinations. Appreciation of these as temporal adverse events associated with COVID-19 spike vaccinations was slow to develop. The etiology of these menstrual adverse events associated with COVID-19 spike vaccination remains unknown.
Methods: The United States Department of Health and Human Services Vaccine Adverse Event Reporting System (VAERS) database was data mined for data reported adverse events affecting menstrual cycles by vaccine type.
Hypothesis: This article proposes the hypothesis that vaccinations can induce a temporary surge in histamine levels immediately post vaccination as part of the innate immune response. Increasing histamine levels is known to increase estrogen levels. Further, it is proposed that this temporary surge in histamine levels causes temporary Histamine Intolerance in some women and causes the menstrual adverse events temporally associated with vaccinations.
Conclusion: Prophylactic and therapeutic treatment of vaccines with diamine oxidase and/or specific antihistamines may reduce the incidence rate and/or severity of menstrual adverse events associated with vaccines with high reactogenicity, including SARS-CoV-2 vaccines and boosters. This model predicts menstrual associated adverse events incidence levels correspond to vaccine reactogenicity.
Keywords: Histamine; Menstruation; Menstrual cycle; Women’s health; mRNA vaccines; COVID-19 vaccine
Abbreviations
COVID-19: Coronavirus Disease 2019; DAO: Diamine Oxidase; HIT: Histamine Intolerance; HPV: Human Papillomavirus; HNMT: Histamine N4-Methyltransferase; LNP: Lipid Nanoparticle; MHRA: Medicines & Healthcare Products Regulatory Agency; NIH: National Institutes of Health; PMS: Premenstrual Syndrome; VAERS: Vaccine Adverse Event Reporting System.
Introduction
In following reports [1,2], some women were experiencing adverse events unexpected vaginal bleeding/intermenstrual bleeding, changes in periods, heavy menstrual bleeding, missed menstruation, irregular menstruation, delayed menstruation, unusual menstrual discomfort, and painful periods (dysmenorrhoea) post COVID-19 spike vaccinations, the United States National Institutes of Health (NIH) funded studies to assess potential effects of COVID-19 vaccination on menstruation. As of April 6, 2022 the United Kingdom Medicines & Healthcare products Regulatory Agency (MHRA) Yellow Card system had 50,916 suspected reactions in 39,670 reports relating to a variety of menstrual disorders including heavier than usual periods, delayed periods, and unexpected vaginal bleeding. Online surveys report 0.98% (Pfizer-BioNTech, N=1,846) and 0.68% (ChAdOx1, N=1,028) [3], 39.4% (N=17,455) [4], 50.9% (N=731) [5], and 66.3% (N=2,269) [6] reporting menstrual changes; in the last survey, the majority of the symptoms resolved within 2 months post-vaccination [6]. A second study reported that half the cases of menstrual irregularities self-resolved within two months [7]. A study of 155 women reported 78% experienced changes in their menstrual cycles [8]. A study of 164 women report menstrual cycle irregularities for 50%-60% following first vaccine dose and 60%- 70% following second vaccine dose [7]. A retrospective study of 3,959 individuals (2,403 vaccinated) characterized that COVID-19 vaccines were associated with less than 1-day change in cycle length but not menses length for both vaccine-dose cycles [9]. A study of 348 women found 4.3% experiencing long-term menstrual disturbances post- COVID-19 vaccination [10]. Any connections between COVID-19 spike vaccinations and disruption of menstrual cycles is unknown. Menstrual abnormalities have previously been reported following Hepatitis B vaccination [11]. No causal association between Human Papilloma Virus (HPV) vaccines and reported menstrual symptoms were found (N=29,846) [12].
The innate immune system responds immediately to vaccinations. Activation of granulocytes and mast cells that release inflammatory molecules including histamine is part of the normal immune response. Histamine exerts its effects primarily by binding to G protein-coupled histamine receptors, designated H1, H2, H3, and H4 (reviewed [13]). Individuals have a threshold over which elevated histamine levels trigger Histamine Intolerance (HIT) syndrome. Drugs [14], foods (cocoa, spinach, tomatoes, beer, wine, cheeses, meat, soy, yogurt, fermented foods, etc. [14,15]), gastrointestinal micro biome [14], and stage of menstrual cycle [14] all affect an individual’s histamine tolerance/intolerance threshold. Histamine is inactivated by Diamine Oxidase (DAO) or Histamine N4-MethylTransferase (HNMT). DAO is expressed in liver hepatocytes, kidney proximal tubular cells [16], digestive tract, and placenta. HNMT is expressed in multiple cell types [15,16]. DAO and HNMT alleles and expression levels may affect rates of histamine metabolism [17]. Low serum DAO activity is associated with histamine intolerance [18,19]. Increases in histamine levels are known to elevated estrogen levels [20] and may play a role in dysmenorrhea [20].
Working hypothesis
This article proposes the hypothesis that elevated histamine levels, from innate immune system response to vaccination, because the menstrual adverse events temporally associated with vaccinations experienced by some female vaccines. This model predicts incidence levels of these menstrual adverse events correlate with vaccine reactogenicity.
Methodology
The Vaccine Adverse Event Reporting System (VAERS) database was data mined for data on reported adverse events affecting menstrual cycles by vaccine type. Adverse event reports of amenorrhoea, dysmenorrhoea, heavy menstrual bleeding, intermenstrual bleeding, menstrual discomfort, menstrual disorder, menstruation delayed, menstruation irregular, oligomenorrhoea, polymenorrhoea, postmenopausal haemorrhage, premenstrual syndrome, premenstrual pain, and vaginal haemorrhage were extracted. The downloaded data include all adverse events reported from 1990 to April 1, 2022. A Ruby program named vaers_slice.rb was developed to tally selected reported vaccine adverse events by vaccine and day of onset. The vaers_slice.rb program takes as input a list of one or more symptoms to summarize and the yearly VAERS Symptoms, Vax, and Data files from 1990 to 2022. The output from vaers_slice.rb consists of five reports: summaries by vaccine, summaries by age of onset of symptoms, summaries by day of onset of symptoms, and two summaries of additional symptoms reported (selected symptoms and all other symptoms). The vaers_slice. rb program can summarize symptoms by either vaccine type or by vaccine name. Microsoft Excel was used create (Figure 1).
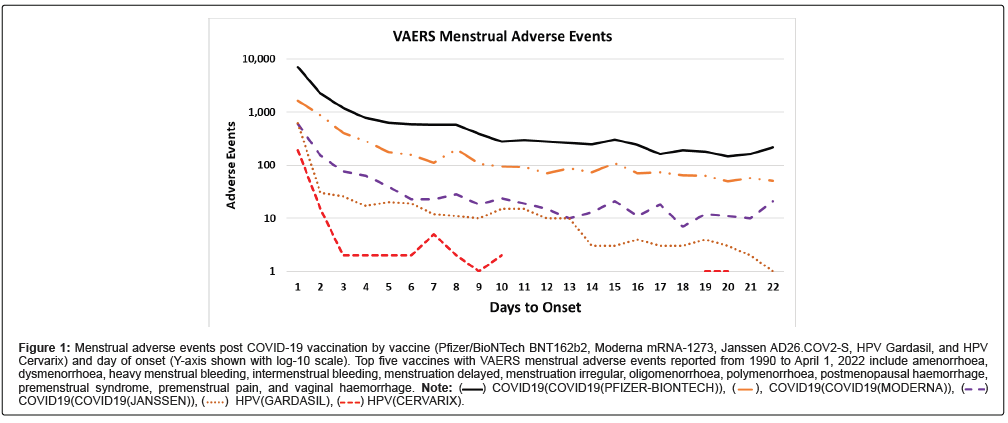
Figure 1: Menstrual adverse events post COVID-19 vaccination by vaccine (Pfizer/BioNTech BNT162b2, Moderna mRNA-1273, Janssen AD26.COV2-S, HPV Gardasil, and HPV Cervarix) and day of onset (Y-axis shown with log-10 scale). Top five vaccines with VAERS menstrual adverse events reported from 1990 to April 1, 2022 include amenorrhoea, dysmenorrhoea, heavy menstrual bleeding, intermenstrual bleeding, menstruation delayed, menstruation irregular, oligomenorrhoea, polymenorrhoea, postmenopausal haemorrhage, premenstrual syndrome, premenstrual pain, and vaginal haemorrhage. Note: COVID19(COVID19(PFIZER-BIONTECH)),
COVID19(COVID19(PFIZER-BIONTECH)),  COVID19(COVID19(MODERNA)),
COVID19(COVID19(MODERNA)), COVID19(COVID19(JANSSEN)),
COVID19(COVID19(JANSSEN)), 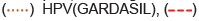 HPV(CERVARIX).
HPV(CERVARIX).
Results
The majority of all menstrual adverse events in VAERS from 1990 to April 1, 2022 are summarized in Table 1. The three vaccines with the largest number of reported adverse events are shown for each reported adverse event description; these vaccines are COVID-19 spike vaccines, and two HPV vaccines (Gardasil and Cervarix). The COVID-19 adverse events ranged from 77.5% for “Amenorrhoea” across all vaccines to as high as 99.1% for “Postmenopausal haemorrhage” and 99.0% for “Intermenstrual bleeding”. The majority of the reported COVID-19 menstrual adverse events reports have immediate onset (Figure 1) for all doses of Moderna mRNA-1273, Pfizer/BioNTech BNT162b2, Janssen Ad26.COV2-S spike, and both HPV (Gardasil and Cervarix) vaccines. The co-occurrences of frequently reported menstrual symptoms for all vaccines reported in VAERS are summarized in Table 2. Other commonly reported symptoms include fatigue, headache, pain, nausea, muscle spasms, thrombosis, pyrexia, and pain in extremity, and haemorrhage (supplemental data, Symptoms table). The vaers_slice. rb reports used in this study are provided in the supplemental data as individual Excel worksheets.
| VAERS Symptom | COVID-19 | HPV (Gardasil) | HPV (Cervarix) | All Vaccines | % COVID-19 |
|---|---|---|---|---|---|
| Heavy menstrual bleeding | 10,578 | 7 | 10,694 | 98.9% | |
| Menstruation irregular | 6,380 | 263 | 118 | 6,954 | 91.7% |
| Dysmenorrhoea | 5,492 | 161 | 97 | 5,869 | 93.6% |
| Menstrual disorder | 4,888 | 188 | 65 | 5,321 | 91.9% |
| Menstruation delayed | 3,409 | 106 | 4 | 3,579 | 95.3% |
| Vaginal haemorrhage | 3,056 | 106 | 29 | 3,598 | 84.9% |
| Intermenstrual bleeding | 2,800 | 2,827 | 99.0% | ||
| Postmenopausal haemorrhage | 1,939 | 2 | 1,956 | 99.1% | |
| Amenorrhoea | 1,617 | 243 | 28 | 2,087 | 77.5% |
| Polymenorrhoea | 1,503 | 15 | 3 | 1,537 | 97.8% |
| Oligomenorrhoea | 946 | 18 | 4 | 993 | 95.3% |
| Premenstrual syndrome | 360 | 4 | 2 | 372 | 96.8% |
| Premenstrual pain | 170 | 173 | 98.3% | ||
| Menstrual discomfort | 85 | 2 | 1 | 91 | 93.4% |
| Menstrual total | 43,223 | 1,113 | 353 | 46,051 | 93.9% |
Table 1: VAERS Menstrual symptoms for COVID-19 (Pfizer/BioNTech BNT162b2, Moderna mRNA-1273, and Janssen AD26.COV2-S), human papillomavirus vaccines (Gardasil and Cervarix), and all VAERS vaccines reported from 1990 to April 1, 2022.
| Adverse event | Amenorrhoea | Dysmenorrhoea | Heavy menstrual bleeding | Hypomenorrhoea | Intermenstrual bleeding | Menstrual disorder | Menstruation delayed | Menstruation irregular | Oligomenorrhoea | Polymenorrhoea |
|---|---|---|---|---|---|---|---|---|---|---|
| Dysmenorrhoea | 89 | |||||||||
| Heavy menstrual bleeding | 158 | 2,937 | ||||||||
| Hypomenorrhoea | 45 | 122 | 151 | |||||||
| Intermenstrual bleeding | 63 | 446 | 734 | 66 | ||||||
| Menstrual disorder | 152 | 897 | 1,594 | 78 | 379 | |||||
| Menstruation delayed | 102 | 559 | 753 | 101 | 157 | 282 | ||||
| Menstruation irregular | 164 | 1,194 | 2,319 | 135 | 507 | 447 | 368 | |||
| Oligomenorrhoea | 21 | 144 | 351 | 12 | 70 | 194 | 53 | 150 | ||
| Polymenorrhoea | 23 | 310 | 645 | 28 | 103 | 195 | 49 | 242 | 28 | |
| Vaginal haemorrhage | 30 | 181 | 332 | 19 | 128 | 155 | 62 | 197 | 21 | 44 |
Table 2: Co-occurrences of reported menstrual symptoms for all VAERS vaccines (predominately COVID-19) reported from 1990 to April 1, 2022.
Discussion
Histamine is known to play a role in the menstrual cycle [15]. This article advances the hypothesis that elevated histamine levels, inducing temporary Histamine Intolerance, causes reported impacts on the menstrual cycle for some women within days post vaccination. Figure 1 shows that the majority of the reported adverse events coincide in time when the histamine levels are predicted to be elevated (i.e., immediately following COVID-19 spike vaccination). The number of these reported menstrual adverse events associated with COVID-19 spike vaccinations significantly exceeds that of all other vaccines reported since 1990, (Table 1). Histamine may be the cause of dysmenorrhea (menstrual pain) associated with decreases in DAO levels at the start of menstruation [21]. Support for the hypothesis that vaccines are inducing elevated histamine levels in affected women can be obtained by measuring histamine and metabolite levels before and after vaccination in clinical studies.
The etiology of long-term menstrual disturbances may be different or additional driving components including extended expression of mRNA or adenovirus encoded Spike proteins. Possible bio distribution of some Lipid Nanoparticles (LNPs) carrying mRNA encoded Spike proteins to ovaries [22] may contribute to these adverse events. Spike protein expression has been proposed to activate histamine release from mast cells or other granulocytes [23]. Ongoing histamine release from mast cells near ovaries could contribute to long-term menstrual adverse events consistent with this histamine etiology model.
This model proposes that elevated histamine is triggering the reported vaccine reactogenicity menstrual adverse events. The phase or status of the menstrual cycle an individual is in when vaccinated may affect the occurrence of menstrual adverse events. The VAERS system also allows reporting of multiple menstrual adverse event symptoms for individuals. Heavy menstrual bleeding is most frequently reported along with dysmenorrhea, menstruation irregular and menstrual disorder (Table 2). Reports of fatigue, headache, and other symptoms are also consistent with the proposed model of vaccine reactogenicity adverse events associated with elevated histamine levels [15,24].
Candidate treatments suggested by elevated histamine model
Evaluation of candidate treatments with the potential to lessen or reduce the number of adverse events can be obtained from case series, and cohort studies of candidate antihistamines and/or DAO treatment prophylactically prior to and post COVID-19 spike vaccination or booster vaccination. Treatment duration could be aligned with observed duration of elevated histamine levels with a predicted treatment course of several days. These clinical results could support the justification for randomized clinical trial(s) of these candidate treatments. Antihistamines have been used to treat dysmenorrhea [25]. For COVID-19 patients, specific H1 and H2 antihistamines are showing efficacy with doses aligned with targeting immune cells [26,27]. DAO and these antihistamines and dosages are potential candidates for initial evaluations: cetirizine (H1) [28,29], dexchlorpheniramine (H1)[29], and high dose famotidine (H2) [26].
Conclusion
The some women are experiencing menstrual adverse events post COVID-19 spike vaccination. Herein, this article proposes that these vaccines associated adverse events, occurring within days of vaccination, are caused by elevated histamine levels from immediate innate immune responses to high reactogenicity vaccines. This hypothesis suggests concurrent treatment with specific antihistamines and/or DAO for several days may lower the incidence rate, reduce severity, or adverse event duration post vaccination with COVID-19 spike vaccines, COVID-19 boosters, and other vaccines with higher reactogenicity.
Consent Statement/Ethical Approval
Not required.
Acknowledgements
None.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authorship
The author attests they meet the ICMJE criteria for authorship.
References
- Male V (2021) Menstrual changes after COVID-19 vaccination. BMJ 374: n2211.
- Kurdoğlu Z (2021) Do the COVID-19 Vaccines Cause Menstrual Irregularities? Int J Womens Health Reprod Sci 9: 158-159.
[Crossref] [Google Scholar] [PubMed]
- Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS (2021) BNT162B2 and chadox1 sars-cov-2 post-vaccination side-effects among saudi vaccinees. Front Med 8: 760047-760047.
[Crossref] [Google Scholar] [PubMed]
- Medina-Perucha L, López-Jiménez T, Holst AS, Jacques-Aviñó C, Munrós-Feliu J, et al. (2021) Self-reported menstrual alterations during the COVID-19 syndemic in spain: A cross-sectional study. Int J Women’s Health 14: 529-544.
[Crossref] [Google Scholar] [PubMed]
- Mersal EA, Morsi AA, Hassanein AM, Alshammri A, Alshammari A, et al. (2022) The Association between COVID-19 Pfizer vaccine and the reported post-vaccination menstrual changes in citizen and resident women in KSA: Results of Riyadh Survey Study. Egypt J Hosp Med 87: 1442-1448.
- Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, et al. (2022) Menstrual symptoms after COVID-19 vaccine: A cross-sectional investigation in the MENA region. Int J Womens Health 14: 395-404.
[Crossref] [Google Scholar] [PubMed]
- Laganà AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, et al. (2022) Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Med 17: 475-484.
[Crossref] [Google Scholar] [PubMed]
- Chavan NN, Boob MM, Simpatwar S, Sakhalkar A, Chavan NN (2022) A survey on the impact of COVID-19 infection on menstrual cycle following second wave of COVID infection in a tertiary care centre in Mumbai. Int J Reprod Contracept Obstet Gynecol 11: 491-495.
- Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, et al. (2022) Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) Vaccination: A U.S. Cohort. Obstet Gynecol 139:481-489.
[Crossref] [Google Scholar] [PubMed]
- Dar-Odeh N, Abu-Hammad O, Qasem F, Jambi S, Alhodhodi A, et al. (2022) Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum Vaccines Immunother 1-7.
[Crossref] [Google Scholar] [PubMed]
- Shingu T, Uchida T, Nishi M, Hayashida K, Kashiwagi S, et al. (1982) Menstrual abnormalities after Hepatitis B vaccine. Kurume Med J 29: 123-125.
- Suzuki S, Hosono A (2018) No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res 5:96-103.
[Crossref] [Google Scholar] [PubMed]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, et al. (2015) International union of basic and clinical pharmacology. XCVIII. Histamine Receptors. Pharmacol Rev 67:601-655.
[Crossref] [Google Scholar] [PubMed]
- Hrubisko M, Danis R, Huorka M, Wawruch M (2021) Histamine Intolerance-The more we know the less we know. A Review. Nutrients 13:2228.
[Crossref] [Google Scholar] [PubMed]
- Kovacova-Hanuskova E, Buday T, Gavliakova S, Plevkova J (2015) Histamine, histamine intoxication and intolerance. Allergol Immunopathol (Madr) 43: 498-506.
[Crossref] [Google Scholar] [PubMed]
- Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, et al. (2008) A genecentric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics 7:2019-2027.
[Crossref] [Google Scholar] [PubMed]
- Ayuso P, García-Martín E, Martínez C, Agúndez JAG (2007) Genetic variability of human diamine oxidase: occurrence of three nonsynonymous polymorphisms and study of their effect on serum enzyme activity. Pharmacogenet Genomics17:687-693.
[Crossref] [Google Scholar] [PubMed]
- Music E, Silar M, Korosec P, Kosnik M, Rijavec M (2011) Serum diamine oxidase (DAO) activity as a diagnostic test for histamine intolerance. Clin Transl Allergy 1: P115.
[Crossref] [Google Scholar] [PubMed]
- Manzotti G, Breda D, Di Gioacchino M, Burastero SE (2016) Serum diamine oxidase activity in patients with histamine intolerance. Int J Immunopathol Pharmacol 29:105-111.
[Crossref] [Google Scholar] [PubMed]
- Bódis J, Tinneberg H-R, Schwarz H, Papenfuß F, Török A (1993) The effect of histamine on progesterone and estradiol secretion of human granulosa cells in serum-free culture. Gynecol Endocrinol 7:235-239.
[Crossref] [Google Scholar] [PubMed]
- Jarish R (2015) Histamine Intolerance in Women. Histamine Intolerance Women, pp: 109-115.
- Kostoff RN, Calina D, Kanduc D, Briggs MB, Vlachoyiannopoulos P, et al. (2021) Why are we vaccinating children against COVID-19?. Toxicol Rep 8: 1665-1684.
[Crossref] [Google Scholar] [PubMed]
- Ricke DO, Malone RW (2021) COVID-19 Arm: Delayed post-vaccination cutaneous hypersensitivity. Clin Dermatol Investig.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, Latorre-Moratalla M, Vidal-Carou MD (2020) Histamine Intolerance: The Current State of the Art. Biomolecules 10.
[Crossref] [Google Scholar] [PubMed]
- Macpherson C (1951) Antihistaminics in Dysmenorrhoea. Can Med Assoc J 64:448-449.
[Google Scholar] [PubMed]
- Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang X-P, et al. (2021) COVID-19:Famotidine, Histamine, Mast Cells, and Mechanisms. Front Pharmacol 12: 216.
[Crossref] [Google Scholar] [PubMed]
- Malone RW (2021) More Than Just Heartburn: Does Famotidine Effectively Treat Patients with COVID-19? Dig Dis Sci 66: 3672-3673.
[Crossref] [Google Scholar] [PubMed]
- Hogan II RB, Hogan III RB, Cannon T, Rappai M, Studdard J, et al. (2020) Dual-histamine receptor blockade with cetirizine-famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm Pharmacol Ther 63: 101942.
[Crossref] [Google Scholar] [PubMed]
- Morán Blanco JI, Alvarenga Bonilla JA, Homma S, Suzuki K, Fremont-Smith P, et al. (2021) Antihistamines and azithromycin as a treatment for COVID-19 on primary health care-A retrospective observational study in elderly patients. Pulm Pharmacol Ther 67: 101989-101989.
[Crossref] [Google Scholar] [PubMed]
Citation: Ricke DO (2022) Etiology Model for Elevated Histamine Levels Driving High Reactogenicity Vaccines (including COVID-19) Associated Menstrual Adverse Events. J Infect Dis Ther S3: 002.
Copyright: © 2022 Ricke DO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1626
- [From(publication date): 0-2022 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 1338
- PDF downloads: 288