Estimation of Above and Belowground Carbon Stocks of Forests:Implications for Sustainable Forest Management and Climate Change Mitigation: A Case Study of Tara Gedam Forest, Ethiopia
Received: 07-Jul-2015 / Accepted Date: 22-Jul-2015 / Published Date: 29-Jul-2015 DOI: 10.4172/2157-7617.1000286
Abstract
The forest ecosystem is an important carbon sink and source containing majority of the above ground terrestrial organic carbon. The status of Tara Gedam forest is declining due to human activities. Sustainable management strategies are necessary to make this forest as carbon sink rather than source. To assess the forest’s carbon source potential, dry biomass is quantified since 50% its part is carbon. This study aims to estimate the biomass and carbon stocks of individual trees based on above and belowground biomass estimation models. Simple random sampling method was carried out for collecting the biophysical data for estimating above and belowground biomass of trees. Diameter at breast height (DBH) was measured at 1.3 m height above the ground. Sample plots were laid along line transects based on altitudinal variation of the study area. A randomly sampling plot of (10 m × 20 m) in each site was established to take vegetation samples. The soil samples were taken from soil depth up to 30 cm at the interval of 10 cm. The collected samples were burnt at 105°C for 24 hours in muffle furnace to calculate carbon content. Likewise, bulk density and soil organic carbon were obtained from the soil samples in the laboratory. The result revealed that, Cordia africana Lam. had the highest above ground biomass, 1799.284 ton/ha and below ground biomass, 359.858 ton/ha among other tree species in the study forest. The carbon sequestration in the soil with depth ranged from 0 cm to 30 cm was found to be 1006.763 ton/ha. 413.9536 ton/ha and 2681.292 ton/ha was the minimum and maximum CO2 values sequestered in soil carbon pool in the study area, respectively. A systematic approach for the inclusion of climate change risk management and adaptation is developed and guidelines for the design of ‘climate-change-proof’ afforestation, reforestation and deforestation avoidance projects are proposed.
Keywords: Adaptation, Carbon sequestration, Climate change, Forest, Soil organic carbon
11564Introduction
Background
The climate change is threatening the economic system, livelihoods and the availability of natural resources in several regions of the world [1] and adaptation to the unavoidable climate change becomes a crucial challenge for a century. Forest ecosystems engage a special position within the debate on adaptation to climate change, as they may act both as a carbon source or sink according to their age, management, environmental conditions and the disturbances that alter their composition [2,3]. The deforestation of tropical forests alone currently contributes 1.5 Gt C/y to the global anthropogenic emission (8.4 Gt C/y from the use of fossil energy sources; Raupach [3]; Canadell [4]).
Plants take up carbon dioxide from the atmosphere and incorporate it into plant biomass through photosynthesis. Some of this carbon is emitted back to the atmosphere but what is left the live and the dead plant parts, above and below ground make up an organic carbon reservoir. Some of the dead plant matter is incorporated into the soil in humus, thereby enhancing the soil organic carbon pool. Soil properties, such as the chemical composition of soil organic matter and the matrix in which it is held, determine the different capacities of the land to act as a store for carbon, which has direct implications for capturing greenhouse gases [5,6]. The fact that many of the highland soils have been degraded means that they are currently far from saturated with carbon and their potential to sequester carbon may be very high.
Furthermore, although growth potential is ultimately rainfall limited, plant growth in highlands is in practice often nutrient limited so that there is opportunity for increasing biomass productivity through improved nutrient management even in below average rainfall season.
The United Nations Framework Convention on Climate Change [7] and the Kyoto Protocol (1997) provide the legal framework for the supranational strive against dangerous climate change. They define several mechanisms of climate change mitigation: the “Activities Implemented Jointly (AIJ)” mechanism, “Clean Development Mechanism (CDM)” and “Joint Implementation (JI)” mechanism. The overall scope of these actions are projects that somehow contribute to emission reduction or carbon sequestration all over the world and thus to climate change mitigation [7-9].
According to the IPCC report on Land Use, Land-Use Change, and Forestry (LULUCF) [10], in the forestry sector, three types of mitigation projects are identified. These are:
Afforestation (A): Conversion of long time non-forested land to forest with free species selection, e.g., using non-native and fastgrowing species.
Reforestation (R): Conversion of recently non-forested land to forest, often with a conservation or landscape protection background, generally, planting rather native species and focusing on restoration of “nature like” ecosystems.
Deforestation avoidance (D): Avoidance of conversion of carbonrich forests to non-forest land, normally driven by land use change and illegal selective logging [11]. These actions can contribute to up to 25% of atmospheric CO2 reduction by 2050 by reducing emissions, increase CO2 removals through sinks at low costs and have synergies with adaptation and sustainable development [12,13]. Climate change is also likely to foster the propagation of invasive species [14], as well as changes of forest fire regimes and the forest susceptibility to fire [15].
A non-climate-related impact of the increasing greenhouse gas concentrations in the atmosphere on forest ecosystems are changes in forest processes driven by the elevated CO2 concentration stimulating net primary productivity of plants [16]. This alone can alter the forest composition as species respond individually to the new growth opportunities and constraints imposed by other lacking nutrients [17,18]. Different forest types also respond differently to these changes; model calculations reveal negative responses from higher CO2 concentrations in the tropics and positive feedbacks in extra tropical regions [19].
Statement of the problems
Highlands of Ethiopia are under constant threat from multiple stresses and challenges, which occur as a result of a complex interplay of natural processes and human-induced processes [20]. Deforestation and forest degradation are also the major cause of global warming, responsible for about 15% of global greenhouse gas emissions, which makes the loss and depletion of forests a major issue for climate change. There is considerable variability and uncertainty in current climate change projections. Nevertheless, there is now reasonable agreement from a number of different models, including the IPCC’s Fourth Assessment Report on Climate Change that Africa is at the highest risk from climate change, given the magnitude of existing stresses in the continent [21]. Increased temperatures are expected to add to water problems by causing additional loss of moisture from the soil. The IPCC’s Fourth Assessment Report estimates that by 2020 between 75 and 250 million people are likely to be exposed to increased water stress and that rain fed agricultural yields could be reduced by up to 50 percent in some countries in Africa if production practices remain unchanged [21]. It is worth noting, though that the impact of increased temperatures on low input agriculture will be minimal as other factors will remain the dominant constraints.
The people living in the highlands of Ethiopia are heavily dependent on ecosystem goods and services directly or indirectly, for their livelihoods. But those goods and services from nutrient cycling, flood regulation and biodiversity, food and fibre are under threat from a variety of human induced causes like urban expansion, deforestation, unsustainable farming practices and settlements. As a result, these fragile soils are becoming increasingly degraded and unproductive. The climate change will aggravate these challenges more and more in the present time.
Communities already have a long record of adaptation to climate variability. However, the impacts of climatic and other man-made stresses have been growing continuously at a rate that often exceeds human and ecosystem tolerance levels. Consequently many traditional adaptive knowledge and livelihood strategies practiced in highlands for centuries are inefficient. Efforts to reduce the vulnerability of highlands populations, therefore, must reinforce their risk management and coping capacities by augmenting existing adaptation mechanisms and supplementing them with new options that are tailored to the unique local contexts.
Objectives
General objective: The overall objective of this study is to assess the above and below ground biomass and carbon stocks of individual trees in Tara Gedam forest.
Specific objectives
• To determine the amount of biomass that can be stored by trees
• To determine the amount of carbon that can be sequestered by trees
• To determine potential value of Tara Gedam forest for climate change mitigation
Materials And Methods
Description of the study area
Geographical location: The study was carried out in Tara Gedam forest located very close to Addis Zemen town and northeast of Lake Tana, northwestern Ethiopia. The study area was set in South Gondar Zone within the Amhara National Regional State. The altitude of Tara Gedam ranges from 2217 to 2457 m.a.s.l. with the highest peak at Wombera Mountain.
Climate: The study area is generally characterized by moderate climate, locally known as woina dega. The area has a mono modal rainfall distribution and the rainy season is from June to August. The dry season extends from December to March. Climatic data obtained from the National Meteorological Services Agency for the study area showed that the mean annual maximum and minimum temperatures are 27.9°C and 11.1°C, respectively, and the mean annual rainfall is from 900 mm to 1,200 mm.
Vegetation cover: The vegetation of Tara Gedam consists of forests, bush lands, shrub lands and mixed/enrichment plantations. There is dense natural forest just around the monastery. Tara Gedam forests consist of different trees and shrubs interspersed with climbers and herbs.
Methods
Delineation of the study site: Delineation of the forest boundaries was the first step in floristic measurement. The boundary of the study forest area was delineated by taking geographic coordinates with GPS at each turning point. The GPS points that were taken from the study site to indicate each sample plots were recorded.
Sampling techniques on the field: Simple random sampling method was used to take samples. Sample plots were laid along line transects based on altitudinal variation of the study area. A randomly sampling plot of (10 m × 20 m) in each site was established. To reveal the tree composition and biomass, all live trees with a diameter ≥ 10 cm were recorded as indicated by Pearson [22]. The diameter was measured at breast height (DBH, 1.3 m height from the ground) to estimate biomass and the size class distribution of trees in a sampling plot.
Data analysis
The data analysis of different carbon pools measured in the forests was organized by arranging and recording the data on the excel data sheet. The data was analyzed by using Statistical Package for Social Science (SPSS) software version 20.
Results
Estimation of above ground and below ground biomass and carbon stocks of trees
Above ground (AGB-AGC) and belowground (BGB-BGC) biomass/carbon pools of collected tree species are given in Table 1.
| Name of trees | Average DBH(cm) | AGB ton/ha | AGC ton/ha | BGB ton/ha | BGC ton/ha |
|---|---|---|---|---|---|
| Acacia senegal L.Wild | 15.095 | 548.69 | 274.346 | 109.739 | 54.869 |
| Acanthus sennii Chiov. | 25.77 | 1117.981 | 558.991 | 223.596 | 111.798 |
| Albizia schimperiana Oliv. | 21.115 | 851.269 | 425.635 | 170.254 | 85.127 |
| Allophylus abyssinicus (Hochst) Radlkofer | 35.43 | 1762.573 | 881.286 | 352.515 | 176.257 |
| Anethum graveolens L. | 13.34 | 469.474 | 234.737 | 93.895 | 46.947 |
| Bersama abyssinicaFresen. | 15.21 | 554.025 | 277.013 | 110.805 | 55.403 |
| Brucea antidysentericaJ.f.Mill. | 25 | 1071.892 | 535.946 | 214.379 | 107.189 |
| Buddleja polystachya Fresen. | 19.185 | 749.063 | 374.531 | 149.813 | 74.906 |
| Calpurnia aurea (Ait) Benth. | 22.66 | 936.626 | 468.313 | 187.325 | 93.663 |
| Carissa spinorum L. | 19 | 739.524 | 369.762 | 147.905 | 73.952 |
| Celtis africana Brum.f. | 13.78 | 488.954 | 244.477 | 97.791 | 48.895 |
| Clausena anisata(willd.) Hook. | 21.34 | 863.504 | 431.752 | 172.701 | 86.350 |
| Combretum molle R.Br.ex G.Don | 15.655 | 574.825 | 287.412 | 114.965 | 57.482 |
| Cordia africana Lam. | 35.93 | 1799.284 | 899.642 | 359.858 | 179.928 |
| Croton macrostachyus Del. | 19.38 | 759.167 | 379.583 | 151.833 | 75.917 |
| Cupressus lusitanica Mill. | 19.45 | 762.806 | 381.403 | 152.561 | 76.281 |
| Dombeya torridaJ.F.Gmel. | 18 | 688.741 | 344.371 | 137.748 | 68.874 |
| Dodonaea angustifolia L.f. | 21.75 | 885.9701 | 442.9851 | 177.194 | 88.597 |
| Dovyalis abyssinica(A.Rich.)Warb. | 15 | 544.301 | 272.150 | 108.860 | 54.430 |
| Ekebergia capensisSparrm. | 17.625 | 670.038 | 335.019 | 134.008 | 67.004 |
| Eucalyptus globulus Labill. | 15.895 | 586.151 | 293.075 | 117.230 | 58.615 |
| Euclea divinorum Hiern. | 13.545 | 478.518 | 239.259 | 95.704 | 47.852 |
| Ficus surForssk. | 18.555 | 716.763 | 358.381 | 143.352 | 71.676 |
| Grewia ferrugineaHochst.ex A.Rich. | 21.495 | 871.972 | 435.986 | 174.394 | 87.197 |
| Hibiscus vitifolius L. | 17.445 | 661.126 | 330.563 | 132.225 | 66.113 |
| Hypericum quartinianum A.Rich. | 25.17 | 1082.001 | 541.000 | 216.400 | 108.2000 |
| Jasminum grandiflorum L. | 25 | 1071.893 | 535.946 | 214.379 | 107.189 |
| Maytenus arbutifolia (A.Rich.)Wilczek. | 17.885 | 682.986 | 341.493 | 136.597 | 68.298 |
| Maytenus gracilipes (Welw.ex Oliv.) Exell. |
17.065 | 642.452 | 321.223 | 128.490 | 64.245 |
| Myrsine africana L. | 18.44 | 710.923 | 355.462 | 142.185 | 71.092 |
| Nuxia congesta R.Br.exFresen. | 17.66 | 671.775 | 335.888 | 134.355 | 67.178 |
| Olea europaea subsp.cuspidata. | 22.94 | 952.431 | 476.216 | 190.486 | 95.243 |
| Osyris quadripartitaDecn. | 24.875 | 1064.484 | 532.242 | 212.897 | 106.448 |
| Phytolacca dodecandra L’Herit | 24.56 | 1045.907 | 522.953 | 209.181 | 104.591 |
| Premna schimperi engl. | 19.115 | 745.448 | 372.724 | 149.089 | 74.545 |
| Phytolacca dodecandra L’Herit | 30.38 | 1410.257 | 705.129 | 282.051 | 141.026 |
| Rosa abyssinica lindly. | 17.875 | 682.486 | 341.243 | 136.497 | 68.249 |
| Schefflera abyssinica (Hochst.ex.A.Rich)Harms | 14.995 | 544.069 | 272.035 | 108.814 | 54.407 |
| Schrebera alata (Hochst.)Welw. | 24.65 | 1051.201 | 525.601 | 210.240 | 105.120 |
| Stereospermum kunthianum Cham. | 18.425 | 710.163 | 355.081 | 142.033 | 71.016 |
| Urtica urens L. | 20.375 | 811.501 | 405.751 | 162.300 | 81.150 |
Table 1: Above ground (AGB-AGC) and below ground (BGB-BGC) biomass/carbon pools of collected tree species.
Soil organic carbon
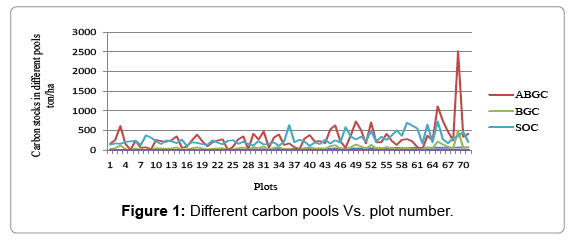
The result showed that, the highest percentage of organic carbon in soil was 35.43% where as 3.95% is the lowest value and the average percentage value of organic carbon in this pool as a whole was found to be 14.25%. On the other hand, the soil was calculated 730.59 ton/ha and 112.6233 ton/ha maximum and minimum values per plot of the study site respectively [23-25]. However, the average values of SOC in the study area was 274.322 ton/ha. The carbon sequestration in the soil with depth ranged from 0 cm to 30 cm was found to be 1006.763 ton/ ha. And also based on the result that obtained, 413.9536 ton/ha and 2681.292 ton/ha was the minimum and maximum CO2 values that is sequestered in the study area respectively [26] (Figure 1).
Conclusion
The present study has estimated the biomass of the above ground and below ground wooded parts of the trees. The estimations regarding carbon emission and sequestration potential of the study forest was made. Different species of plants were collected, of which Cordia africana Lam. had the highest above ground biomass and below ground biomass among other tree species in the study forest.
The determination of a baseline by which to assess carbon sequestration is critical as it provides the frame of reference for determining how carbon sequestration projects are contributing to the net carbon sink. Tree plantations would enhance carbon sequestration, policy makers must also initiate promoting carbon friendly goods and services so as to reduce carbon excess budget. In this regard, encouragement to public transport over private, subsidy to organic farming over synthetic etc. measures should be considered, so that emission levels will also reduce.
References
- Hansen AJ, Sato M, Ruedy R (2006)Â Global temperature change. PNAS 103: 14288-14293.
- Rosenbaum KL, Schöne D, Mekouar A (2004). Climate change and the forest sector – Possible national and subnational legislation. FAO Forestry Paper. FAO, Rome.
- Dale VH, Joyce LA, McNulty S (2001) Climate change and forest disturbances. Biosci 51: 723-734.
- Raupach MR, Marland G, Ciais P (2007) Global and regional drivers of accelerating CO2 emissions. PNAS 104: 10288-10293.
- Canadell JG, Kirschbaum MUF, Kurz WA (2007) Factoring out natural and indirect effects on terrestrial carbon sources and sinks. Environ Sci Policy 10: 370-384.
- Food and Agriculture Organisation of the United Nations (2007) State of the world’s forests. FAO, Rome.
- United Nations Framework Convention on Climate Change (2007) Revised approved afforestation and reforestation baseline methodology AR-AM0001 – “Reforestation of degradedlandâ€.
- Aukland L, Moura-Costa P, Bass S (2002) Laying the foundation for clean development: preparing the land use sector. A quick guide to the Clean Development Mechanism IIED, London.
- Stuart MD, Moura–Costa P (1998) Climate change mitigation by forestry: a review of international initiatives. IIED, London
- Watson RT, Noble IR, Bolin B (2000) Land use, land-use Change, and forestry. A Special Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK
- Asner GP, Knapp DE, Broadbent EN (2005) Selective logging in the Brazilian Amazon.
- Niles JO, Brown S, Pretty J (2002)Â Potential carbon mitigation and income in developing countries from changes in use and management of agricultural and forest lands. Phil Trans R SocLond 360: 1621-1639
- Barker T, Bashmakov I, Bernstein L (2007) Technical Summary. In: B Metz, OR Davidson, PR Bosch (edn) Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK and New York, USA.
- Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? TREE 14: 135-139.
- Westerling AL, Hidalgo HG, Cayan DR (2006) Warming and earlier spring increases Western U.S. forest wildfire activity. Sci 313: 940-943
- Boisvenue C, Running SW (2006) Impacts of climate change on natural forest productivity – evidence since the middle of the 20thcentury. Global Change Biol 12: 862-882.
- Reich PB, Hobbie SE, Lee T (2006)Â Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440: 922-925
- Yarie J, Parton B (2005) Potential changes in carbon dynamics due to climate change measured in the past two decades. Can J For Res 35: 2258-2267
- Berthelot M, Friedlingstein P, Ciais P (2002) Global response of the terrestrial biosphere to CO2 and climate change using a coupled climate-carbon cycle model. Global Biochem Cycles 16:31.1-31.16.
- Mahrenholz P, Georgi B (2005) Braucht Klimaschutz biologische Vielfalt? In: Korn H, Schliep R, Stadler J (edn) Biodiversität und Klima – Vernetzung der Akteure in Deutschland – Ergebnisse und Dokumentation des Auftaktworkshops. BundesamtfürNaturschutzSkripten 131, Germany
- Intergovernmental Panel on Climate Change (2007) Highlights from Climate Change. The Physical Science Basis: Summary for Policy Makers. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Institute of Terrestrial Ecology, Edinburgh, pp.545-552.
- Pearson T, Walker, Brown S (2005) Source book for land-use, land-use change and forestry projects. Winrock International and the Bio-carbon fund of the World Bank. Arlington, USA, pp. 19-35.
- Capoor K, Ambrosi P (2006) State and trends of the carbon market (2006).The World Bank, USA.
- F Evrendilek (2004)An inventory-based carbon budget for forest and woodland ecosystems of Turkey. J Environ Monitoring 6: 26-30.
- F Evrendilek, MK Wali (2001) Modelling long-term C dynamics in croplands in the context of climate change: a case study from Ohio. Environ Model Softw16:361-375.
- Evrendilek F, Berberoglu S, Gulbeyaz O,Ertekin V (2007) Modeling potential distribution and carbon dynamics of natural terrestrial ecosystems: a case study of Turkey. Sensors 7:2273-2296.
Citation: Gedefaw M (2015) Estimation of Above and Belowground Carbon Stocks of Forests: Implications for Sustainable Forest Management and Climate Change Mitigation: A Case Study of Tara Gedam Forest, Ethiopia. J Earth Sci Clim Change 6: 286. DOI: 10.4172/2157-7617.1000286
Copyright: © 2015 Gedefaw M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15963
- [From(publication date): 7-2015 - Jul 14, 2025]
- Breakdown by view type
- HTML page views: 11145
- PDF downloads: 4818