Essential Oil and Fatty Acid Constituents of Buccholzia coriacea (Wonderful Kola) Seeds Harvested in Nigeria
Received: 09-Feb-2018 / Accepted Date: 30-Apr-2018 / Published Date: 07-May-2018 DOI: 10.4172/2168-9652.1000233
Keywords: Essential oils; Fatty acids; Buchholzia coriacea; Medicinal plants; Bioactive
Introduction
The application of herbal formulations as substitutes for orthodox medicine is gaining remarkable recognition because of availability, cost and accessibility all over the world. Medicinal plants are extensively used as solutions for numerous diseases because they contain relevant chemical constituents of therapeutic appraisal [1]. The medicinal and therapeutic potentials of plants have long been credited to the observed bioactive compounds inherent in them [2,3]. Majority of the populace in Nigeria and other developing countries subscribe to the utilization of herbal medicines and formulation. Agricultural products like seeds are essential sources of oils of pharmaceutical, industrial and nutritional significance [4]. The appropriateness of oil for a specific purpose is determined by its characteristics, volatile constituents and to a large extent its fatty acid compositions. Oil from diverse sources varies in their fatty acid composition and a single oil source cannot be specifically suitable for all purposes [5].
Fatty acids and essential oils are employed in a widespread variety of end-use industries like rubber, cosmetics, medicine, food, plastics and pharmaceuticals [6] and as a matter of fact they account for the majority of raw materials utilized in chemical industries [7]. Nevertheless, the sources of fats and oils are shrinking, indicating the increasing need for novel sources of oil to enhance the prevailing ones.
Wonderful kola, known as Buccholzia coriacea in the scientific world is mostly utilized as herb in Nigeria and has acclaimed tremendous medicinal prowess. Previous studies Nwaichi and Olua [8] had demonstrated the abundance of phytochemicals of therapeutic benefits in the seeds of Buchholzia coriacea. It has been reported by numerous authors [9-11] Ajaiyeoba et al. [1] to be effective in ameliorating numerous diseases like sinusitis, convulsion in children, earache, nasal congestion, syphilis, headache, smallpox and gonorrhea. The seeds are sometimes consumed to enhance memory retention [1]. It has been employed for countless medicinal purposes and is domicile in most countries in Africa like Gabon [1], where it has been utilized to treat smallpox and cure skin itches and Ivory Coast where the seeds are used to alleviate sinusitis, nasal congestion and headache [12]. Application of the pulped bark to the chest helps in the treatment of boils and chest pains. They seeds have anti-helminthic potentials and is conventionally used in Liberia for the treatment of skin eruption [12]. The anti-fungal and antibacterial potentials have been reported by Mbata et al. [13], while Anowi et al. [12] recorded the anti-spasmodic and anti-diarrhoea characteristics. Most biological activities have been principally credited to the fixed and volatile oils inherent in the seeds. To the best of our knowledge there is no detailed characterization of the chemical composition of the volatile and fatty acid constituents of the seeds of Buchholzia coriacea.
Taking into consideration the enormous potentials of some prevailing herbal formulations, this study evaluates the essential oil and fatty acid composition of oils from seeds of Buchholzia coriacea in order to complete their chemical characterization and further underscore and corroborate their potentials for use in the aforementioned industries. This exploration will be expedient to identify the bioactive compounds of the oils, which may be culpable for the therapeutic potentials of the seeds.
Materials and Method
Methods
Sample collection: Fresh samples of Buccholzia coriacea was purchased from the herb shop at Mile 1 Market in Port Harcourt, Rivers State, Nigeria. These collected samples were transported to the Department of Plant Science and Biotechnology of the University of Port Harcourt Nigeria, where the Curator identified them. Identified samples were air dried and milled in a clean Blender.
Extraction of dried samples: Milled samples (10 g each) were extracted in 20 mL dichloromethane (DCM) after soaking for five days in a well stoppered bottle. The mixtures were vigorously agitated and were left to stand for five days. The crude extract was gathered by sieving into a quartz beaker, the process was repeated two more consecutive times. The combined aliquot collected was concentrated on a steam bath to about 5 ml. This was purified by passing through a pasture pipette on a membrane and air dried to about 2 ml for gas chromatographic analysis [14].
Gas chromatography-mass spectrometry analysis: The extract of the sample was subjected to GC/MS analysis, this group of powerful instruments interfaced helped to characterize the various compositions. The gas chromatographic Model: 7890A (GC) analysis was performed on an Agilent Technologies interfaced with Mass Selective Detector model: 5975C (MSD). The electron ionization was at a 70 V with an ion source temperature at 250°C. Highly pure helium gas (99.9% purity) was used as carrier gas, while HP-5 (30 mm × 0.25 mm × 0.320 μm) was used as the stationary phase. The oven temperature was at 60°C held for 0.5 min and ramped to 140°C at the rate of 4°C/min holding for a minute, then ramped to 280° while holding for 5 min at the rate of 8°C/min. 1 μ/l was auto injected. The split ratio was 1:5. Retention indices for all components were determined according to the Van Den Dool method [15], using n-alkanes as standard. Identification of the components was based on the comparison of their mass spectra with those of internal (computer) library, NIST libraries and some reference compounds and those described by Adams [16].
Fatty acid methylation and analysis: Using 30 g L-1 sodium methylate in methanol, total fatty acids were transformed into their analogous methyl esters according to the technique illustrated by Cecchi et al. [17]. For the quantification of fatty acids, heptadecanoic acid methyl ester was employed as an internal standard. Before analysis, the fatty acid methyl esters were aspirated and reduced using nitrogen gas. The fatty acid analysis was carried out with a TRACE GC Ultra gas chromatograph coupled to ITQ mass 1100 mass spectrometer. Chromatographic separation was performed on a 105 m × 0.25 mm I.D., 0.20 mm film thickness Rtx-2330 capillary column. Helium (purity 99.99%) was utilized as the carrier gas at constant flow 2.8 ml.min. Initial oven temperature was set at 60°C, followed by a linear ramp to 250°C at a rate of 5°C/min. Subsequently, the temperature was raised to 265°C at a rate of 10°C/min. A split-spitless injector set at 250°C was always used and injections of 1 μl were performed in the splitless mode. Transfer line temperature was set at 260°C and the source temperature at 260°C. The mass spectrometer was operated in the electron impact mode (EI, 70 eV). In order to facilitate identification, the retention times of various fatty acids were compared to those of the standards.
Results
Data from this study uncovered the presence of sixteen essential oils and ten fatty acids. From the results 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester (74.88%), was the abundant essential oil constituents of B. coriacea seed oil followed by 9-Octadecenoic acid methyl ester (E) (3.08%), while 1-Heptacosanol (0.21%) was the least essential oil. The fatty acid contents of B. coriacea showed the presence of saturated, monounsaturated and polyunsaturated fatty acids which are demonstrated in Table 1. Pentadecanoic acid was the fatty acid detected in the largest amount in this investigation, with a value of 35.57% followed by Oleic acid and Palmitic acid at values of 26.05% and 20.185, respectively.
| S/N | Name of Compound | Molecular Formula | % Total |
|---|---|---|---|
| 1 | Lauric acid | C12 | 5.01 |
| 2 | Mystric acid | C14 | 1.35 |
| 3 | Pentadecanoic acid | C15 | 34.88 |
| 4 | Palmitic acid | C16 | 20.18 |
| 5 | Palmitoleic acid | C16:1 | 1.44 |
| 6 | Margaric acid | C17 | 3.1 |
| 7 | Oleic acid | C18:In9t | 26.05 |
| 8 | Linoleic acid | C18:2n6t | 1.65 |
| 9 | Linolenic acid | C18:3n6 | 5.9 |
| 10 | Lauric acid | C22:6n3 | 0.78 |
Values are means of triplicate determinations
Table 1: Fatty acid constituents of Buccholzia coriacea.
Discussion
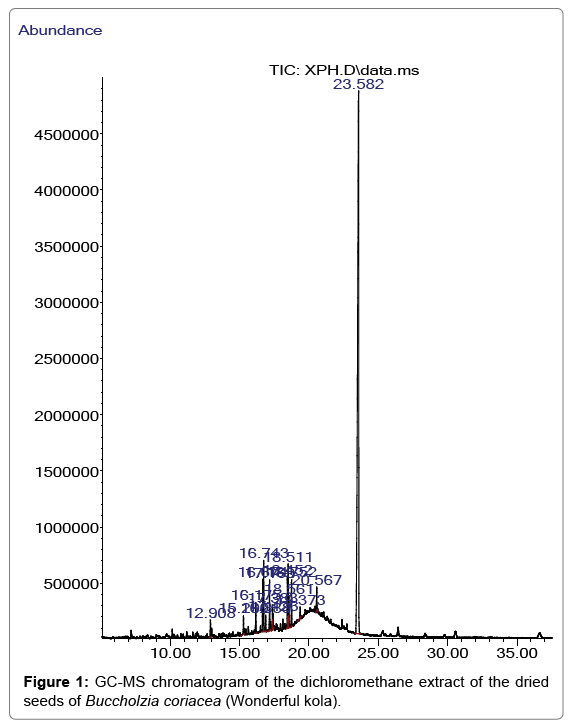
The GC/MS characterization of B. coriacea seeds revealed the existence of fifteen compounds which amounted to 100% of the total essential oil constituents. The identified compounds of the dried seeds of B. coriacea, their retention times, percentage composition, molecular formula, molecular weight and their structures are given in Table 1. The GC/MS chromatogram displayed in Figure 1 demonstrates the retention time in minutes of the identified compounds on the X-axis and the respective percentage peaks and the intensity of various compounds at their respective retention times on the Y-axis.
The compounds in the essential oil revealed were 1,2-benzene dicarboxylic acid, mono (2-ethylhexyl) ester (74.88%), 1-Hexadecene (0.79%), 1-Octadecene (1.58%), Phthalic acid, isobutyl nonyl ester (1.63%), 1,2-Benzenedicarboxylic acid, butyl ester (2.52%), Hexadecanoic acid methyl ester (3.22%), Phthalic acid, isobutyl undecyl ester (0,74%), Dibutyl phthalate (2.52%), 1,2-Benzenedicarboxylic acid butyl decyl ester (1.16%), 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (2.51%), 9-Octadecenoic acid methyl ester (E) (3.08%), -9-Octadecenoic acid (Z)-methyl ester (1.54%), Octadecanoic acid methyl ester (2.21%), 1-Heptacosanol (0.21%), Phthalic acid dodecyl octyl ester (1.32%).
These identified compounds have numerous activities biologically predominantly in the pharmaceutical industry. From this study, 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester (Table 2) was the major abundant compound in the dichloromethane extract subjected to GC/MS characterization and this compound has been reported to possess anti-inflammatory, anti-fungal, anti-diabetic, antioxidant, anti-cancer, anti-retroviral, anti-scabies biological activities [18-20] as well as strong in vitro cytotoxic activity [21]. More recently, in vitro cytotoxicity assays of the compound against human hepatocellular cancer cell lines revealed that 1,2-benzene dicarboxylic acid, mono (2-ethylhexyl) ester demonstrated cytotoxic activities on the cancer cell lines [21].
| S/N | Compound | Retention time (min) | Total (%) | Molecular formula | Molecular weight (gmol-1) |
|---|---|---|---|---|---|
| 1 | 1-Hexadecene | 12.9 | 0.79 | C16H32 | 224.42 |
| 2 | 1-Octadecene | 15.29 | 0.93 | C18H36 | 252.47 |
| 17.42 | 0.65 | C18H36 | 252.47 | ||
| 3 | Phthalic acid isobutyl nonyl ester | 16.17 | 1.63 | C21H32O4 | 348.47 |
| 4 | 1,2-Benzenedicarboxylic acid, butyl ester | 16.67 | 2.52 | C13H16O4 | 236.26 |
| 5 | Hexadecanoic acid, methyl ester | 16.74 | 3.22 | C17H34O2 | 270.45 |
| 6 | Phthalic acid, isobutyl undecyl ester | 16.88 | 0.74 | C23H36O4 | 376.53 |
| 7 | Dibutyl phthalate | 17.16 | 2.52 | C16H22O4 | 278.34 |
| 8 | 1,2-Benzenedicarboxylic acid, butyl decyl ester | 17.38 | 1.16 | C22H34O4 | 362.5 |
| 9 | 9,12-Octadecadienoic acid (Z,Z)- methyl ester | 18.45 | 2.51 | C19H34O2 | 294.47 |
| 10 | 9-Octadecenoic acid, methyl ester, (E)- | 18.56 | 3.08 | C19H36O2 | 296.48 |
| 11 | 9-Octadecenoic acid (Z)-, methyl ester | 18.56 | 1.54 | C19H36O2 | 296.48 |
| 12 | Octadecanoic acid, methyl ester | 18.75 | 2.21 | C19H34O2 | 294.47 |
| 13 | 1-Heptacosanol | 19.37 | 0.21 | C27H56O | 396.73 |
| 14 | Phthalic acid, dodecyl octyl ester | 20.56 | 1.32 | C28H46O4 | 446.66 |
| 15 | 1,2-Benzenedicarboxylic acid, mono (2-ethylhexyl) ester | 23.58 | 74.88 | C16H22O4 | 278.34 |
The individual Essential oils and their structures were determined using GC/MS and their structures and molecular weight was confirmed using (NIST) National Institute of Standard Test data base
Table 2: Constituent oils in Buccholzia coriacea seeds.
Hexanedecanoic acid methyl ester (Table 2), the second most abundant compound was reported by Hema et al. [22] to possess hypocholesterolemic, anti-oxidant, anti-fungal and nematicidal activities and recently Nwaichi et al. [1] demonstrated the antihyperlipidemic potential of B. coriacea seeds in Wistar rats. Also, Syeda et al. [18] and Hema et al. [22] reported that 9-Octadecenoic acid, methyl ester (Table 2), among phytocompounds detected in this study possesses anti-oxidant and anti-carcinogenic activities.
Furthermore, Di-n-butyl phthalate (Table 2) identified as one of the phytochemicals in this study possesses anti-microbial as well as anti-malarial and anti-fungal activities biologically [23]. On the other hand it is also used as a solvent for dye, employed as a plasticizer in the production of plastics and for myriad products [24]. However, Shanna et al. [25] said that phthalate esters cause the decline of human sperm counts and testicular atrophy in experimental rats [26]. Di-n-butyl phthalate is easily emitted into the environment during the industrial process in the conversion of phthalate into plastics; hence, human populations are exposed to phthalates through medical materials, consumer products and foods [27]. Moreover, Octadecanoic acid methyl ester (Table 2) has also been reported as a potent anti-fungal, anti-bacterial and anti-microbial material at low pH [28]. As presented in Table 1 saturated fatty acids were established as the predominant fatty acids in Buccholzia coriacea seed oil. The saturated fatty acids detected in the largest amount constituted 65.96% of total FAMEs in B. coriacea seed oil and they were represented in descending order by Pentadecanoic (C15), Palmitic (C16) Lauric (C12), Margaric (C17), Palmitoleic (C16:1) and Mystric (C14) acids in the amounts 34.88%, 20.18%, 5.01%, 3.10%, 1.44%, 1.35%, respectively. Pentadecanoic acid (C15) was the predominant saturated fatty acid 34.88%.
As a matter of fact, several investigations [29,30] established diverse impacts of saturated fatty acids on human health. It has been reported that mystric acid and lauric acid increase total cholesterol levels in the plasma, the former due to elevation of both low density lipoprotein and high density lipoprotein concentrations, the latter as a result of an increase in low density lipoprotein concentration. The results of this study is not in consonance with Kostik et al. [31], who reported high values of lauric acid for coconut oil and palm oil, while the content of mystric acid was also lower than the reports for cashew and melon seed oil. However, the levels of the aforementioned fatty acids are relatively low in the sample as shown in Table 1 and may not likely portend danger upon consumption. However, other authors Mensink et al. [32] and Lawrence et al. [33] reported that the ratio of total cholesterol to high density lipoprotein is a better predictor of coronary heart disease than the value of low density lipoprotein cholesterol. Oils abundant in lauric acid reduced the ratio of total cholesterol to high density lipoprotein cholesterol. Conversely, palmitic acid and myristic acid affected this ratio in a small proportion. The palmitic acid content (Table 1) of this present study is comparable to those of D. mespiliformis, C. pulcherrima and A. lebbeck seeds as reported by Adewuyi and Oderinde [34]. The palmitoleic acid content of the seeds of B. coriacea was low but was higher than the value reported for pumpkin seed oil and sunflower evaluated by Orsavova et al. [35].
Furthermore, the Mediterranean diet is renowned all over the world as a diet with negligible sum of saturated fatty acids with elevated consumption of olive oil. Avocados, red meat, high fat fruits and nuts, etc., are some of the natural sources of monounsaturated fatty acids. Oleic acid was the abundant monounsaturated fatty acid in the amount of 26.05% and this concentration was high in comparison to those observed for melon seed oil (14.73%) and rubber seed oil (23.74%) as reported by Bello and Anjorin [36] but was however lower than those observed by Bello and Anjorin [36] for cashew seed oil, ground nut oil and pumpkin seed oil. According to FAO/WHO [37] monounsaturated fatty acids might increase high density lipoprotein and decrease low density lipoprotein levels in the blood. Oleic acid unlike polyunsaturated fatty acids might encourage insulin resistance (FAO/WHO) [37] and has been documented as an anti-inflammatory agent and anti-apoptotic agent [38].
According to Mišurcová [39] the principal source of polyunsaturated fatty acids like Linoleic acid and Linolenic acid is marine phytoplankton and algae producing the focal part of fish feed while leafy vegetables, seeds and nuts etc. accounts for the terrestrial sources [40]. The most abundant polyunsaturated fatty acid discovered in B. coriacea seed oil is Linolenic acid (5.90%) and it was lower than those reported by Rajah [41] for pumpkin seed. The importance of polyunsaturated fatty acids on humans in the prevention of hypertension, coronary heart diseases, renal disease, cardiovascular diseases etc. has been emphasized in recent studies [42] and thus the presence of this fatty acid and Hexanedecanoic acid methyl ester (Table 2) may have mediated the antihyperlipidemic potentials of the seed as reported by Nwaichi et al. [1].
Conclusion
It is concluded that some of the identified compounds contribute beneficial role to human beings and have been considered as new source for different supplement and curative for many ailments with low risk for resistance development by pathogenic microorganisms due to natural origin. From obtained results, Buccholzia coriacea could be suggested as a new potential source for natural therapeutic use in many remedies and could be a potential source for useful drugs like anticancer types.
Acknowledgement
The authors would like to acknowledge the financial assistance from UNESCO L’Oreal For Women in Science.
Funding
Cost of this study was borne by the authors and in part by UNESCO L’Oreal FWIS.
References
- Nwaichi EO, Osuoha JO, Monanu MO (2017) Nutraceutical potential of Tetracarpidium conophorum and Buccholzia coriacea in diet-induced hyperlipidemia. J Chem Health Risks 7: 157-170.
- Himal PC, Nisha SY, Jyoei S (2008) Phytochemical and antimicrobial evaluations of some medicinal plants of Nepal. J Appl Sci Eng Technol 1: 49-54.
- Nwaichi EO, Igbinobaro O (2012) Effects of some selected spices on the biochemical profile of Wistar Albino rats. Am J Environ Eng 2: 8-11.
- Alvarez AMR, Rodriguez MLG (2000) Lipids in pharmaceutical and cosmetic preparations. Gras Aceites 51: 74-96
- Dagne K, Jonsson A (1997) Oil content and fatty acid composition of seeds of Guizotia cass (Compositae). J Sci Food Agric 73: 274-278
- Gunstone FD (1996) Fatty acid and lipid chemistry. In: Blackie Academic and Professional (1eds). London, UK.
- Biermann U, Friedt W, Lang S, Luhs W, Machmuller G, et al. (2000) New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew Chem Int Ed Engl 39: 2206-2224.
- Nwaichi EO, Olua V (2015) Antioxidants and phytochemical profile of various extracts from Buccholzia coriacea. Indian J Appl Res 5: 415-420.
- Ajaiyeoba EO, Onocha,PA, Olarenwaju OT (2001) In vitro anti-helmintic properties of Buchholzia coricea and Gynandropsis gynandra extracts. Pharm Biol 39: 217-222.
- Ajaiyeoba EO, Onocha PA, Nwozo SO, Sama W (2003) Antibacterial activities and preliminary phytochemical screening of four medicinal plants. J Appl Sci 7: 4228-4338.
- Ezekiel OO, Onyeoziri NF (2009) Preliminary studies on the antimicrobial properties of Buhholzia coriacea (Wonderful kola) Afr J Biotechnol 8: 472-474.
- Anowi CA, Chibeze I, Emma E (2012) The phytochemical, anti-spasmodic and anti-diarrhoea properties of the methanol extract of the leaves of Buchholzia coriacea family Capparaceae. IJCPR 4: 52.
- Mbata TI, Duru C M, Onwumelu HA (2009) The antibacterial activity of crude seed extracts of Buchholzia coriacea on some pathogenic bacteria. J Dev Biol Tissue Eng 1: 1-5.
- IOA Instituute of Agrophysics (2014) GC-MS Laboratory validated procedure/protocol for extraction of dry plant-part materials.
- Dool VD, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11: 463-471.
- Adams R (1995) Identification of essential oil components by gas chromatography/mass spectroscopy, Allured Publishing Co., Carol stream, Illinois, USA.
- Cecchi G, Biasini S, Castano JM (1985) Ethanolyse rapide des huiles en solvant. note de laboratoire. Rev Fr Corps Gras 4: 163-164.
- Syeda FA, Habib-Ur-Rehman, Choudahry MI, Atta-Ur-Rahman (2011) Gas chromatography-mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bioassays of crude extract of Iris germanica. Int J Genet Mol Biol 3: 95-100.
- Balachandran C, Lakshmi RS, Duraipandiyan V, Ignacimuthu S (2012) Antimicrobial activity of Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil from Chennai, India. Bioresour Technol 112: 83-90
- Bagavathi PE, Ramasamy N (2012) GC/MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Res l4: 11-14.
- Kannabiran K, Abirami M, Subashini J (2014) Cytotoxic activity of bioactive compound 1,2-benzenedicarboxylic acid, mono 2-ethylhexyl ester extracted from a marine derived Streptomyces sp. VITSJK. Int J Mol Cell Med 3: 246-254.
- HemaR, Kumaravel S, Alagusundaram (2011) GC/MS determination of bioactive components of Murraya koenigii. J Am Sci 7
- Elija K, Vaishali B, Adsul MK, Deshpande NR, Kashalkar RV (2012) Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J Pharm Res 5: 150-152
- Tsutsumi T, Ichihara T, Kawabe M (2004) Renal toxicity induced by folic acid is associated with the enhancement of male reproductive toxicity of di (n-butyl) phthalate in rats. Reprod Toxicol 18: 35-42.
- Shanna H, Eric P, Laura F (1997) Have sperm density declined? Are analyses of global trend data? Environ Health Perspect 105: 1228-1232.
- Srivastava SP, Srivastava S, Saxena DK, Chandra SV, Seth PK (1990) Testicular effects of di-n-butyl phthalate (DBP): Biochemical and histopathological alterations. Arch Toxicol 64: 148-152.
- Hauser R, Duty S, Godfrey-Bailey L, Calafat AM (2004) Medications as a source of human exposure to phthalates. Environ Health Perspect 112: 751-753.
- Gehan MA, Hanan AE, Hassan AHI, Okbah MA (2009) Marine natural products and their potential applications as anti infective agents. World Appl Sci J 7: 872-880.
- Denke MA, Grundy SM (1992) Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am J Clin Nutr 56: 895-898.
- Zock PL, de Vries JHM, Katan MB (1994) Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler Thromb Vasc 14: 567-575.
- Kostik V, Memeti S, Bauer B (2013) Â Fatty acid composition of edible oils and fats. J Hyg Eng Des 4: 112-116.
- Mensink RP, Zock PL, Kester ADM, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials 1-3. Am J Clin Nutr 77: 1146-1155.
- Lawrence GD (2013) Dietary fats and health: Dietary recommendations in the context of scientific evidence. Adv Nutr 4: 294-302.
- Adewuyi A, Oderinde R A (2014) Fatty acid composition and lipid profile of Diospyros mespiliformis, Albizia lebbeck and Caesalpinia pulcherrima seed oils from Nigeria. Int J Food Sci
- Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J (2015) Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci
- Bello EI, Anjorin SA (2012) Fatty acid composition of six Nigeria’s vegetable oils and their methyl esters. Res J Eng Appl Sci 1: 166-170.
- Kim H, Youn K., Yun EY, Hwang JS, Jeong WS (2015) Oleic acid ameliorates Aβ-induced inflammation by down regulation of COX-2 and iNOS via NFκB signaling pathway. J Funct Foods 14: 1-11.
- Mišurcová L, Vávra Ambrožová J, Samek D (2011) Seaweed lipids as nutraceuticals. Adv Food Nutr Res 64: 339-355.
- De Caterina R, Basta G (2001) n-3 Fatty acids and the inflammatory response biological background. Eur Heart J Suppl 3: D42-D49.
- Rajah KK (2002) Fats in food technology. In: Sheffield Academic Press, Sheffield, UK, pp: 1-379.
- Vávra Ambrožová J, MiÅ¡urcová L, VÃcha R, Machů L, Samek D, et al. (2014) Influence of extractive solvents on lipid and fatty acids content of edible freshwater algal and seaweed products and green microalga Chlorella kessleri and cyanobacteria Spirulina platensis. Molecules 19: 2344-2360
Citation: Nwaichi EO, Osuoha JO (2018) Essential Oil and Fatty Acid Constituents of Buccholzia coriacea (Wonderful Kola) Seeds Harvested in Nigeria. Biochem Physiol 7: 233. DOI: 10.4172/2168-9652.1000233
Copyright: © 2018 Nwaichi EO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6923
- [From(publication date): 0-2018 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 6045
- PDF downloads: 878