Esophageal Cancer: 10 Year Survival after Surgery
Abstract
Objective: 10 Year survival (10 YS) after radical surgery for esophageal cancer (EC) patients (ECP) (T1-4N0-2M0) was analyzed.
Methods: We analyzed data of 551 consecutive ECP (age=56.5 ± 8.9 years; tumor size=6 ± 3.5 cm) radically operated (R0) and monitored in 1975-2021 (m=411, f=140; esophagogastrectomies (EG) Garlock=284, EG Lewis=267, combined EG with resection of pancreas, liver, diaphragm, aorta, VCS, colon transversum, lung, trachea, pericardium, splenectomy=154; adenocarcinoma=314, squamous=227, mix=10; T1=128, T2=115, T3=181, T4=127; N0=278, N1=70, N2=203; G1=157, G2=141, G3=253; early EC=109, invasive=442; only surgery=423, adjuvant chemoimmunoradiotherapy-AT=128: 5-FU+thymalin/taktivin+radiotherapy 45 Gy-50 Gy. Multivariate Cox modeling, clustering, SEPATH, Monte Carlo, bootstrap and neural networks computing were used to determine any significant dependence.
Results: Overall life span (LS) was 1881.1 ± 2230.6 days and cumulative 5 year survival (5 YS) reached 52.1%, 10 years 45.9%, 20 years 33.7%. 184 ECP lived more than 5 years (LS=4308.7 ± 2413.3 days), 99 ECP more than 10 years (LS=5883 ± 2296.6 days). 226 ECP died because of EC (LS=628.3 ± 319.9 days). AT significantly improved 5 YS (68.8% vs. 48.5%) (P=0.00025 by log rank test). Cox modeling displayed that 10 YS of ECP significantly depended on: phase transition (PT) N0-N12 in terms of synergetics, cell ratio factors (ratio between cancer cells CC and blood cells subpopulations), T, G, histology, age, AT, localization, blood cells, prothrombin index, hemorrhage time, residual nitrogen, protein (P=0.000-0.021). Neural networks, genetic algorithm selection and bootstrap simulation revealed relationships between 10 YS and PT N0-N12 (rank=1), healthy cells/CC (2), PT early invasive EC (3), thrombocytes/CC (4), erythrocytes/CC (5), lymphocytes/CC (6), eosinophils/CC (7), stick neutrophils/CC (8), segmented neutrophils/CC (9), monocytes/CC (10). Leucocytes/CC (11). Correct prediction of 5 YS was 100% by neural networks computing (area under ROC curve=1.0; error=0.0).
Conclusion: 10 Year survival after radical procedures significantly depended on: (1) PT “early invasive cancer”; (2) PT N0- N12; (3) Cell Ratio Factors; (4) blood cell circuit; (5) biochemical factors; (6) hemostasis system; (7) AT; (8) EC characteristics; (9) tumor localization; (10) anthropometric data; (11) surgery type. Optimal diagnosis and treatment strategies for EC are: (1) screening and early detection of EC; (2) availability of experienced thoracoabdominal surgeons because of complexity of radical procedures; (3) aggressive en block surgery and adequate lymph node dissection for completeness; (4) precise prediction; (5) adjuvant chemoimmunoradiotherapy for ECP with unfavorable prognosis.
Keywords: Esophageal cancer; Surgery; 10 year survival; Prognosis
Introduction
Esophageal cancer (EC) is the global problem in the world in the structure of mortality from malignant neoplasms. Nevertheless, there is practically no analysis of 10 year survival (10 YS) of EC patients (ECP) in the literature. But information on 10 YS rate is extremely important in optimizing the treatment and diagnostic process in oncology and especially for extremely aggressive cancer EC. The high mortality rate associated with EC is primarily due to the high incidence of late stage and the lack of curative management for the majority of ECP. Up to 80%-90% of ECP present with stage III-IV disease. The role of adjuvant chemotherapy or chemo immunotherapy after complete esophagogastrectomies in ECP with stage II-IV remains controversial [1-3]. Moreover, the optimal treatment plan in general and optimal approach for adjuvant chemotherapy in particular has not been defined and long term prognosis of ECP especially with stage III-IV remains poor, because of local relapse and distant metastases, with the real 5 year survival rate after radical procedures only 20%-30% [4,5]. One of the approaches developed involves aggressive enblock surgery and complete lymphadenectomy. Another of the modern approaches developed to enhance the efficacy of surgery is the combination of chemotherapy and immunotherapy or gene therapy which offers the advantage of exposing EC cell population for drugs and immune factors thus obviating cancer cell cycle cytotoxic and host immunoprotective effects [6-8]. Nevertheless, very few studies have demonstrated convincing clinical results. We developed optimal treatment strategies that incorporate bolus chemotherapy, radiotherapy and immunotherapy after radical, aggressive en-block surgery [9].
Patients and Methods
We conducted this study from 1975 to 2021. 551 consecutive ECP (male 411, female 140; age=56.5 ± 8.9 years, tumor size=6 ± 3.5 cm) (mean ± standard deviation) entered this trial. All ECP were white Europeans. Patients were not considered eligible if they had stage IV, previous treatment with chemotherapy, immunotherapy or radiotherapy or if there were two primary tumors of the time of diagnosis. Patients after non-radical procedures, postoperative died ECP were excluded to provide a homogeneous patient group. The preoperative staging protocol included clinical history, physical examination, complete blood count with differentials, biochemistry and electrolyte panel, chest X-rays, roentgenoesophagogastroscopy, abdominal ultrasound, bronchoscopy, fibroesophagogastroscopy, electrocardiogram. Computed tomography, NMR-tomography scan of upper abdomen, liver and bone radionucle scan were performed whenever needed. All ECP were diagnosed with histologically confirmed EC. All had measurable tumor and ECOG performance status 0 or 1. Before any treatment each patient was carefully examined by medical panel composed of thoracoabdominal surgeon and chemotherapeutist to confirm the stage of disease. All patients signed a written informed consent form approved by the local Institutional Review Board.
The initial treatment was started with radical procedures. We performed two types of procedures: 267 complete esophagogasrectomies with lesser and partially major omentum with preservation of right gastroepiploic vessels and lymph node dissection through separate abdominal and right thoracic incision (Lewis, Lewis-McKeown) and 284 through left thoracoabdominal incision (Garlock). The present analysis was restricted to ECP with complete resected tumors with negative surgical resection margin and with N1 and celiac lymph node metastases (N2). Complete surgical resection consisted of esophagogastrectomy with one stage intrapleural esophagogastrostomy in 361 (Lewis, Garlock), and with anastomosis on the neck in 190 (Lewis-McKeown, Garlock). EC was localized in lower third of esophagus in 361, middle third in 61, upper third in 75, total 54. Among these, 154 ECP underwent combined and extensive radical procedures with resection of pancreas, liver, diaphragm, aorta, VCS, colon transversum, lung, trachea, pericardium, splenectomy. The extent of lymphadenectomy in the upper abdominal compartment and lower posterior mediastinum was identical for all surgical approaches and comprised a suprapancreatic lymphadenectomy, including all lymph nodes along the common hepatic artery, celiac axis, and splenic artery toward the splenic hilum. The left gastric artery was always transected at its origin and remained with the specimen. Also included were all lymph nodes along the proximal two thirds of the lesser gastric curvature and the gastric fundus, left and right paracardiac nodes, distal paraesophageal nodes, and nodes in the lower posterior mediastinum up to the tracheal bifurcation. Patients with the right thoracoabdominal approach had an additional formal extended mediastinal lymphadenectomy comprising all nodes at the tracheal bifurcation along the left and right main stem bronchi, the upper mediastinal compartment, and along the left recurrent nerve. A systematic cervical lymphadenectomy was performed routinely for ECP with neck anastomosis (190). Routine twofield lymphadenectomy (in terms of EC surgery) was performed in 361, three fields in 190. The present analysis was restricted to ECP with complete resected tumors with negative surgical resection margin (R0) and with N1-N2 nodes. All ECP were postoperatively staged according to the TNM-classification. Histological examination showed adenocarcinoma in 314, squamous cell cancer 227 and mixed carcinoma in 10 patients. The pathological T stage was T1 in 128, T2 in 115, T3 in 181, T4 in 127 cases; the pathological N stage was N0 in 278, N1 in 70, N2 in 203 patients. The tumor differentiation was graded as G1 in 157, G2 in 141, G3 in 253 cases. Early EC was in 109 patients, invasive EC in 442. We understand as the early cancer the tumor up to 2 cm in diameter, witch invades submucosa without lymph node and distant metastases [10]. After surgery postoperative chemoimmunoradiotherapy were accomplished ECP in ECOG performance status 0 or 1.
All patients (551 ECP) were divided between the two protocol treatments: (1) surgery and adjuvant chemoimmunoradiotherapy (128 ECP group A); (2) surgery alone without any adjuvant treatment 423 ECP group (B) the control group.
All 128 patients completed adjuvant chemoimmunoradiotherapy (group A): 1 cycle of bolus chemotherapy was initiated 10 days-14 days after complete resections and consisted of fluorouracil (5-FU) 500 mg/ m2 intravenously (IV) for 5 days. Immunotherapy consisted thymalin or taktivin 20 mg intramuscularly on days 1, 2, 3, 4 and 5. These immunomodulators produced by Pharmaceutics of Russian Federation (Novosibirsk) and approved by Ministry of Health of Russian Federation. Thymalin and taktivin are preparations from calf thymus, which stimulate proliferation of blood T-cell and B-cell subpopulations and their response [11]. The importance must be stressed of using immunotherapy in combination with chemotherapy, because immune dysfunctions of the cell mediated and humoral response were induced by tumor, surgical trauma, radiotherapy and chemotherapy [5,10]. Such immune deficiency induced generalization of EC and compromised the long term therapeutic result. In this sense immunotherapy shielded human organism from side and adverse effects of basic treatment. 4-5 courses of adjuvant chemoimmunotherapy were repeated every 28 day. Concurrent radiotherapy (60 CO; ROKUS, Russia) with a total tumor dose 45 Gy-50 Gy starting 5 weeks-7 weeks after surgery. Radiation consisted of single daily fractions of 180 cGy- 200 cGy 5 days weekly. The treatment volume included the ipsilateral hilus, the supraclavicular fossa and the mediastinum from the incisura jugularis to 8 cm below the carina. The lower mediastinum and upper abdomen were included in cases of primary tumors in the lower third of esophagus or N2. The resected tumor bed was included in all patients. Parallel opposed AP-PA fields were used. All fields were checked using the treatment planning program COSPO (St. Petersburg, Russia). Doses were specified at middepth for parallel opposed technique or at the intersection of central axes for oblique technique. No prophylactic cranial irradiation was used. During chemoimmunotherapy antiemetics were administered. Gastrointestinal side effects, particularly nausea and vomiting, were mild, and chemoimmunotherapy was generally well tolerated. Severe leukopenia, neutropenia, anemia and thrombocytopenia occurred infrequently. There were no treatment related deaths.
A follow up examination was; generally, done every 3 months for the first 2 years, every 6 months after that and yearly after 5 years, including a physical examination, a complete blood count, blood chemistry, chest roentgenography. Endoscopy and abdominal ultrasound were done every 6 month for the first 3 years and yearly after that. Zero time was the data of surgical procedures. No one was lost during the follow up period and we regarded the outcome as death through personal knowledge, physician's reports, and autopsy or death certificates. Survival time (days) was measured from the date of surgery until death or the most recent date of follow up for surviving patients.
Variables selected for 10 YS and life span study were sex, age, TNM, cell type, tumor size. Survival curves were estimated by the Kaplan Meier method. Differences in curves between groups of GCP were evaluated using a log rank test. Multivariate proportional hazard Cox regression, structural equation modeling (SEPATH), Monte Carlo simulation and neural networks computing were used to determine any significant dependence [12-16]. Neural networks computing, system, biometric and statistical analyses were conducted using CLASS MASTER program (Stat Dialog, Inc., Moscow, Russia), SANI program (Stat Dialog, Inc., Moscow, Russia), STATISTICA and STATISTICA Neural Networks program (Stat Soft, Inc., Tulsa, OK, the USA), DEDUCTOR program (BaseGroup Labs, Inc., Riazan, Russia), SPSS (SPSS Inc., Chicago, IL, USA), Table Curve 3D (Systat Software Inc., San Hose, CA, USA), SIMSTAT2.6 (Provalis Research, Montreal, Canada). All tests were considered significant when the resulting P value was less than 0.05.
Results
For the entire sample of 551 patients’ overall life span (mean ± standard error) was 1881.1 ± 2230.6 days (95% CI, 1694.4-2067.8; median=854) and cumulative 5 year survival reached 52.1%, 10 year survival 45.9%, 20 year survival 33.7% (Figure 1). 184 ECP (life span=4308.7 ± 2413.3 days) lived more than 5 years and 99 (life span=5883 ± 2296.6 days) more than 10 years without any features of EC progressing. 226 ECP (life span=628.3 ± 319.9 days) died due to the cancer generalization within the first 5 years after complete gastrectomies.
It is necessary to pay attention on the five very important prognostic phenomenons. First, 95.7% 10 year survival (10 YS) for ECP with the early cancer as against 32.1% for the others ECP after esophagogastrectomies (P=0.000 by log rank test) (Figure 2).
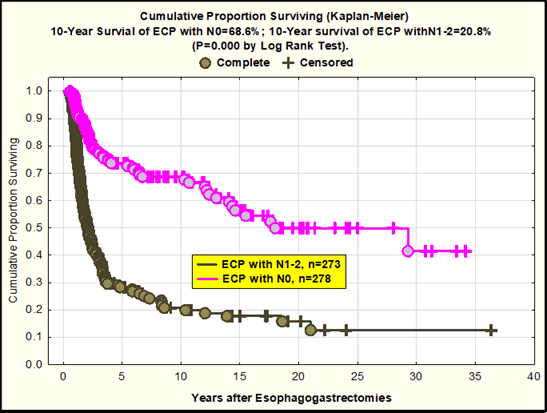
Second, good 10 YS for ECP with N0 (68.6%) as compared with ECP with N1-2 (10 year survival was 20.8%) after radical procedures (P=0.000 by log rank test) (Figure 3)
Third, for the 128 ECP in adjuvant chemoimmunoradiotherapy (AT) arm (group A) cumulative 10 YS reached 68.8% vs. 48.5% (group B) (P=0.00025 by log rank test) (Figure 4)
Fourth, we revealed 61.4% 10 YS for ECP with upper third of esophagus versus 42.4% for other ECP after surgery (P=0.00339 by log rank test) (Figure 5)
In the fifth we found 60.6% 10 YS for ECP versus 30.2% for cardioesophageal cancer patients after surgery (P=0.000 by log rank test) (Figure 6).
All clinicopathologic characteristics and treatment modalities were evaluated in traditional Cox multivariate prognostic factor analysis. In accordance with Cox regression model, the 17 variables significantly explained ECP 10 YS with N0-2 (n=551) after complete esophagogastrectomies. Cox modeling displayed that 10 YS of ECP significantly depended on: phase transition (PT) N0-N12 in terms of synergetics, cell ratio factors (ratio between cancer cells CC and blood cells subpopulations), G1-3, T1-4, AT, prothrombin index, protein, hemorrhage time, residual nitrogen, age, histology, tumor localization (P=0.000-0.021) (Table 1).
| Cox Regression n=551 | Parameter Estimate | Standard Error | Chi-square | P value | 95% Lower CL | 95% Upper CL | Hazard Ratio |
|---|---|---|---|---|---|---|---|
| Hemorrhage Time | 0.0014 | 0.000402 | 12.18223 | 0.000482 | 0.00062 | 0.002192 | 1.0014 |
| Residual Nitrogen | 0.05156 | 0.01111 | 21.54182 | 0.000003 | 0.02979 | 0.073339 | 1.05292 |
| Protein | 0.02145 | 0.008664 | 6.12841 | 0.013303 | 0.00447 | 0.038428 | 1.02168 |
| Prothrombin Index | 0.02606 | 0.006403 | 16.55867 | 0.000047 | 0.01351 | 0.038605 | 1.0264 |
| T1-4 | 0.43221 | 0.08598 | 25.26934 | 0 | 0.26369 | 0.600727 | 1.54066 |
| N0---N12 | 0.59826 | 0.161755 | 13.67911 | 0.000217 | 0.28122 | 0.91529 | 1.81894 |
| Age | 0.02845 | 0.007746 | 13.48557 | 0.00024 | 0.01326 | 0.043627 | 1.02885 |
| Histology | -0.28768 | 0.12496 | 5.30016 | 0.021323 | -0.5326 | -0.042767 | 0.75 |
| G1-3 | 0.42034 | 0.088794 | 22.41002 | 0.000002 | 0.24631 | 0.594377 | 1.52248 |
| Adjuvant Chemoimmunoradiotherapy | -0.93274 | 0.197277 | 22.35461 | 0.000002 | -1.31939 | -0.546082 | 0.39348 |
| Segmented Neutrophils/Cancer Cells | 4.04524 | 1.582385 | 6.53527 | 0.010576 | 0.94382 | 7.146654 | 57.12473 |
| Localization | -0.53528 | 0.190887 | 7.86335 | 0.005045 | -0.90941 | -0.161147 | 0.58551 |
| Leucocytes/Cancer cells | -4.11797 | 1.590909 | 6.70002 | 0.009641 | -7.2361 | -0.999847 | 0.01628 |
| Eosinophils/Cancer Cells | 4.26504 | 1.671458 | 6.51111 | 0.01072 | 0.98904 | 7.541033 | 71.1675 |
| Stick Neutrophils/Cancer Cells | 4.33745 | 1.642549 | 6.97321 | 0.008274 | 1.11812 | 7.55679 | 76.51243 |
| Lymphocytes/Cancer Cells | 3.93332 | 1.604682 | 6.00815 | 0.01424 | 0.7882 | 7.078439 | 51.07626 |
| Monocytes/Cancer Cells | 4.34006 | 1.643347 | 6.97483 | 0.008266 | 1.11916 | 7.560962 | 76.71226 |
Table 1: Results of multivariate proportional hazard Cox regression modeling in prediction of ECP 10-year survival after esophagectomies (n=551).
All of these differences and discrepancies were further investigated by structural equation modeling (SEPATH) as well as Monte Carlo simulation. For more exact analysis, 141 patients were excluded from sample, which were alive less than 10 years after complete esophagogastrectomies without relapse (Figure 7). It was revealed that the eleven clusters significantly predicted 10 YS and life span of ECP with N0-2 status (n=410): (1) PT early invasive EC; (2) PT N0-N12; (3) Cell Ratio Factors; (4) EC characteristics; (5) blood cell circuit; (6) biochemical homeostasis; (7) hemostasis system; (8) surgery type; (9) adjuvant chemoimmunoradiotherapy; (10) anthropometric data; (11) tumor localization.
Figure 7: Significant networks between ECP (n=410) 10 year survival, life span, cancer characteristics, blood cell circuit, cell ratio factors, hemostasis system, biochemic and anthropometric data, phase transition “early cancer—invasive cancer”, phase transition “cancer with N0—cancer with N1-N2” and treatment protocols (SEPATH network model).
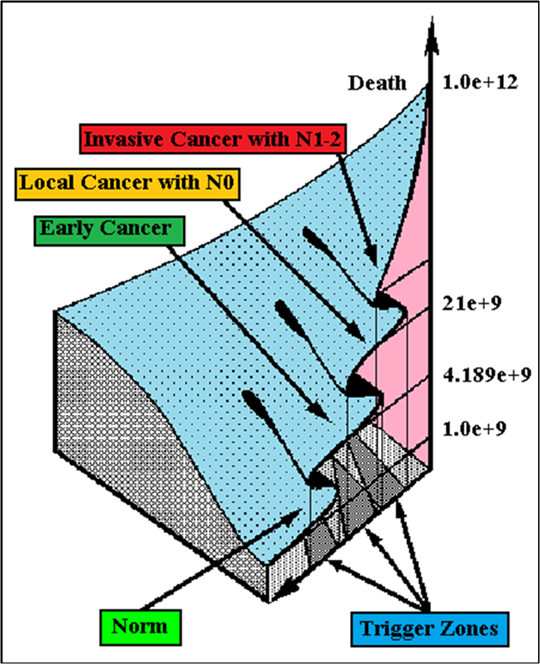
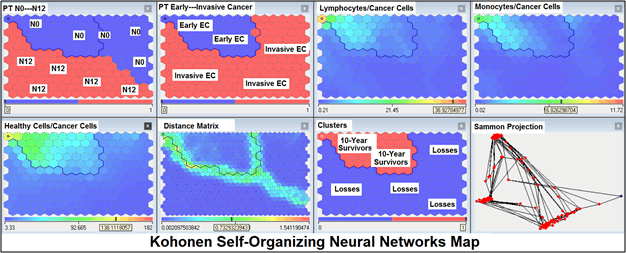
For comparative purposes, clinicopathological factors of ECP (n=410) were tested by neural networks computing (4 layer perceptron) (Table 2). Obviously, analyzed data provide significant information about EC prediction. High accuracy of classification (10 year survivors vs. losses) was achieved 100% (baseline error=0.000, area under ROC curve=1.0). In other words, it remains formally possible that at least 11 of these factors might predate neoplastic generalization: PT N0-N12 (rank=1), healthy cells/CC (2), PT early invasive EC (3), thrombocytes/CC (4), erythrocytes/ CC (5), lymphocytes/CC (6), eosinophils/CC (7), stick neutrophils/CC (8), segmented neutrophils/CC (9), monocytes/CC (10), leucocytes/CC (11). Moreover, bootstrap simulation confirmed the paramount value of Cell Ratio Factors, PT N0-N12 and PT early-Invasive GC (Table 3). It is necessary to note a very important law: both transitions of the early cancer into the invasive cancer, as well as the cancer with N0 into the cancer with N1-N2, have the phase character. These results testify by mathematical and imitating modeling of system “EC-patient homeostasis” in terms of synergetics (Figures 8-16). This also proves the first results received earlier in the work [10]. Presence of the two phase transitions is evidently shown on Kohonen self-organizing neural networks maps (Figure 17).
| Factors: Correct Classification Rate=100%; Error=0.0; Area under ROC Curve=1.0 | Rank | Sensitivity |
|---|---|---|
| Phase Transition N0---N12 | 1 | 14768 |
| Healthy Cells/Cancer Cells | 2 | 11853 |
| Phase Transition Early---Invasive Cancer | 3 | 10080 |
| Thrombocytes/Cancer Cells | 4 | 8933 |
| Erythrocytes/Cancer Cells | 5 | 8326 |
| Lymphocytes/Cancer Cells | 6 | 7595 |
| Eosinophils/Cancer Cells | 7 | 6406 |
| Stick Neutrophils/Cancer Cells | 8 | 5490 |
| Segmented Neutrophils/Cancer Cells | 9 | 3938 |
| Monocytes/Cancer Cells | 10 | 2943 |
| Leucocytes/Cancer Cells | 11 | 2658 |
Table 2: Results of neural networks computing in prediction of 10-year survival of ECP after esophagectomies (n=410).
Discussion
Central goal of the present research was to estimate the efficiency of adjuvant chemoimmunoradiotherapy after complete esophagogastrectomies. The importance must be stressed of using complex system analysis, artificial intelligence (neural networks computing), statistical methods and simulations in combination, because the different approaches yield complementary pieces of prognostic information [3,10,16,17].
Although there is no consensus on adjuvant treatment followed by radical procedures two of the most commonly employed strategies are surgery alone and adjuvant chemo radiotherapy with or without immunotherapy.
Actually surgical removal of tumor and its metastases remains basic management of this very aggressive cancer giving the real chance for recovery in spite of quite intensive researches developed during the last 30 years in terms of chemotherapy, radiotherapy and immunotherapy [18,19]. Unfortunately, the effectiveness of complete esophagogastrectomies (Lewis, Garlock, Lewis McKeown) has already reached its limit and leaves much to be desired: the average real 5 year survival rate of radically operated ECP even after combined and extensive procedures is 20%-30% and practically is not improved during the past 50 years, as the great majority of patients has already EC with stage II-III [9,20].
In the last 10 years-20 years a number of new drugs have been shown to have good activity against EC, including mitomycin C, UFT, epirubicin, etoposide, cisplatin, doxetacel, irinotecan, etc. [21-23]. On the other hand new immunomodulators, checkpoint inhibitors, new adoptive immunotherapeutic modalities with lymphokine activated killer cells, tumor infiltrating lymphocytes and high dose interleukins have been developed and antitumor effect have been successfully demonstrated in advanced malignancies, including EC [7,24-26].
Theoretically chemoimmunoradiotherapy is most effective when used in patients with a relatively low residual malignant cell population (approximately 1 billion cancer cells per patient) in terms of hidden micrometastases [10]. This is typical clinical situation at ECP with stage II-III after complete esophagogastrectomies (R0). Present research only confirmed this axiom.
In summary, when adjuvant chemoimmunoradiotherapy is applied to complete esophagogastrectomies for EC, the following benefits should be considered: (1) possibility of total elimination of residual hidden micrometastases; (2) surgery and chemoradiotherapy can result immunosuppressive state, which can be improved by immunotherapy; (3) radical operated ECP with stage II-III are thought to be potentially good candidates for adjuvant chemoimmunoradiotherapy as the majority of these patients would be expected to have EC progressing.
Further investigations will be required to determine efficiency, compatibility and tolerance of new drugs, immunomodulators and checkpoint inhibitors after esophagogastrectomies. The results of the present research will offer guidance for the design of future studies.
Conclusion
10 Year survival after radical procedures significantly depended on: (1) PT “early invasive cancer”; (2) PT N0-N12; (3) Cell Ratio Factors; (4) blood cell circuit; (5) biochemical factors; (6) hemostasis system; (7) AT; 8) EC characteristics; (9) tumor localization; (10) anthropometric data; (11) surgery type.
Optimal diagnosis and treatment strategies for EC are: (1) screening and early detection of EC; (2) availability of experienced thoracoabdominal surgeons because of complexity of radical procedures; (3) aggressive en block surgery and adequate lymph node dissection for completeness; (4) precise prediction; (5) adjuvant chemoimmunoradiotherapy for ECP with unfavorable prognosis.
Conflicts of Interests
I do not have any financial, commercial, legal, or professional relationship with other organizations, or with the people working with them, that could influence my research.
References
- Ku GY, Ilson DH (2007) Esophageal cancer: adjuvant therapy. Cancer J 13:162-7.
- Lin D, Leichman L (2014) The current status of neoadjuvant therapy for esophageal cancer. Semin Thorac Cardiovasc Surg 26:102-9.
- Kshivets O (2008) Esophageal cancer: Optimization of management. Cardio and Thora Surg J 1:1-11.
- Xiao X, Hong HG, Zeng X, Yang YS, Luan SY, et al. (2020) The efficacy of neoadjuvant versus adjuvant therapy for resectable esophageal cancer patients: A systematic review and meta-analysis. World J Surg 44:4161-4174.
- Kshivets O (2017) Optimization of management for esophageal cancer patients with stage T1-4N0-2M0. Inter J of Clinc Med Res 4:30-37.
- Kelly RJ (2019) Emerging multimodality approaches to treat localized esophageal cancer. J Natl Compr Canc Netw 17:1009-1014.
- Kono K, Nakajima S, Mimura K (2020) Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer 23:565-578.
- Akin Telli T, Bregni G, Camera S, Deleporte A, Hendlisz A, et al. (2020) PD-1 and PD-L1 inhibitors in oesophago-gastric cancers. Cancer Lett 469:142-150.
- Egyud MR, Tseng JF, Suzuki K (2019) Multidisciplinary therapy of Esophageal Cancer. Surg Clin North Am 99:419-437.
- Kshivets O (1995) Expert system in diagnosis and prognosis of malignant neoplasms. Dissertation for Sc.D., Tomsk 10:639.
- Morozow VG, Chavinson VC (1981) Isolation, refinement and identification of immunomodulated polypeptide from calf and human thymus. Biochemistry 9:1652-59.
- Odom-Maryon T (1996) Biostatistical methods in oncology. Cancer management: A multidisciplinary approach. Huntington 1:788-802.
- Mirkin BG (1990) A sequential fitting procedure for linear data analysis models. J Classification 7:167-196.
- Joreskog KG, Sorbom D (1982) Recent development in structural equation modeling. J Marketing Research 19:404-416.
- Bostwick DG, Burke HB (2001) Prediction of individual patient outcome in cancer: comparison of artificial neural networks and Kaplan-Meier methods. Cancer 91:1643-1646.
- Husmeier D (2000) The Bayesian evidence scheme for regularizing probability-density estimating neural networks. Neural Comput 12:2685-2717.
- Fujita H (2020) Ways and tradition of Japan in esophageal surgery for cancer. Gen Thorac Cardiovasc Surg 68:1187-1192.
- Yamasaki M, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, et al. (2014) Perioperative therapy for esophageal cancer. Gen Thorac Cardiovasc Surg 62:531-40.
- Kshivets O (2010) Significant Impact of Phase Transitions and Cell Ratio Factors For 5-Year Survival of Cardioesophageal Cancer Patients after Surgery. Anna of Surg Oncogy 17:312-29.                                                          Â
- Ikebe M, Morita M, Yamamoto M, Toh Y (2016) Neoadjuvant therapy for advanced esophageal cancer: The impact on surgical management. Gen Thorac Cardiovasc Surg 64:386-94.
- Visser BC, Venook AP, Patti MG (2003) Adjuvant and neoadjuvant therapy for esophageal cancer: A critical reappraisal. Surg Oncol 12:1-7.
- Gao SJ, Park HS, Corso CD, Rutter CE, Kim AW, et al. (2017) Role of adjuvant treatment in esophageal cancer with incidental pathologic node positivity. Ann Thorac Surg 104:267-274.
- Mimura K, Yamada L, Ujiie D, Hayase S, Tada T, et al. (2018) Immunotherapy for esophageal squamous cell carcinoma: A review. Fukushima J Med Sci 64:46-53.
- Sohda M, Kuwano H (2017) Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg 23:1-11.
- Ku GY, Ilson DH (2013) Adjuvant (postoperative) therapy for esophageal cancer. Thorac Surg Clin 23:525-33.
- Park R, Williamson S, Kasi A, Saeed A (2018) Immune therapeutics in the treatment of advanced gastric and esophageal cancer. Anticancer Res 38:5569-5580.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1478
- [From(publication date): 0-2021 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 1072
- PDF downloads: 406