Epidermal Growth Factor Immunotherapy: Exploring the Effects of NB-UVB Exposure.
Received: 25-Jun-2018 / Accepted Date: 12-Jul-2018 / Published Date: 17-Jul-2018 DOI: 10.4172/2161-0681.1000348
Keywords: NB-UVB; HaCaT; Cytokines; Cetuximab
Introduction
The Epidermal growth factor receptor (EGFR) is over-expressed in many solid tumors and is associated with poorer prognosis and outcomes [1]. The growing investigation on EGF inhibitors application, in anticancer therapy, has been related to their high specificity for EGFR.
Target therapies for EGFR, with inhibitors, determine a downregulation of EGFR signal pathway, regulating cell differentiation, proliferation, migration, angiogenesis and apoptosis [2,3] improving the safety profile compared to conventional chemotherapeutic agents, generally interfering with DNA and RNA synthesis.
EGFR-inhibitors can be classified into two categories, the monoclonal antibodies (mAb) and the tyrosine kinase inhibitors (TKI). mAbs acts by a competition with the endogenous ligands, epidermal growth factor (EGF) and transforming growth factor alpha (TGF), leading to receptor dimerization and inhibition of EGFR activity, with a reduction in cancer proliferation, invasion, metastasis, angiogenesis and inhibition of apoptosis [4]. In contrast, TKI mechanism is represented by the competition with the adenosine triphosphate (ATP) to bind the tyrosine kinase region, determining intracellular catalytic activity inhibition of the receptor [5,6].
Cetuximab, an anti-EGFR, is a chimeric human-murine immunoglobulin (IgG1) approved by the American Food and Drug Administration (FDA) for the treatment of EGFR positive metastatic colon rectal cancer, either alone or in combination with chemotherapy [4,7]. Cetuximab, can be used in patients without mutations in RAS gene, since it was demonstrated to inhibit the downstream RASsignaling cascade and ERK activation [8].
Patients, treated with immunotherapy, show dermatological side effects, with an incidence ranging from 80% to 86% of cases [3,5]. These could be motivated by the highly EGFR expression in the epidermis, at the basal cell layer. Since EGF may control skin immunity [4], the dermatological side effects may be due to an inflammatory response mediated by keratinocyte-derived cytokines, macrophages, mast cells and granulocytes recruitment, followed by long-term cutaneous effects development. Due to its role, in the regulation of barrier function, innate host defense and inflammatory reaction, in epidermal keratinocytes, EGFR inhibition induces the release of inflammatory mediators and consequently infiltration of immune cells.
The most common form of cutaneous adverse events is the papulopustular acneiform rash [9,10] associated with mAb anti-EGFR, followed by xerosis, fissures, pruritus, paronychia, and blepharitis [11,12].
The papulopustular acneiform rash generally occurs after 1-2 weeks from the onset of therapy (on average 8 to 10 days), peaking at 3 weeks after treatment initiation and decreases in severity after 6-8 weeks. Post-inflammatory skin alterations, such as erythema and hyperpigmentation, are long-standing sequel that can last for months or years. Typically seborrheic areas (face, scalp, trunk) and/or sunexposed, are preferred, while rarely found in the upper and lower extremities of the abdomen, gluteal region, saving palm plantar sites [3,9]. The histopathological evaluation of papules and pustules shows an aseptic supportive folliculitis: in the earliest stages of treatment with anti-EGFR, the inflammatory infiltrate is mainly composed of T lymphocytes, which is subsequently replaced by an inflammatory infiltration composed mainly of neutrophils distributed in the dermis, particularly around the follicles. Microbiological tests have demonstrated the absence of infection, confirming that this is a sterile process [10]. Papulopustular skin rash may be accompanied by postinflammatory hyperpigmentation, telangiectasia, and erythema [9]. These features develop as the dose-dependent effect, and the severity of anti-EGFR inhibitors associated rash, was classified using the US Common Terminology Criteria for Adverse Events (CTCAE), in 5 grades [9]. Moreover, a strong correlation between the presence and severity of the cutaneous effects with patient compliance and survival is reported. Patients with dermatological rash appear to be the most responsive to the anti-EGFR treatments [13,14]. Dermatological side events are persistent during life and have a deep impact on the patient’s quality of life (QoL) and may result in a significant physical and emotional discomfort. Studies have suggested that 30% of patients needs to stop therapy due to cutaneous adverse events [15,16].
Sun exposure could exacerbate anti-EGFR toxicities, as suggested by clinical and experimental data, through the deregulation of cell cycle and cells proliferation induction [17-19].
Based on wavelengths, the sun ultraviolet radiations are classified as ultraviolet A (UVA), ultraviolet B (UVB) and ultraviolet C (UVC). UVB, ranging from 295 to 315 nm is one of the main components of solar UV that reach only the very superficial layers of skin, composed predominantly of keratinocytes [20]. The UVB exposure may be responsible for a dose-related effect, such as a premature skin aging, local and systemic immunosuppression, and promotion of different biological events [17] such as sunburn, inflammation, cell and/or tissue damage, cell death and skin cancer [18,21,22]. Their immunosuppressive properties are due to the interaction with DNA leading to single-stranded DNA breaks and intra-stranded DNA basecross linking via formation of several photoproducts [21] with immunosuppressive role and production of reactive oxygen species (ROS) responsible for epigenetic alterations [21-25]. In cancer cells, UVB-exposure may be responsible for a conformational change in EGFR, causing an altered EGFR signaling pathway transduction. Moreover, the 280-315 nm wavelengths are commonly used in the clinical application for the treatment of cutaneous conditions due to their immunosuppressive action [21]. Immunomodulatory effect of UVB on the skin is widely used for the treatment of psoriasis and eczema. Phototherapy represents the main and often a favorite therapeutic option for several dermatoses. In vitro studies were conducted to evaluate the beneficial effects of UV-irradiation on skin cells, suggesting that UV-treatment on cell culture activates genes related to cell division and immune responses. UV-exposure might be beneficial for wound healing and restoration of skin homeostasis, besides its anti-inflammatory, antioxidant, and also acting as modulator of keratinocyte-melanocyte cross-talks [26,27]. The balance between negative and positive effect UVB-exposure, remain to be clarified.
It is well known that keratinocytes are able to produce a variety of cytokines and chemokines, according to the inducing stimulus.
In this study, we investigated whether a specific range of light, called “narrow-band ultraviolet” (NB-UVB) that theoretically can reach the optimum response minimizing skin redness if compared with the “broad-band” (BB-UVB) in the range of UVB [28].
Despite NB-UVB has been developed more than 30 years ago, the exact mechanism of its therapeutic action remains poorly understood.
The 90% of NB-UVB irradiation is absorbed by epidermis, making keratinocytes the most important players in mediating biological activity, moreover NB-UVB also induce systemic effects on different cells. Lymphocytes or Langerhans cells are a primary target of NBUVB systemic action [21].
Most of NB-UVB effects are attributed to its influence on the immune system, with immunosuppressive effect than BB-UVB on natural killer cell activity, cytokine production and peripheral blood mononuclear cells responses. NB-UVB irradiation it has been shown to determine the reduction in circulating Th1, Th17 and CD8+ cytotoxic T cell levels with increase in Treg levels, in psoriasic patients compared to halthy control [29].
NB-UVB irradiation is involved in the balance in cytokines network that plays a critical role in the health and in body’s response to stimulus. The ability of NB-UVB in suppression of cytokines production, is involved in the shift of the immune response toward Thelper (Th)2-like responses. Indeed the expression of TNF-α, IL-17A, IL-6, IL-1 β, INF-γ, and IL-10, the most important cytokines involved in precesses as wound healing, infections, inflammation, autoimmune and inflammatory skin conditions can be downregulated by NB-UVB therapy [30].
NB-UVB exposure could modulate the expression and production of inflammatory cytokines and chemokines, in human model of keratinocytes, in presence of Cetuximab.
Materials and methods
HaCaT cell culture and treatments
The human immortalized non-tumorigenic keratinocytes cell line (HaCaT) were supplied by Cell Lines Service (CLS, Germany) and growing in High-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/ml of streptomycin (Sigma-Aldrich, USA), in humidified atmosphere containing 5% CO2 at 37°C. For all experiments were used the same passage of HaCaT cells and over-night in serum starving condition, before starting treatments and incubations. Cells were incubated for 24 hrs in presence of EGF (4 nM) or Cetuximab (10 μg/ml) whether or not UVB-irradiation (0.06 J/cm2).
Western Blotting
In HaCaT whole cells lysate, proteins were extracted using ice-cold RIPA lysis buffer (Sigma-Aldrich, USA) added with protease inhibitor cocktail (Sigma-Aldrich, USA). 50 μg of proteins were separated to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE 10%) and then transferred for 1hr to a nitrocellulose membrane (Bio-Rad, USA). EGFR immunoreactive proteins were evaluated using the following antibodies: EGFR 1005 (Santa Cruz Biotechnology, USA), EGFR phosphotyrosine-1068 (Cell Signaling Technology, USA) and horseradish peroxidase-conjugated secondary antibodies, goat anti-rabbit (Santa Cruz Biotechnology, USA). Specific signals were detected and analyzed with ChemiDocTMTouch Imaging System (Bio- Rad, USA) using a chemiluminescence system, ClarityTM-ECL Western Blotting Substrate (Bio-Rad, USA).
UVB-exposure
A Sunlamp 70 230 V (CIRCA)-0.8 A-50 Hz; NB-UVB, containing three Philips 20 W ultraviolet-B TL20/01RS bulbs was used for cells irradiation. According to the literature and after serial irradiation dose-response experiments, the frequency of 0.06 J/cm2, was selected and controlled using a UV light-meter Vario-control (Waldmann, Germany) positioned at the same distance from the UV source as the cells. HaCaT cells were grown in 6-well microplates (Eppendorf, Germany) until confluence. Cells were exposed to UVB-irradiation (0.06 J/cm2), replacing the culture medium with 1 ml of Dulbecco’s PBS, w/o CaCl2 and MgCl2 (Sigma-Aldrich, USA). Non-irradiated cell controls were also covered with Dulbecco’s PBS for the same time. After irradiation, the culture medium has been replenished and cells were grown for 24 hrs prior testing.
Measurement of cell viability and ROS production
HaCaT cells viability was determined by using the 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, USA). HaCaT were plated in 96-well microplates in quadruplicate and exposed to the three different treatments, EGF (4 nM), Cetuximab (10 μg/ml) and UVB (0.06 J/cm2), for 24 hrs. After treatments, the MTT-based assay was developed according to the procedures indicated by the manufacturer. Color absorbance was quantified at 570 nm using the spectrophotometer module of the GloMax®-Multi Microplates Detection Reader (Promega Corporation, USA). Cell viability was also estimated using a Bürker counting chamber with trypan blue, vital dye. To assess, reactive oxygen species (ROS), 10 μM of carboxy-2’,7’-dichloro-dihydro-fluorescein diacetate (DCHF-DA) fluorescent probe has been added to the cells treated with Cetuximab. The plate was irradiated with UVB (0.06 J/cm2) and intracellular ROS production was evaluated at 485 nm (excitation) and 527 nm (emission) wavelengths with the GloMax®-Multi Microplates Detection Reader (Promega Corporation, USA).
Enzyme-linked immunosorbent assay
Cytokine and chemokine concentration, in the supernatant obtained from HaCaT cells, after NB-UVB and Cetuximab treatments, were determined using ELISA assay. The Quantikine ELISA kit for Human CXCL8/IL8 and CCL5/RANTES (R&D Systems, USA) were used following manufacturer’s instructions. Relative absorbance was measured at 450 nm and the cytokines and chemokines concentration calculated using a reference standard curve.
Extraction of nucleic acid, cDNA synthesis, and real-time PCR
Total RNA was extracted from HaCaT according to the classical protocol phenol-chloroform, using QIAzol Lysis Reagent (Qiagen, Germany). After extraction, the total RNA concentration and purity were assessed using 1 μl of the sample and performed with NanoDrop 2000 (Thermo Scientific, USA). 1 μg/μl of cDNA, in a total volume of 20 μl, was obtained using the QuantiTect Reverse Transcription Kit (Qiagen, Germany) following the manufacturer's instruction. Template for the Real-time PCR was 1:2 diluted and evaluated for gene expression of IL-1β, IL-6, IL-8, CCL-2, and CCL-5, using RPS18 as housekeeping gene (Table 1). All primers sequences were designed using the AlleleID Primer Biosoft International (Premier Biosoft, USA) and IDT SciTools, Inc. (Tema Ricerca, Italy). Real-time PCR was carried out using the Mastercycler® ep realplex (Eppendorf, Germany) according to the manufacturer’s protocol. The cycle conditions for realtime PCR were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Each reaction was performed in triplicate.
| Forward Primer Sequence [5'-3'] | Reverse Primer Sequence [5'-3'] | Amplicone size | |
|---|---|---|---|
| RPS18 | CTTTGCCATCACTGCCATTAAG | TCCATCCTTTACATCCTTCTGTC | 199 bp |
| EGF-R | CCTGACTCCGTCCAGTATTG | GCTTGTTACTCGTGCCTTG | 169 bp |
| IL-1 | TGAGGATGACTTGTTCTTTGAAG | GTGGTGGTCGGAGATTCG | 115 bp |
| IL-6 | GTACATCCTCGACGGCATC | ACCTCAAACTCCAAAAGACCAG | 198bp |
| IL-8 | GTGTAAACATGACTTCCAAGCTG | GTCCACTCTCAATCACTCTCAG | 183bp |
| RANTES | CTGCTGCTTTGCCTACATTG | AGACGACTGCTGGGTTGG | 95 bp |
| MCP1 | AACTGAAGCTCGCACTCTCG | GAGTGAGTGTTCAAGTCTTCGG | 337 bp |
Table 1: Gene sequences
Statistical analysis
All data were summarized as the mean and standard deviation (SD) from three independent experiments. For each Real-time experiment to give an indication of the precision of the fold change, calculated with 2-ΔΔCt method, the 95% confidence interval (95% CI) was determined. Statistical comparison between values from exposed to non-exposed cells was analyzed by Student t-test for unpaired data. Pvalues were corrected for multiple comparisons when appropriated.
Analysis of variance (ANOVA) test was applied to evaluate statistical significance differences in CCL-5 and IL-8 release between different experimental conditions.
In all statistical tests, the threshold of statistical significance will be assumed equal to p=0.05. Data were analyzed using R-2.9.2 (R Core Development System: http://www.r- project.org).
Patients
Ten patients with colorectal or pulmonary carcinoma, candidates for Cetuximab-treatment were recruited at the Department of Oncology of the Clinical “SS. Annunziata” Hospital of Chieti. Eligibility criteria included patients who were scheduled to start firstline chemotherapy (oxaliplatin-/irinotecan-based chemotherapy) combined with Cetuximab, administered on a weekly schedule, with a 400 mg/m2 initial loading dose, followed by 250 mg/m2 weekly infusions until disease progression, and presenting skin Fitzpatrick's Phonotype less than IV, as assessed by the Dermatological Clinic of the SS Annunziata Hospital University of Chieti. This study was conducted in accordance with the guidelines from the International Conference on Harmonization-Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the Ethical Committee of the University “G d’Annunzio”, Chieti-Pescara, Italy. All patients provided written informed consent before participating in the study.
Evaluation of photosensitivity
To determine the photosensitivity of the patients, a solar simulator (model 16S SolarLight Co) was used. The device is capable of delivering an intensity of 2 mW/cm2 to simulate a solar exposure at doses gradually increasing of UVB on the surface of the forearms. Patients undergoing stimulation with the solar simulator had phenotype according to Fitzpatrick <IV. The timing of NB-UVB exposure was reported in Table 2. Evaluation of photosensitivity was carried out by handheld skin color probe (CORTEX TECHNOLOGY) for melanin detection (pigment present volume index), erythema (reddening index based on the amount of hemoglobin) and CIE*L*a*B*, three relative parameters for complexity (CIE*L) and skin coloring (CIE* and CIE*B). Color Space Color Lab (CIELAB) software is used to read colorimetric data.
| J/cm2 | 0,12 | 0,16 | 0,2 | 0,25 | 0,31 | 0,39 | 0,49 |
|---|---|---|---|---|---|---|---|
| Time | 1’ | 1’20’’ | 1’40’’ | 2’ | 2’35’’ | 3’15’’ | 4’ |
Table 2: Timing of irradiation
Results
EGFR phosphorylation in HaCaT cells
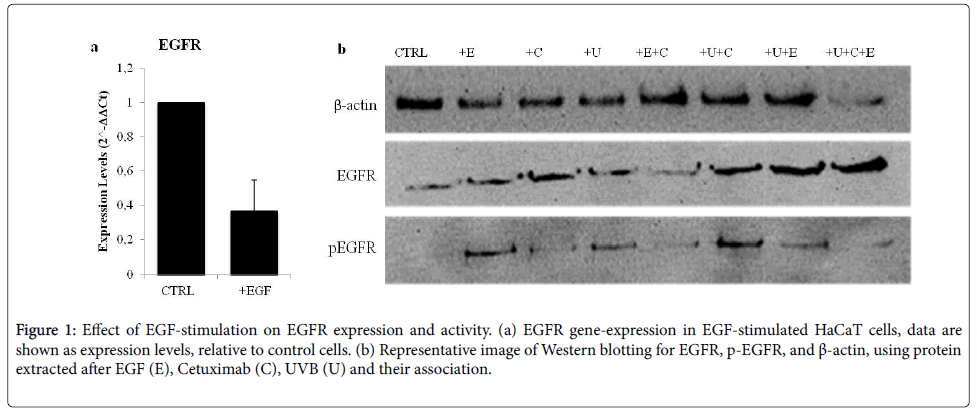
Presence and functionality of EGFR in HaCaT cells were assessed after stimulation with EGF (4 nM), UVB (0.06 J/cm2) exposure and Cetuximab-treatment. Figure 1a shows EGFR expression that appears to be down-regulated after EGF-treatment. Instead, EGFR phosphorylation (EGFR phosphotyrosine-1068), evaluated by Western Blotting analysis, was increased. UVB exposure enhanced levels of phosphorylation of EGFR were observed. No effects after Cetuximabtreatment were noticed. Interestingly, a combination of NB-UVB exposure and Cetuximab resulted in increased EGFR activation, higher than Cetuximab or NB-UVB alone (Figure1b). Our results indicate that EGFR is constitutively expressed in HaCaT cells and they represent a good model to study, in vitro , the effects of Cetuximabtreatment.
Figure 1: Effect of EGF-stimulation on EGFR expression and activity. (a) EGFR gene-expression in EGF-stimulated HaCaT cells, data are shown as expression levels, relative to control cells. (b) Representative image of Western blotting for EGFR, p-EGFR, and ß-actin, using protein extracted after EGF (E), Cetuximab (C), UVB (U) and their association.
Cells viability and ROS production
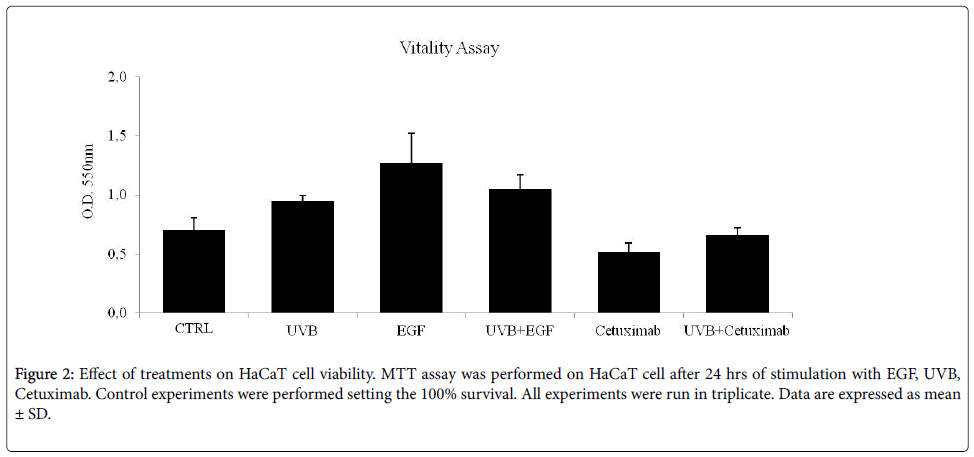
Cells viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay, and by cell counts by Bürker chamber in HaCaT cells, as described above. EGF-treatment, NB-UVB and their combination induce an increased cell survival and proliferation, concurrently inducing the increase of EGFR phosphorylation. Cetuximab-treatment causes a reduction of cells growth, and the association between NB-UVB and Cetuximab show no variation in cell viability (Figure 2). Taken together, these results suggest that NB-UVB can counteract the Cetuximab-dependent reduction of cells viability.
Oxidative stress and production of ROS, was also investigated. NBUVB exposure stimulates ROS generation, while Cetuximab-treatment causes about a 5% of the reduction in NB-UVB induced ROS generation (data not shown).
Cytokines and chemokines gene expression in NB-UVB exposed and cetuximab-treated cells
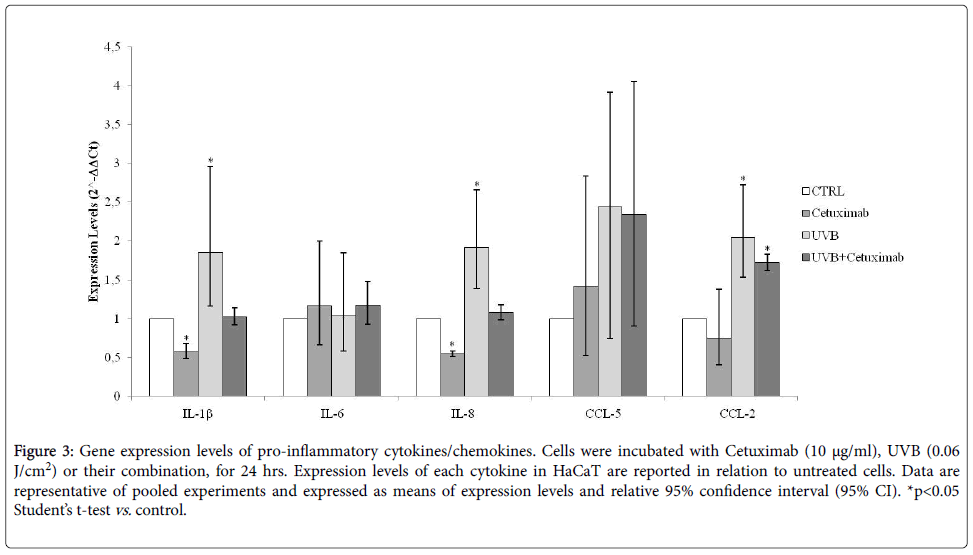
The expression levels of pro-inflammatory cytokines and chemokines, in HaCaT cells treated with Cetuximab, alone or in association to NB-UVB, were evaluated by Real-time PCR assay, after 24 hrs. Cetuximab-treatment reduces the expression of IL (interleukin)-1β (p=0.0001), IL-8, CCL(chemokine C-C motif ligand)-2 and CCL-5, a very slow increase of IL-6 expression, while NB-UVB exposure is responsible for the opposite expression, increasing significantly IL-1β (p=0.003), IL-8 (p=0.002) and CCL-5 (p=0.001), in relation to unexposed cells. Association between NBUVB and Cetuximab-treatments on keratinocytes cell culture, increased significantly CCL-2 expression, compared to control cells. IL-1β, IL-6, IL-8 and CCL-5 expression levels were not influenced (Figure 3).
Figure 3: Gene expression levels of pro-inflammatory cytokines/chemokines. Cells were incubated with Cetuximab (10 µg/ml), UVB (0.06 J/cm2) or their combination, for 24 hrs. Expression levels of each cytokine in HaCaT are reported in relation to untreated cells. Data are representative of pooled experiments and expressed as means of expression levels and relative 95% confidence interval (95% CI). *p
Chemokines quantification
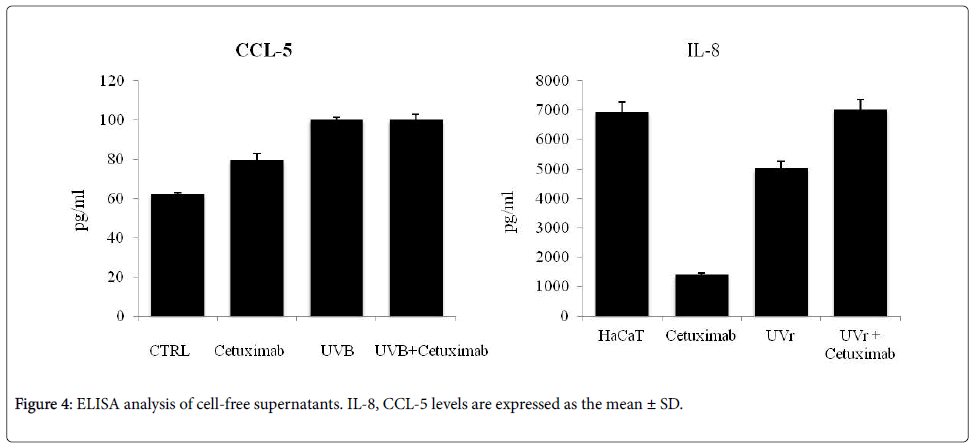
CCL-5 and IL-8, the major chemokines involved in the chemotaxis and inflammatory process, supporting acneiform reactions, were also quantized in cell cultures supernatant. The mean concentration of IL-8 decrease in presence of Cetuximab, compared to untreated cells, on the other hand, increased production of CCL-5 was observed. After NBUVB exposure, levels of IL-8 and CCL-5 concentrations, any significant variation was detected. Co-stimulation is responsible for an increased amount of both chemokines (Figure 4).
Photosensitivity of patients
In order to assess the sensitivity to solar light (Solar simulator model 16S Solar Light Company, USA), a pivotal experiment on oncologic patients, was performed. Patients enrolled were evaluated for photosensitivity before, during and after Cetuximab-treatment. We did not find differences in the intensity of erythema after NB-UVB exposure in all patients (n=10). The minimal erythema dose (MED) did not change in any patients, from baseline thought out the treatment time (Table 3).
| Exposure time | T2-T1 | p-value |
|---|---|---|
| 1’ | -0.4 | 0.55 |
| 1’20’’ | -0.4 | 0.92 |
| 1’40’’ | 0.5 | 0.64 |
| 2’ | 1.5 | 0.22 |
| 2’35’’ | 1.3 | 0.39 |
| 3’15’’ | 0.2 | 0.89 |
| 4’ | 1.4 | 0.63 |
Table 3: MED evaluation
Discussion
The anti-EGFR drugs represent a recognized treatment for a variety of tumour types and have established efficacy as a single agent and in combination with chemo/radio/hormone therapy. The efficacy of anti- EGFR therapies was related to EGFR and the activated signalling cascades involved in cell proliferation, differentiation, and apoptosis. These drugs are able to increase the progression-free survival, overall patient survival, to improve clinical outcome and quality of life in cancer patients. Unfortunately, besides its needed effects, also some unwanted effects were observed during anti-EGFR therapy. The adverse events may interfere with the quality of life of patients, reducing the compliance of the therapeutic regimen [16]. Sometimes, due to the severity of side effects, temporary suspension of the biological therapy could be recommended and, in 8%-17% of the cases, the complete suspension of the therapy is required. Anti-EGFR dose reduction or discontinuation, affect the treatment outcomes.
Since EGFR was expressed in the keratinocytes, the follicular epithelium, the most common side effects of anti-EGFR drugs are skin problems such as an acne-like rash on the face and chest during treatment. To ensure patient compliance, it could be helpful to identify the anti-EGFR role in cutaneous adverse events development. Treatments for dermatological side effects may include topical [17] and systemic antibiotic, steroids or topical immune modulator agents [31,32].
Although NB-UVB phototherapy represents one of the main treatments for an inflammatory skin condition, such as psoriasis and vitiligo, with minimal systemic toxicities [33]. According to different guidelines [33] and patient-oriented skin, adverse effects care treatments, exposure to UVB is not recommended in patients during immunotherapy. Several studies reported that following Cetuximab or others anti-EGFR treatment, patients may be sensitized to UVB exposure [3,34,35], and solar ultraviolet exposure has been linked to the increased skin inflammatory observed in these patients. Recently, the specific pathways activated by UVB-phototherapy, have been better understood, highlighting the link with the alteration of the cytokines profile at systemic level [36]. It is possible to explain that the increased sensitization could be due to the ability of the UVB to determine the inflammation onset, with associated vasodilatation and immune cells
recruitment at the skin level. UV-radiation also activates VEGF signalling involving EGF/PI3K pathway [37,38] and MAPK pathway [3].
The HaCaT cells, due to the common characteristic with normal keratinocytes, may allow a widely used in vitro model for skin pattern, producing a well-organized and differentiated pseudo-stratified epithelium [16,34].
To evaluate if NB-UVB exposure may modulate inflammatory mediators in anti-EGFR treated cells, we have analyzed the expression of pro-inflammatory cytokines in HaCaT cells, exposed to NB-UVB or Cetuximab alone and in association.
Our study indicates that the NB-UVB and Cetuximab treatments play a different role in defining the activation and functionality of keratinocytes. Following NB-UVB exposure there is increase cells viability and an important reduction of Cetuximab-induced cell death. First, we underline the higher EGFR phosphorylation UVB-induced, and then the induction of intracellular ROS formation. In accord with Yoshizumi et al. [39], the increase of IL-1, IL-8, CCL-2 and CCL-5 production and expression in HaCaT cells, was observed after 24 hrs of NB-UVB exposure. Otherwise the inhibition of EGFR by incubation with Cetuximab, in our in vitro skin model showed a reduction in cytokines and chemokines expression and release [40]. Inhibition of EGFR reduced also ROS production, likely with the downstream of Ras/Nox1 cascade [41,42]. Thus, in our model, Cetuximab reduced inflammatory mediators, in accord with previous studies conducted by El-Abaseri et al. and other researchers we observed that NB-UVB exposure may revert the Cetuximab-dependent EGFR protein reduction, followed by restoration of IL-1, IL-6 and IL-8 expression to basal levels [3,19,23].
CCL-5 and CCL-2, involved in the early inflammatory response [43-45] and in neutrophils, basophils, and monocytes recruitment in the inflammatory site, was not significantly reduced by the combination of NB-UVB exposure and Cetuximab-treatment.
Collectively, these data shows the tighter regulation of inflammatory response following NB-UVB exposure in Cetuximab-treated cells. The effect of NB-UVB, as immune-modulator, during Cetuximabtreatment, may open new perspectives for patients treated with EGF immunotherapy.
Our in vitro results are supported by the preliminary in vivo evaluation of the MED. Evaluation of patient’s phenotype and photosensitivity, before and after Cetuximab administration and exposure to the solar simulator device, show any additional harmful effects.
In conclusion, our in vivo and in vitro result, seem to deny the additive effect of NB-UVB exposure on dermatological side effects, during anti-EGFR therapy. Nevertheless, to better understanding the pathogenesis of skin reactions during anti-EGFR treatment, and effects of NB-UVB exposure, further in vivo studies must be performed. The knowledge of the pathogenic mechanisms of skin reactions could promote new strategies to prevent and manage adverse reaction in cancer patients, improving their QoL and favoring therapeutic adherence.
Acknowledgment
It is gratifying to honor the memory of Dr. Giovina Vianale with this work, which was substantially inspired by long dialogues with her. This study was supported by grants from the Università degli Studi G. D’Annunzio di Chieti-Pescara (Italy). The following statements should be used: E.C. and P.A. conceived and designed the experiments; M.D.T. and C.N. collected the samples; C.D.A and E.C. performed experiments; M.A. performed the in vivo exposure; M.R. and P.A. analyzed the data; M.R. and P.A. contributed reagents/materials/ analysis tools; E.C., P.A., M.R. wrote the paper; R.M. contributed to discussion.
References
- Xu MJ, Johnson DE, Grandis JR (2017) EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev 36: 463–473.
- Yewale C, Baradia D, Vhora I, Patil S,Misra A (2013) Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials 34: 8690-707.
- Fakih M, Vincent M. (2010) Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol 17: S18-30.
- Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW (2007) Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol 56: 317-26.
- Lupu I, Voiculescu VM, Bacalbasa N, Prie BE, Cojocaru I, et al. (2015) Cutaneous adverse reactions specific to epidermal growth factor receptor inhibitors. J Med Life 8: 57-61.
- Mendelsohn J, Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21: 2787-99.
- Prenen, H, De Schutter J, Jacobs B, De Roock W, Biesmans B, et al. (2009) PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin. Cancer Res 15: 3184–3188.
- VanCutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, et al. (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29: 2011–2019.
- Chanprapaph K, Vachiramon V, Rattanakaemakorn P (2014) Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract 2014: 734249
- Cignola S, Gonella S, Alessandra B, Palese A (2016) Monoclonal antibody-induced papulopustular rash: Clinical course, communication to health-care professionals and reactive measures as reported by patients. Eur J Oncol Nurs 20: 133-9.
- Urban C, Anadkat MJ (2013) A review of cutaneous toxicities from targeted therapies in the treatment of colorectal cancers. J Gastrointest Oncol 4: 319-327.
- Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, et al. (2006) Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55: 657-67.
- Tiwari S, Goel V, John MC, Patnaik N, Doval DC (2016) Efficacy and toxicity of cetuximab with chemotherapy in recurrent and metastatic head and neck cancer: A prospective observational study. Indian J Cancer 53: 487-492.
- Abdel-Rahman O, Fouad M (2015) Correlation of cetuximab-induced skin rash and outcomes of solid tumor patients treated with cetuximab: a systematic review and meta-analysis. Crit Rev Oncol Hematol 93: 127–135.
- Joshi SS, Ortiz S, Witherspoon JN, West DP, Anderson R, et al. (2010) Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 116: 3916–3923.
- De Tursi M, Zilli M, Carella C, Auriemma M, et al. (2017) Skin toxicity evaluation in patients treated with cetuximab for metastatic colorectal cancer: a new tool for more accurate comprehension of quality of life impacts. Onco Targets Ther 10: 3007-3015.
- Lacouture ME (2006) Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6: 803-12.
- Amerio P, Carbone A, Auriemma M, Varrati S, Tulli A (2009) UV induced skin immunosuppression. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry 8: 3-13.
- El-Abaseri TB, Fuhrman J, Trempus C, Shendrik I, Tennant RW, et al. (2005) Chemoprevention of UV light-induced skin tumorigenesis by inhibition of the epidermal growth factor receptor. Canc Res 65: 3958-65.
- Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A (2015) Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol 72: 203-18.
- Reich A, Mędrek K (2013) Effects of narrowband UVB (311 nm) irradiation on epidermal cells. Int J Mol Sci 14: 8456-66.
- Berneburg M, Röcken M, Benedix F (2005) Phototherapy with narrowband vs broadband UVB. Acta Dermato-Venereologica 85: 98-108.
- Correia M, Thiagarajan V, Coutinho I, Gajula GP, Petersen SB, et al. (2014) Modulating the structure of EGFR with UV light: new possibilities in cancer therapy. PLoSOne 9: e111617.
- Newton-Bishop JA, Chang YM, Elliott F, Chan M, Leake S, et al. (2011) Relationship between sun exposure and melanoma risk for tumors in different body sites in a large case-control study in a temperate climate. Eur J Cancer 47: 732-41.
- Watson M, Holman DM, Maguire-Eisen M (2016) Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin Oncol Nurs 32: 241-54.
- Rennekampff HO, Busche MN, Knobloch K, Tenenhaus M (2010) Is UV radiation beneficial in postburn wound healing?. Med Hypotheses 75: 436.
- Gupta A, Avci P, Dai T, Huang YY, Hamblin MR (2013) Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Adv Wound Care (New Rochelle) 2: 422-437.
- Chen X, Yang M, Cheng Y, Liu GJ, Zhang M (2013) Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database of Systematic Reviews. 10: CD009481.
- Fattouh M, El-Din AN, El-Hamd MA (2017) Role of Lymphocyte Subpopulations in The Immunopathogenesis of Psoriasis and The Effect of Narrow Band UVB Phototherapy on The Immunological Profile of Psoriasis Patients. Egypt J Immunol. 24: 105-117.
- Batycka-Baran A, Besgen P, Wolf R, Szepietowski JC, Prinz JC (2016) The effect of phototherapy on systemic inflammatory process in patients with plaque psoriasis. J Photochem Photobiol B. 161: 396-401.
- Perez-Torres M, Guix M, Gonzalez A, Arteaga CL (2006) Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. J Biol Chem 281: 40183-92.
- Palm MD, O'Donoghue MN (2007) Update on photoprotection. Dermatol Ther 20: 360-76.
- Lee E, Koo J, Berger T (2005) UVB phototherapy and skin cancer risk: a review of the literature. Int J Dermatol 44: 355–360.
- Jaka A, Gutiérrez-Rivera A, López-Pestaña A, del Alcázar E, Zubizarreta J, et al. (2015) Predictors of Tumor Response to Cetuximab and Panitumumab in 116 Patients and a Review of Approaches to Managing Skin Toxicity. Actas Dermosifiliogr 106 483-92.
- Schoop VM, Mirancea N, Fusenig NE (1999) Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J.Invest Dermatol 112: 343-53.
- Wong T, Hsu L, Liao W (2013) Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg 17: 6-12.
- Di Girolamo N, Coroneo M, Wakefield D (2005) Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am J Pathol 167: 489-503.
- Li Y, Bi Z, Yan B, Wan Y (2006) UVB radiation induces expression of HIF-1alpha and VEGF through the EGFR/PI3K/DEC1 pathway. Int J Mol Med 18: 713-719.
- Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, et al. (2008) Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int 32: 1405-11.
- Paul T, Schumann C, Rüdiger S, Boeck S, Heinemann V, et al. (2014) Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor-associated skin toxicity in cancer patients. Eur J Cancer 50: 1855-63.
- Dahan L, Sadok A, Formento JL, Seitz JF, Kovacic H (2009) Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br J Pharmacol 158: 610–620.
- Teppo HR, Soini Y, Karihtala P (2017) Reactive Oxygen Species-Mediated Mechanisms of Action of Targeted Cancer Therapy. Oxid Med Cell Longev 2017: 1485283.
- Finke J, Ferrone S, Frey A, Mufson A, Ochoa A (1999) Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today 20: 158-60.
- Mascia F, Mariani V, Girolomoni G, Pastore S (2003) Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol 163: 303-12.
- Frey AB, Monu N (2008) Signaling defects in anti-tumor T cells. Immunol Rev 222: 192-205.
Citation: Costantini E, Angelo C, Muraro R, Tursi M, Natoli C, et al. (2018) Epidermal Growth Factor Immunotherapy: Exploring the Effects of NB-UVB Exposure. J Clin Exp Pathol 8:348. DOI: 10.4172/2161-0681.1000348
Copyright: © 2018 Costantini E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3963
- [From(publication date): 0-2018 - Jul 08, 2025]
- Breakdown by view type
- HTML page views: 3123
- PDF downloads: 840