Epidemiology of Nosocomial Candida Species, Resistance Patterns, and the Associated Crude Mortality
Received: 27-Jan-2020 / Accepted Date: 11-Feb-2020 / Published Date: 18-Feb-2020 DOI: 10.4172/2332-0877.1000415
Abstract
Background: To study the nosocomial Candida epidemiology, susceptibility patterns, and associated cude mortality.
Methods: A multicenter prospective study. Laboratory logbooks were reviewed. Candida species considered were isolates or invasive. Patients' records were queried for the characteristics and demography. Statistical analysis was by Fisher's Exact Test for categorical and continuous variables, mean and ANOVA where appropriate.
Results: 307 Candida species were collected; total 15.2% and 2.48% were invasive infections percent admission. The mean length of hospital stay was 19 days (95% CI, 15.87 – 22.18, trimmed mean 15.18 days). C. albicans accounted for 34.5% and Non-albicans Candida 65.5% (p=0.000). No difference between C. albicans and Non-albicans candida in gender (p =0.148), age (p=0.305) and comorbidities (p=0.194), neither was the type of surgery (p=0.166) nor white blood cell count (p=0.595) 79 patients died; 31 in C. albicans and 48 in the non-albicans Candida (p=0.337). C. albicans susceptibility to fluconazole was 94.5%, C. glabrata 68.8%. Voriconazole was 100% active for all Candida species. C. albicans was 100% susceptible to echinocandins; C. glabrata 97.8%. Candida species were 93% susceptible to Amphotericin-B. The invasive candidiasis-associated crude mortality was 50%.
Conclusion: Non-albicans Candida was more prevalent than C. albicans. The antifungal resistance rates were high, and the crude mortality rates were similar for Candida species. No single case of C. auris was documented or suspected.
Keywords: Candida mortality; Antifungal resistance; Candida prevalence; Nosocomial candida
Introduction
Nosocomial Candida colonization may lead to higher rates of infections and invasive infections, frequently occur in patients with prolonged hospital stay and high-risk groups including the immunocompromised patients who are steadily growing like patients with Hematological malignancy, solid organ transplant patients, immunosuppressed patients, patients on chemotherapeutic agents, on steroids and antibacterial therapies [1-6]. Candida may colonize the oral, esophageal, vaginal and cutaneous structures, and it may cause invasive infections like bloodstream infection, hepato-splenic candidiasis, and peritonitis carrying a poor prognosis [7-9]. The epidemiology of Candida species has been changing in the last decades with the decrease in the prevalence of C. albicans augmented by the wide scale use of fluconazole as prophylaxis [10], allowing the relative increase in the prevalence of non-albicans Candida species, leading to more difficult-to-treat infections [11]. The growing candida resistance to antifungal agents are alarming, in a "USA-SENTRY" based study, nosocomial Candida was more resistant than community-onset candida stains, especially to the triazoles and echinocandins reaching rates somewhat above 5%, and some strains of C. glabrata were resistant to both classes [12]. Furthermore, multidrug-resistant species emerged and being reported from different countries, a new resistant strain i.e. C. auris was reported from Japan in 2009, it demonstrates resistance to several antifungal classes, it was associated with patients who had diabetes mellitus, recent surgery, a central venous catheter, patients receiving systemic antifungal therapy, and a patient with immotile cilia syndrome (primary ciliary dyskinesia) and bronchiectasis. Later reports showed that C. auris may have been misidentified as C. haemulonii and C. famata [13-19]. A nosocomial outbreak in a cardiac surgery center in London reported a genotypically closely related C. auris infection in the period 2015–2016, possible or proven C. auris constituted 44% (22/50) including Candidemia 18% (9/50) [20].
Mortality from bloodstream candidiasis is high, reaching up to 31.8%, especially if was associated with delayed empiric treatment, and failure to remove the causative central venous catheter [21,22]. The study aims to shed light on Candida in Jordan; its nosocomial prevalence, susceptibility, and the associated mortality (then after crude mortality) adding to the knowledge about this common yeast in the region.
Materials and Methods
Settings and study conduct
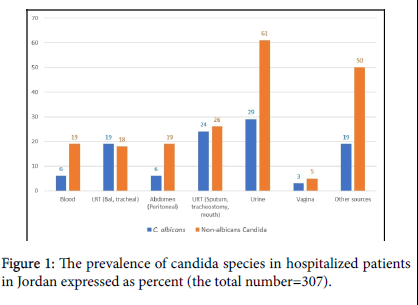
A multicenter prospective study focusing on the epidemiology, susceptibility, and in-hospital mortality of patients with candidiasis. The study was conducted in the period January 2018 to November 2019, it was conducted in Al Takhassusi, Al Khalidi and Jordan Hospitals, all located in Amman – Jordan, they encompass 650 beds including 60 ICU beds and serve as referral hospitals for patients coming from within and nearby countries, they contain operating rooms designed for cardiac surgery, kidney, and liver transplant surgery (Jordan hospital). The internal review and ethics board of each hospital approved the study. Laboratory logbooks and patients' records were reviewed for Candida growth, candida was considered as an isolate, except blood-growth candida or when isolated from a sterile body site then it was considered as invasive candidiasis. Patients' records were queried for other clinical data, including, the characteristics and demography of patients, diagnoses, prescribed antibacterial agents, comorbidities, antifungal agents prescribed as prophylaxis or treatment, duration of treatment or prophylaxis, the body site from which candida was isolated, white blood cell counts, and the site of surgery (Figure 1).
*Note that Candida albicans makes up 34.5% (106) Non-albicans Candida makes up 65.5% (201) of the isolated Candida species.
Outcome measures
To identify the epidemiology of nosocomial Candida infections, the prevalence of the regional candida species including the notorious C. auris should it suspected or uncovered, their susceptibility patterns, and the mortality associated with isolating Candida or invasive candidiasis [23,24].
Candida identification and susceptibility
General-purpose media that are commonly used for fungal culture, Sabouraud dextrose agar is used here. The standard temperature for incubation of fungi is 300C and cultures are incubated in a humidified environment. Once colonies are visible, they were inspected carefully for their morphology and germ tube. An updated version 8.1 software VITEK-2 (BioMérieux Marcy l'Etoile. 376, Chemin de l'Orme. 69280 Marcy l'Etoile. France) is used for yeast identification and susceptibility. A reagent card for the identification of different organism classes is used. A Suspension is prepared, a sterile swab or an applicator stick is used to transfer a sufficient number of colonies of a pure culture and the microorganisms were suspend in 3.0 ml of sterile saline (aqueous 0.45% to 0.50% NaCl, pH 4.5 to 7.0) in a 12 × 75 mm clear plastic (polystyrene) test tube. The turbidity is adjusted for yeast at I.80-2.20, accordingly turbidity is measured using a turbidity meter: the DensiChekTM (BioMérieux).
Statistical analysis
The total prevalence of Candida species isolates and invasive strains was calculated as means, medians and trimmed mean per the number of admissions. Also, calculating the numbers and the rates of Candida and their susceptibility, and most common morbidities among the isolates and the invasive strains (descriptive statistics), Chi-square (Χ2) and 2-sided Fisher's Exact Test is used to assess the differences in the categorical and continuous variables and ANOVA for the significant differences among means. Data were collected by upload to a Google cloud form, an excel sheet is generated and imported to an SPSS version 22 (IBM corporation) for analysis.
Results
There were 307 non-duplicate Candida isolates, the prevalence of Candida species calculated per admission was 15.2% and the invasive species was 2.48%. The overall Candida mean length of hospital stay was 19 days (95% CI, 15.87 – 22.18, trimmed mean 15.18 days), for C. albicans the mean was 18.53 days (95% CI 17.4–19.65) and for Non-albicans Candida the mean was 20.46 days (95% CI 19.77 ‒ 21.15).
C. albicans accounted for 34.5% (106) and Non-albicans Candida 65.5% (201) (p=0.000). No significant gender difference was found between C. albicans and non-albicans Candida (p=0.148), and no significant difference in age (p=0.305). Comorbidities; haemato-malignancy, solid tumors, diabetes mellitus, central venous catheter use, the used antimicrobials, and the different immunodeficient states, were not significantly different between C. albicans and non-albicans Candida (p=0.194), neither were the rates of the surgical intervention (p=0.166). Low white blood cell count did not differ significantly between the two Candida groups (p=0.595). Seventy-nine patients died; 31 in C. albicans group and 48 in the non-albicans Candia group, but there was no significant difference (p=0.337) (Table 1).
| Number (%) | C. albicans | Non-albicans candida | &p value | |
|---|---|---|---|---|
| Total | 307 (100) | 106 (34.5) | 201 (65.5) | 0 |
| Gender | ||||
| Male | 169 (55) | 52 | 117 | 0.148 |
| Female | 138 (45) | 54 | 84 | |
| Age mean@ | ||||
| Male | 59 | 60.4 | 58.8 | 0.305 |
| Female | 54 | 50.5 | 57.4 | |
| Haemato-malignancy | 4(1.3) | 1 | 3 | 1 |
| Solid Tumor | 52 (16.9) | 17 | 35 | 0.873 |
| Diabetes mellitus | 147 (47.9) | 45 | 102 | 0.187 |
| Central Venous Catheter | 63 (20.5) | 21 | 42 | 0.883 |
| Used Antibacterials | 252 (82.1) | 84 | 168 | 0.352 |
| Immunodeficient states* | ||||
| Renal failure** | 54 | 19 | 35 | 0.194 |
| Renal transplant$ | 15 | 1 | 14 | |
| Steroids | 42 | 12 | 30 | |
| Rheumatological illness | 3 | 1 | 2 | |
| Others$$ | 52 | 22 | 30 | |
| Surgery | ||||
| Abdominal | 69 (22.5) | 20 | 49 | 0.166 |
| Extremities | 16 (5.2) | 3 | 13 | |
| Head and Neck | 7 (2.3) | 1 | 6 | |
| Thoracic | 14 (4.6) | 7 | 7 | |
| White blood cells count | ||||
| <200 | 3 (1) | 0 | 3 | 0.595 |
| 201 – 500 | 17 (5.5) | 7 | 10 | |
| 501 – 1000 | 61 (19.9) | 18 | 43 | |
| 1001 – 3000 | 52 (16.9) | 19 | 33 | |
| >3001 | 174 (56.7) | 62 | 112 | |
| #Death | 79 (25.7) | 31 (29.2) | 48 (23.3) | 0.337 |
&p value is for the difference between C. albicans and Non-albicans Candida; 2- sided Statistical significance was tested by Fisher’s Exact Test (X2) except where indicated;
@ 2-sided significance by ANOVA; *Immune deficient: **7 renal failures were on steroids; $Renal transplant on Cyclosporine, MMF, Tacrolimus, steroids; $$Others: One HIV, Bed ridden, 2 hemoglobinopathies; 1 complement deficiency and bed ridden patients; # See table 3 for nested analysis.
Table 1: The features and characteristics of patients with 307 Candida species.
Candida was collected from several sources; numerically C. albicans was more than each other Candida species, but as a group Non-albicans Candida was numerically higher than C. albicans (Table 2 and Figure 1). The non-albicans candida were; C. glabrata 55 (17.9%), C. tropicalis 39 (12.7%), C. ciferii 20 (6.5%) and C. famata 14 (4.6%). The other were C. krusei 3.3%, C. parapsilosis 2.9%, C. kefyr 2.6% and C. lusitanae 2%. The unidentified Candida species were collected before the update of VITEK 2 database to version 8.01, the database update may have identified some of the "Others" including C. auris (Figure 2). The invasive strains; blood 6 C. albicans and 19 Non-albicans, the peritoneum recovered 6 invasive C. albicans and 19 Non-albicans Candida (Table 2). There was no significant proportion difference within both Candida groups between invasive strains and isolates (p=0.104). Associated mortality among patients was significantly more in all patients with invasive Candidiasis rather than isolates (p=0.000). Nested analysis demonstrated mortality to significantly differ within non-albicans Candida between isolates and invasive infection (p=0.000) but not for C. albicans (p=0.150), (Table 3).
| Candida | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sources | Albicans | Glabrata | Tropicalis | Parapsilosis | Krusei | Lusitanae | All Non-albicans | Total |
| Blood | 6 | 6 | 2 | 1 | 2 | 0 | 19 | 25 |
| LRT | 19 | 6 | 5 | 1 | 0 | 0 | 18 | 37 |
| Abdomen | 6 | 6 | 3 | 1 | 1 | 1 | 19 | 25 |
| URT | 24 | 2 | 4 | 1 | 1 | 2 | 26 | 55 |
| Urine | 29 | 16 | 11 | 3 | 3 | 3 | 61 | 88 |
| Vagina | 3 | 1 | 0 | 0 | 0 | 0 | 5 | 8 |
| Other | 19 | 18 | 14 | 2 | 3 | 0 | 50 | 69 |
| Total | 106 | 55 | 39 | 9 | 10 | 6 | 201 | 307 |
LRT (lower respiratory tract): BAL (bronchoalveolar lavage) and tracheal; URT (upper respiratory tract): Sputum, tracheostomy and mouth. Abdominal is limited to the peritoneal samples. Other sources: skin, pressure ulcers and wounds. Other Non-albicans: 8 C. kefyr, 1 C. spherica, 20 C. ceferii, 1 C. duoboshaemulonii, 14 C. famata; C. glabrata : 6 were blood, 6 peritoneal, and 6 were lower respiratory tract isolates recovered by BAL; C. albicans : 6 were blood, 6 peritoneal and 19 were lower respiratory tract isolates recovered by BAL.
Table 2: The distribution of all Candida species isolated from different sources.
| C. albicans or Non-albicans | *Death | p value | ||
| Yes | No | |||
| Candida albicans | Invasive | 6a | 6a | |
| Isolate | 25a | 69a | 0.105 | |
| 31 | 75 | |||
| Non-albicans Candida | Invasive | 19a | 19b | |
| Isolate | 29a | 134b | 0.000 | |
| 48 | 153 | |||
| Total | Invasive | 25a | 25b | |
| Isolate | 54a | 203b | 0.000 | |
| 79 | 228 | |||
Each subscript letter (a or b) denotes a subset of Death categories whose column proportions do not differ significantly from each other at the .05 level; * There was no significant mortality difference for the proportions of the invasive strains between C. albicans (6/31=19.35%) and Non-albicans Candida (19/48=39.58%) (p >0.05).
Table 3: The mortality rates among invasive C. albicans and non-albicans infections compared with colonizers.
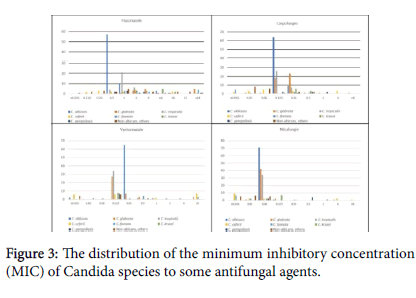
Among 73 tested C. albicans species 94.5% were susceptible to Fluconazole with MIC distribution for all strains ≤ 1 µg/mL but one isolate (5.5%) was resistant (16 µg/mL). Among 35 C. tropicalis isolates, one (2.8%) was fluconazole-resistant. C. glabrata were 16 isolates with 68.8% susceptibility, and among 7 isolates of C. parapsilosis 85.7% were susceptible to fluconazole. Among 68 C. albicans species susceptibility to voriconazole was 100%, and the same 100% susceptibility for C. tropicalis (n=34), C. krusei (n=8) and C. parapsilosis (n=7). For echinocandins, C. albicans was 100% susceptible (n=72 for micafungin and 73 for Caspofungin). Also, 100% susceptibility for both echinocandins; C. tropicalis (n=34 and 33), C. krusei (n=9 and 9), and C. parapsilosis (n=7 and 7) respectively, but susceptibility in C. glabrata was 97.8% for micafungin, and 95.6% for caspofungin (n=46 and 46) respectively (Figure 3).
Numbers next to Candia names denote the CLSI breakpoint; those without numbers next to them have no established CLSI breakpoints.
CLSI: clinical laboratory institute
There are no Amphotericin-B CLSI breakpoints for Candida species (25), however, the CLSI most frequent low MIC for Amphotericin-B is (0.5-2) µg/mL that includes 92% - 99% of species. Our isolates showed that Amphotericin-B MIC of <2 µg/mL for C. albicans was 93.6% (3/47) and for Non-albicans Candida 92.9% (7/99), the 7 out of range (unsusceptible) species were: 3/16 C. Ciferii, 2/25 C. glabrata , 2/10 C. famata (Figure 4). No multidrug-resistant Candida was observed when tested by several antifungal agents.
*Note that are no CLSI breakpoints for Amphotericin B for Candida species.
Discussion
Nosocomial Candida infections are increasing in hospitalized patients. With the worldwide growth in the immunocompromised population, they account for 7% – 10% of patients. In our patients colonization accounts for 15.2% of admitted patients and the invasive infections accounted for 2.48% (considering blood and peritoneum as a proven invasive infection), the lower rates in our population may be due to exclusion of other sources, as the study design is not powered to identify invasive strains from all sources, and importantly due to the nature of the patients population where sicker patients and those who need longer hospital stay are being treated in public hospitals, and a referral cancer center in Jordan [26].
There were no significant distribution differences between C. albicans and the non-albicans Candida in gender, age, or for the other comorbidities (p>0.05). Though surgery is a known risk for candida infections [27] the type of surgery did not significantly differ in the two Candida groups (p>0.05), as well as the white blood cells count (Table 1). Like elsewhere the non-albicans Candida was significantly (p=0.000) dominant accounting for 65.5%, and C. glabrata and C. tropicalis were commonly isolated from most sources, C. glabrata vaginal isolates were 12.5% (1/8) (Table 2), this is (Figure 2) due to the low vaginal isolates (n=8) and the female population at two study hospitals were dominated by a healthy parturient women with a routine vaginal swabs before delivery [28]. A study from Mexico quoted higher rate for C. glabrata (19%), but the study included women with symptoms of vaginal candidiasis [29].
The susceptibility of Candia species (Figure 3) against the tested antifungal agents are all positively skewed, where the bulk of the MIC values are skewed to lower values. Fluconazole has a descent activity against C. albicans and C. tropicalis, but it was poor for C. glabrata; resistance rates in C. albicans was 5.5% (n=73), similar to an earlier report [12], and higher than other reports of 2%. C. tropicalis resistance rate was 2.8% (n=35) versus other reports of 9%. Resistance in C. parapsilosis was 14.3 % (n=7) versus up to 6%, and C. glabrata resistance here was 30.2% (n=16) versus up to 13%. Evidently, our resistance patterns are higher than what was reported, possibly driven by the wide-scale use of fluconazole and the oral antifungal gel/solution in the country in addition to the small sample size [30]. Other Candida species with no CLSI breakpoints have variable susceptibilities to fluconazole, and no comment can be done at the moment.
The susceptibility to voriconazole was 100% in C. albicans, C.tropicalis, C. Krusei and C. parapsilosis similar to what was reported from our region [31]. Candida susceptibility to the tested two echinocandins was 100% except for C. glabrata; for micafungin it was 97.8% and caspofungin 95.8% [32]. Though there are no clear CLSI breakpoints used for Amphotericin B, again the MIC distribution for Candia species is positively skewed, where the majority of the tested isolates were in the anticipated susceptibility region; C. albicans was 93.6% susceptible and non-albicans was 92.9% Susceptible (Figure 4).
No single isolate of C. auris was identified or suspected by Vitek 2 with the new updated software (version 8.1 YST-AST card) though it has its limitations in identifying C.auris [(https://www.cdc.gov/fungal/candida-auris/recommendations.html), (https://www.cdc.gov/fungal/diseases/candidiasis/pdf/Testing-algorithm-by-Method-temp.pdf). However, there was no single isolate that was a multidrug-resistant among the few resistant cases to a single agent, including C. duobushaemulonii, C. haemulonii, or C. famata [33]
The associated mortality rate with Candida isolation among our patients was 25.7%; with C. albicans was 29.2% and non-albicans Candida was 23.3% (p=0.337). The associated mortality with the invasive candidiasis was (25/50) 50%, this high rate may be due to the selection of blood and peritoneum as invasive, while in a review it was 40% possibly due to the consideration of other sources [34]. The mortality associated with the non-invasive isolates of candida was (54/257) 21.0%, but there was no significant mortality difference between isolates and invasive strains for C. albicans (p=0.105), though there was a significant difference for the non-albicans Candida associated mortality (p=0.000). Also, there was no significant mortality difference for the proportions of the invasive strains between C. albicans (6/31=19.35%) and non-albicans Candida (19/48=39.58%) (p>0.05).
Conclusion
Non-albicans Candida was more in prevalence than C. albicans; C. galbrata, C. tropicalis and C. ciferii are the most predominant, followed by C. famata, C. krusei and C. parapsilosis. Our antifungal resistance rates are higher than what was reported, and the mortality from Non-albicans was not significantly higher than C. albicans. No single case of C. auris was documented or suspected in our Candida species.
Acknowledgement
We thank the help provided by: Deema Al Jammal, Nisreen Al Radaydeh, Marwa Abu Saad, Rasmiyyeh Abu Kwaik, and Walid Jamal from the microbiology laboratories.
Conflict of Interest
JW receives honoraria for his lectures from Pfizer, MSD, Gilead and Hikma pharmaceuticals. All other authors have nothing to disclose.
References
- Wright WL, Wenzel RP (1997) Nosocomial Candida: Epidemiology, transmission, and prevention. Infect dis clin of North America 11:411-425.
- Fridkin SK, Jarvis WR (1996) Epidemiology of nosocomial fungal infections. Clin Microbiol Reviews 9:499-511.
- Jarvis WR (1995) Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 20:1526-1530.
- Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, et al. (2012) ESCMID guideline for the diagnosis and management of Candida diseases: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol and Infect 18:53-67.
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, et al. (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091-1100.
- Neofytos D, Fishman JA, Horn D, Anaissie E, Chang CH, et al. (2010) Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transplant Infect Dis 12: 220-229.
- Kett DH, Azoulay E, Echeverria PM, Vincent JL (2011) Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Critical Care Med 39: 665-670.
- Montravers P, Mira JP, Gangneux JP, Leroy O, Lortholary O, et al. (2011) A multicentre study of antifungal strategies and outcome of Candida spp. peritonitis in intensive-care units. Clin Microbiol and Infect 17:1061-1067.
- Rammaert B, Desjardins A, Lortholary O (2012) New insights into hepatosplenic candidosis, a manifestation of chronic disseminated candidosis. Mycoses 55:74-84.
- Nguyen MH, Peacock JJE, Morris AJ, Tanner DC, Nguyen ML, et al. (1996) The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. The American J of Med 100:617-623.
- Miceli MH, DÃaz JA, Lee SA (2011) Emerging opportunistic yeast infections. The Lancet Infect Dis 11:142-151.
- Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M, et al. (2011) Candida bloodstream infections:comparison of species distributions and antifungal resistance patterns in community-onset and nosocomial isolates in the SENTRY Antimicrobial Surveillance Program. Antimicrobial Agents and Chemotherapy 55:561-566.
- Chowdhary A, Kumar VA, Sharma C, Prakash A, Agarwal K et al. (2014) Multidrug-resistant endemic clonal strain of Candida auris in India. European J of Clin Microbiol & Infect Dis 33:919-926.
- Borman AM, Szekely A, Johnson EM (2016) Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. MSphere.
- Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, et al. (2015) Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J of Clin Microbiol 53:1823-1830.
- Magobo RE, Corcoran C, Seetharam S ,Govender NP (2014) Candida auris–associated candidemia, South Africa. Emerging Infect Dis 20:1250.
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, et al. (2017) Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clinic Infect Dis 64:134-140.
- Morales López SE, Parra-Giraldo CM, Ceballos-Garzón A, MartÃnez HP, RodrÃguez GJ et al. (2017) Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis 23:162.
- Emara M, Ahmad S, Khan Z, Joseph L, Al Obaid IM et al. (2015) Candida auris candidemia in Kuwait Emerg Infect Dis.
- Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, et al. (2016) First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrobi Res & Infect Control 5:35.
- Morrell M, Fraser VJ, Kollef MH (2005) Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrobial Agents and Chemotherapy 49:3640-3645.
- Labelle AJ, Micek ST, Roubinian N, Kollef M H (2008) Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Critical Care Med 36: 2967-2972.
- Puig Asensio M, Padilla B, Garnacho Montero J, Zaragoza O, Aguado JM, et al. (2014) Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a populationâ€based surveillance in Spain. Clin Microbiol and Infect 20:245-254.
- Wenzel RP (1998) Perspective: attributable mortality-the promise of better antimicrobial therapy. The J of Infect Dis 178:917-919.
- CLSI [Clinical and Laboratory Standards Institute]. Performance standards for antifungal susceptibility testing of yeasts M60 (1st edn), 2017.
- Khan HA, Baig FK, Mehboob R (2017) Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pacific J of Trop Biomed 7:478-482.
- Eggimann P, Bille J, Marchetti O (2011) Diagnosis of invasive candidiasis in the ICU. Annals of Intensive Care 1:37.
- Rai M, Poudel TP, Gurung K, Neupane GP, Durga BC, et al. (2017) Prevalence of Candida albicans in Genital Tract of Pregnant Women Attending Antenatal Clinic of Nepalgunj Medical College Hospital. J of Nepalgunj Med Col 15:13-17.
- Tiran Saucedo J, Garza H, Leon Y, Godinez-Burgos JA (2019) The Search for Vaginal Candida Resistant Infections, A Descriptive Study [15R]. Obstetrics & Gynecology 133:195.
- Berkow EL, Lockhart SR (2017) Fluconazole resistance in Candida species: a current perspective. Infect and Drug Res 10:237.
- Amr GE, Atef DM, Salah AM (2019) Identification and Anti-Fungal Resistance Profile of Different Candida Species Isolated from Patients in ICUs. Int J Curr Microbiol App Sci 8(6):564-573.
- Aigner M, Erbeznik T, Gschwentner M, Lass-Flörl C (2017) Etest and Sensititre Yeast One susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrobial Agents and Chemotherapy 61:512-517.
- Ambaraghassi G, Dufresne PJ, Dufresne SF, Vallières É, Muñoz J F, et al. (2019) Identification of Candida auris by use of the updated Vitek 2 yeast identification system, version 8.01: A multilaboratory evaluation study. J of Clin Microbiolk 57:884-919.
- Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. New England J of Med 373:1445-1456.
Citation: Al Ramahi JW, Pharm AB, Bashir N, Bazara A,Shami D, et al. (2020) Epidemiology of Nosocomial Candida Species, Resistance Patterns, and the Associated Crude Mortality. J Infect Dis Ther 8: 415. DOI: 10.4172/2332-0877.1000415
Copyright: © 2020 Al Ramahi JW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3544
- [From(publication date): 0-2020 - Dec 01, 2025]
- Breakdown by view type
- HTML page views: 2696
- PDF downloads: 848