Electronic Clinical Decision Support (eCDS) Intervention to Increase Hepatitis C Virus (HCV) Screening and Linkage to Care among Baby Boomers in Urban Safety-Net Health System
Received: 28-Jan-2019 / Accepted Date: 07-Feb-2019 / Published Date: 11-Feb-2019 DOI: 10.4172/2161-0711.1000646
Abstract
Objective: An estimated 3.5 million Americans are living with hepatitis C virus (HCV) infection and the majority is unaware of their infection. HCV causes significant morbidity and mortality and is one of the leading causes of hepatocellular carcinoma (HCC) and other liver complications. Baby boomers (born between 1945 and 1965) account for approximately 75% of people chronically infected with HCV. The CDC and USPSTF recommend universal one-time screening of all Baby Boomers.
Methods: Cook County Health (CCH) implemented an electronic clinical decision support (eCDS) tool in September 2016 to increase HCV screening among baby boomers throughout its outpatient clinic network. We evaluated the impact of the eCDS tool on screening and the successive stages of the care continuum by analyzing the proportion of patients who completed 1) anti-HCV screening, 2) HCV RNA confirmatory testing, 3) HCV RNA detectable result, 4) liver staging, and 5) treatment in the 12-month periods pre- and post-implementation.
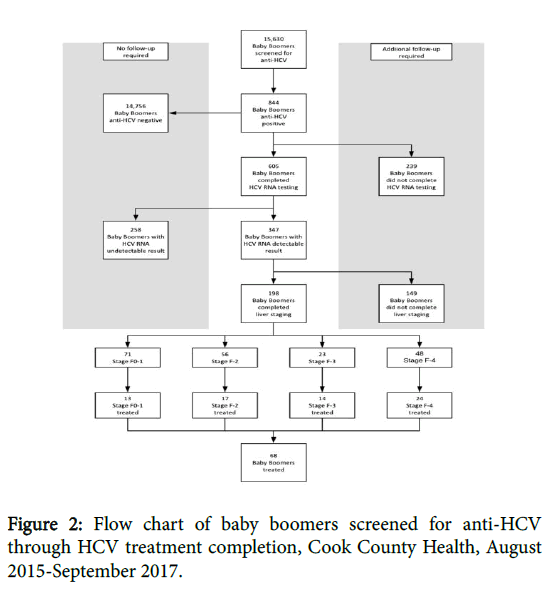
Results: The number of baby boomers newly tested system-wide each month increased by 344% over the 24-month evaluation period. Of 15,630 patients tested for anti-HCV, 844 (5.4%) tested positive. Patients with anti-HCV positive results were predominantly male (59%), between the ages of 52 and 64 (70%), Black/African American (71%) and Non-Hispanic (86%). 605 (72%) of anti-HCV positive patients completed HCV ribonucleic acid (RNA) testing; of those patients, 347 (58%) had confirmed HCV infection. Of 347 patients with confirmed HCV infection, 198 completed liver staging and 68 initiated treatment.
Conclusions: Implementation of eCDS tools across large urban safety-net health systems is an effective strategy for ensuring adherence to national guidelines for HCV screening among baby boomers. The high prevalence of HCV infection in this primarily male, Black/African American baby boomer population highlights the urgency of universal screening programs at similar institutions.
Keywords: Centers for disease control and prevention; Hepatitis C virus; Hepatitis C-chronic; Mass screening; Decision support techniques
Introduction
An estimated 3.5 million people in the United States are living with chronic hepatitis C infection (HCV) [1-4]. Approximately threequarters of persons chronically infected with HCV are baby boomers (born between 1945 and 1965), and the majority are unaware of their HCV status [1]. As a result, the US Centers for Disease Control and Prevention (CDC) and US Preventive Services Task Force (USPSTF) recommend universal one-time HCV screening for baby boomers [1,5,6]. Prior to these recommendations, most health systems used risk-based HCV screening to identify chronic infections, which often misses patients who do not self-report risk factors or have not engaged in risk behaviors for many years [5].
Primary care providers have reported several barriers to implementing recommended screening practices, including time constraints, insufficient training on screening guidelines, and misconceptions about the CDC recommendations [7]. To address these barriers, several studies have concluded that implementation of electronic clinical decision support (eCDS) tools is a low-cost and effective strategy for increasing age cohort screening in outpatient clinic networks [8-12]. Additional clinical support available in primary care clinics, including disclosure of test results by a known and trusted provider, integrated support services, and systems for tracking patients from point of diagnosis to cure, indicate that this setting is optimal for HCV screening [10]. While several studies have disseminated findings from enhanced screening interventions, few studies have reported on patient outcomes from the point of screening to treatment completion.
Methods
Screening prompt
Cook County Health (CCH) consists of two hospitals, a large network of more than a dozen community health centers, the Ruth M. Rothstein CORE Center, and Cermak Health Services, which provides health care to individuals in the Cook County correctional system. CCH serves approximately 300,000 patients annually, the majority of whom are served at one of the outpatient clinics of the Ambulatory and Community Health Network (ACHN). We created prompts for HCV testing using the clinical decision support system embedded in our electronic health record (Cerner Inc., Kansas City, MO).
Prompt implementation required approval from the Chief Medical Officer and a standing Decision Support Committee. Through this vetting process, we focused our rules on outpatient primary care providers, excluding emergency departments and specialty providers. The rule was triggered for patients (1) born during 1945 through 1965, who (2) had never had an anti-HCV test, and who (3) had any order for a laboratory blood test. We obtained final approval in February 2016 and implemented the prompt in September 2016. In June 2017, we obtained approval from the Division Chair of the Microbiology and Virology Lab to implement HCV RNA reflex testing.
Data collection
Data was abstracted from the electronic medical record for two time periods: August 1, 2015 through August 31, 2016 (pre-implementation of the eCDS tool) and September 1, 2016 through September 30, 2017 (post-implementation of the eCDS tool). The eCDS tool was implemented across all 13 CCH ACHN outpatient sites during September 2016. Data abstraction included age, sex, race and ethnicity.
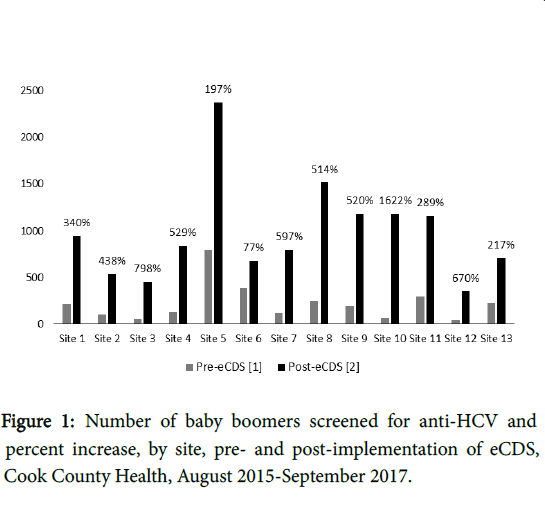
Additional data abstraction for patients who received anti-HCV positive results included completion and result of HCV RNA confirmatory testing, completion of liver staging assessment, and treatment status. This data was abstracted from a separate patient database maintained by the Ruth M. Rothstein CORE Center Hepatitis C Clinic team. Linkage to care data for patients who received HCV staging and treatment at outside institutions is not included in this analysis (Figures 1 and 2).
Linkage to care
Patients with confirmed chronic HCV infection were referred to the Ruth M. Rothstein CORE Center for Fibroscan staging and physician consult. Referrals submitted to the HIV/HCV Care Coordinator were reviewed to ensure all required laboratory tests were completed and then placed in a patient queue for scheduling. On average, patients received a Fibroscan appointment within three weeks of referral. Once completed, Fibroscan results were scanned into the EMR and/or faxed to external providers for ongoing HCV care. CCH patients were scheduled for initial physician assessments in the Hepatitis Clinic. On average, patients received an appointment for an initial physician assessment within three months.
Due to highly restrictive Medicaid reimbursement policies in Illinois, insurance coverage and fibrosis staging significantly determined which patients initiated the prior authorization process and started HCV treatment. Patients deemed ineligible for treatment due to low fibrosis staging, substance use, or unmanaged comorbidities were monitored and contacted periodically for reassessment. Patients deemed eligible for HCV treatment met with a team of insurance specialists, pharmacists and prescribing providers to complete the prior authorization process, receive counseling on medication adherence and drug interactions, and schedule viral load monitoring appointments. Patients completed blood draws 4 weeks post-initiation, at treatment completion, and 12 weeks post-completion. Sustained virologic response (SVR) data was not collected for patients who did not complete lab work 12 weeks post-completion.
Results
Screening
The HCV eCDS prompt was implemented system-wide in September 2016. We report on 24 months of screening and linkage to care data from August 2015 to September 2017 (12 months preimplementation of eCDS and 12 month post-implementation of eCDS). During the 24-month evaluation period, 15,630 patients were screened, with a greater proportion of women compared with men (56% vs . 44%) and Blacks/African Americans representing the single largest racial group (60%), followed by Whites (29%). 80% of screened patients were born between 1944 and 1953, with the mean age of 60.5 years and median age of 60.0 years. The number of those newly tested each month increased from 2,874 in the 12 months preimplementation to 12,756 in the 12 months post-implementation, representing a 344% increase. The percent of eligible patients tested increased by 24% across all sites. Some sites considered lowperforming prior to implementation (i.e., Site 3 and Site 10) experienced significant increases in the percent of eligible patients screened (38% and 33%, respectively) while some high-performing sites (i.e., Site 6) experienced more modest increases before reaching a screening plateau (20% vs. 26% of eligible patients screened). The number of patients previously screened (defined as the number of registered patients with a documented HCV test result in their health record, thereby excluding them from the prompt) increased by 22% across all sites. As this metric only captures the number of patients who were excluded from the prompt among those with registered visits during the evaluation period, it does not accurately depict the number of patients previously tested for HCV. Most notably, the number of anti-HCV reactive patients identified increased from 265 patients in the pre-implementation period to 579 in the post-implementation period, representing a 118% increase across sites. A previously lowyield site (Site 2) experienced a 500% increase in anti-HCV reactive patient’s post-implementation, highlighting the value and effectiveness of increased screening among age-based cohorts (Table 1 and 2).
| Patient Characteristics | Anti-HCV screened (%) | Anti-HCV positive (%) | Completed HCV RNA (%) | HCV RNA detected (%) | Staged (%) | Treated (%) |
|---|---|---|---|---|---|---|
| Total | 15,630 | 844 | 605 | 347 | 198 | 68 |
| Mean Age | 60.5 | 61.2 | 61.6 | 59.9 | 61.8 | 61.9 |
| Age range | ||||||
| 52-64 years | 3,098 (19.8) | 589 (69.8) | 431 (71.2) | 251 (72.3) | 141 (71.2) | 45 (66.2) |
| 65-74 years (Medicare eligible) | 12,532 (80.2) | 255 (30.2) | 174 (28.8) | 96 (27.7) | 57 (28.8) | 23 (33.8) |
| Sex | ||||||
| Male | 6,809 (43.6) | 495 (58.6) | 357 (59.0) | 230 (66.3) | 129 (65.2) | 45 (66.2) |
| Female | 8,819 (56.4) | 349 (41.4) | 248 (41.0) | 117 (33.7) | 69 (34.8) | 23 (33.8) |
| Race | ||||||
| American Indian/ Alaska Native | 315 (2.0) | 4 (0.5) | 3 (0.5) | 2 (0.6) | 2 (1.0) | 2 (2.9) |
| Asian | 796 (5.1) | 23 (2.7) | 14 (2.3) | 6 (1.7) | 3 (1.5) | 3 (4.4) |
| Black/African American | 8,905 (60.0) | 601 (71.2) | 430 (71.1) | 270 (77.9) | 150 (75.8) | 49 (72.1) |
| Other/Unknown | 1,081 (6.9) | 27 (3.2) | 22 (3.6) | 4 (1.1) | 2 (1.0) | 1 (1.5) |
| White | 4,532 (29.0) | 189 (22.4) | 136 (22.5) | 65 (18.7) | 41 (20.7) | 13 (19.1) |
| Ethnicity | ||||||
| Hispanic/Latino | 3,955 (25.3) | 115 (13.6) | 84 (13.9) | 29 (8.4) | 20 (10.1) | 7 (10.3) |
| Not Hispanic/Latino | 11,675 (74.7) | 729 (86.4) | 521 (86.1) | 318 (91.6) | 178 (89.9) | 61 (89.7) |
Table 1: Baby boomer HCV care continuum by patient characteristic, cook county health, August 2015-September 2017.
| Patient Characteristics | Staged (%) | Stage 0-1 (%) | Stage 2 (%) | Stage 3 (%) | Stage 4 (%) |
|---|---|---|---|---|---|
| Total | 198 | 71 | 56 | 23 | 48 |
| Mean Age | 61.8 | 52.7 | 61.9 | 62.1 | 62.4 |
| Age Range | |||||
| 52-64 years | 141 (71.2) | 54 (76.1) | 43 (77.2) | 15 (65.2) | 29 (60.4) |
| 65 -74 years (Medicare eligible) | 57 (28.8) | 17 (23.9) | 13 (22.8) | 8 (34.8) | 19 (39.6) |
| Sex | |||||
| Male | 129 (65.2) | 41 (57.7) | 42 (75.0) | 16 (69.6) | 30 (62.5) |
| Female | 69 (34.8) | 30 (42.3) | 14 (25.0) | 7 (30.4) | 18 (37.5) |
| Race | |||||
| American Indian/Alaska Native | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.2) |
| Asian | 3 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (6.3) |
| Black/African American | 150 (75.8) | 57 (80.3) | 45 (80.4) | 18 (78.3) | 30 (62.5) |
| Other/Unknown | 2 (1.0) | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| White | 41 (20.7) | 13 (18.3) | 11 (19.6) | 5 (21.7) | 12 (25.0) |
| Ethnicity | |||||
| Hispanic/Latino | 20 (10.1) | 6 (8.5) | 6 (10.7) | 1 (4.3) | 7 (14.6) |
| Not Hispanic/Latino | 178 (89.9) | 65 (91.5) | 50 (89.3) | 22 (95.7) | 41 (85.4) |
Table 2: Liver fibrosis staging by patient characteristic, cook county health, August 2015-September.
Linkage to care
Between August 2015 and September 2017, 844 patients were identified as HCV-antibody reactive. Patients identified as HCVantibody reactive were predominantly male (59%), between the ages of 52 and 64 (70%), Black/African American (71%) and Non-Hispanic (86%). Despite representing only 20% of patients screened, patients in the 52-64-year-old age group represented 70% of patients identified as HCV-antibody positive. Similarly, despite lower representation of males (44%), Black/African Americans (60%) and Non-Hispanics (75%) among the total screened population, males, Black/African Americans and Non-Hispanics disproportionately accounted for 59%, 71% and 86% of HCV-antibody reactive patients, respectively. There were no noticeable differences across age, sex, race and ethnicity as to which patients completed HCV RNA confirmatory testing. Of those who completed HCV RNA testing, males were more likely than females to receive an HCV RNA detectable result (64% vs. 47%, P<0.001) and Blacks/African Americans were more likely than Whites to receive a HCV RNA detectable result (63% vs . 48%, P<0.005). Across ethnicity groups, Non-Hispanics were more likely than Hispanics to receive an HCV RNA detectable result (62% vs. 35%, P<0.001). Males were more likely than females to complete a Fibroscan assessment (26% vs. 20%, P<0.05). Of the 198 patients who completed a Fibroscan liver staging assessment, 71 (36%) scored F-01, 56 (28%) scored F-2, 23 (12%) scored F-3 and (24%) scored F-4. American Indian/Alaska Native and Asians were more likely to receive a score of F-4, while African American/Black and Whites were more likely to receive a score of F0-1. Due to restrictive Medicaid reimbursement policies, only 44% of patients with a fibrosis stage lower than F-3 received HCV treatment.
Discussion
Implementation of an HCV eCDS tool led to a marked increase in HCV testing among birth cohort patients across ambulatory sites, demonstrating the effectiveness of EHR interventions to promote adherence to guideline-based recommendations throughout a large urban health system.
Acceptance of the eCDS prompt was likely facilitated by (1) buy-in from system and medical leadership, (2) on-site provider and medical staff trainings by Hepatitis staff, and (3) dissemination of site-specific data metrics to demonstrate progress toward goals and areas for improvement. Though provider adherence to the eCDS prompt immediately post-implementation were promising, prompt fatigue and competing provider obligations ultimately led to a plateau effect approximately eight months post-implementation. Implementation of HCV RNA reflex testing significantly increased the number of HCV antibody reactive patients who received confirmatory testing. Additionally, reflex testing ultimately decreased wait times and increased patient capacity in Hepatitis Clinic appointments, as only patients with confirmed chronic HCV infection received referrals for physician consults.
A lower HCV RNA positivity (58%) was also observed. Without extensive chart abstraction, it is difficult to determine whether this reflects spontaneous clearance trends or treatment completion at an external provider prior to HCV antibody screening at CCH. Notably, approximately one-quarter of patients who completed fibrosis staging received a result of F-4, indicating that many had been living with HCV infection for several years. This highlights the urgency of universal screening for Baby Boomer patients as a tool to prevent disease progression and complications, including liver decompensation and hepatocellular carcinoma (HCC).
Limitations
This study had several limitations. The number of patients previously tested was derived from exclusionary criteria for the EMR prompt; monthly data grabs reported on the number of patients who met all other eligibility criteria for the prompt but had previously been tested. Because this metric does not distinguish between duplicated and unique patients, it should not be interpreted as an exact count of patients previously tested for HCV. Rather, this metric demonstrates that the number of patients excluded from the prompt due to previous testing has increased significantly since prompt implementation, indicating a good prompt saturation level throughout the patient population.
Additionally, data on the number of patients who completed stages of care continuum beyond RNA confirmatory testing was only available for patients who continued care at CCH. We were unable to capture linkage data on patients who received HCV care elsewhere. As a result, linkage to care and treatment outcomes were likely underreported.
Finally, we collected and analyzed this data before Illinois Medicaid removed all disease severity restrictions for HCV treatment coverage. Additional studies should be conducted to determine the impact on age, gender, and racial and ethnic disparities in access to HCV treatment after removal of these restrictions.
Conclusions
Implementation of eCDS tools across large urban safety-net health systems is an effective strategy for ensuring adherence to national guidelines for HCV screening among baby boomers. RNA reflex testing and timely access to liver staging assessments reduce the likelihood that patients are lost to care at subsequent stages of the care continuum. The large proportion of patients with advanced liver fibrosis identified through this intervention underscores the urgency of universal screening to prevent disease progression, improve patient outcomes, and reduce future healthcare costs.
Acknowledgements
This project was supported by Gilead Sciences FOCUS funding under Regional Lead Lora Branch.
References
- Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, et al. (2014) Chronic hepatitis C virus infection in the United States, national health and nutrition examination survey 2003 to 2010. Ann Intern Med 160: 293-300.
- Ward JW, Valdiserri RO, Koh HK (2012) Hepatitis C virus prevention, care, and treatment: From policy to practice. Clin Infect Dis 55: 58-63.
- Ward JW, Lok AS, Thomas DL, El-Serag HB, Kim WR (2012) Report on a single-topic conference on “chronic viral hepatitis-strategies to improve effectiveness of screening and treatment.†Hepatology 55: 1652-61.
- Denniston MM, Klevens RM, McQuillan GM, Jiles RB (2012) Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National health nutrition examination 2001-2008. Hepatology 55: 1652-61.
- Smith BD, Morgan RL, Beckett GA, et al (2012) Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 61: 1-32.
- Moyer VA (2013) Screening for hepatitis C virus infection in adults: U.S preventive services task force recommendation statement. Ann Intern Med 159: 349-57.
- Jewett A, Garg A, Meyer K, Wagner LD, Krauskopf K, et al (2015) Hepatitis C virus testing perspectives among primary care physicians in four large primary care settings. Health Promot Pract 16: 256-63.
- Coyle C, Viner K, Hughes E, Kwakwa H, Zibbell JE, et al. (2015) Identification and linkage to care of HCV-infected persons in five health centers-Philadelphia, Pennsylvania, 2012-2014. MMWR Morb Mortal Wkly Rep 64: 459-63.
- Coyle C, Kwakwa H (2016) Dual-Routine HCV and HIV testing: Seroprevalence and linkage to care in four community health centers in Philadelphia, Pennsylvania. Public Health Rep 131: 41-52.
- Coyle C, Kwakwa H, Viner K (2016) Integrating routine HCV testing in primary care: Lessons learned from five federally qualified health centers in Philadelphia, Pennsylvania, 2012-2014. Public Health Rep 131: 65-73.
- Sidlow R, Msaouel P (2015) Improving hepatitis C virus screening rates in primary care. J Healthc Qual 37: 319-323.
- Miller LS, Rollin F, Fluker S, Lundberg KL, Park B, et al. (2016) High-yield birth-cohort hepatitis C virus screening and linkage to care among underserved African Americans, Atlanta, Georgia, 2012-2013. Public Health Rep 131: 84-90.
Citation: Armstrong H, Gonzalez-Drigo M, Adeyemi O, Trick W, Diep L, et al. (2019) Electronic Clinical Decision Support (eCDS) Intervention to Increase Hepatitis C Virus (HCV) Screening and Linkage to Care among Baby Boomers in Urban Safety-Net Health System. J Community Med Health Educ 9: 646. DOI: 10.4172/2161-0711.1000646
Copyright: © 2019 Armstrong H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3467
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 2705
- PDF downloads: 762