Epidemiology and Risk Factors for Chlamydia trachomatis, Treponema pallidum,Hepatitis B Virus and Hepatitis C Virus in the Marajó Archipelago, Brazilian Amazon
Received: 06-Dec-2018 / Accepted Date: 21-Jan-2019 / Published Date: 23-Jan-2019 DOI: 10.4172/2161-0711.1000643
Abstract
Background: The dynamics of hepatitis B virus, hepatitis C virus, Treponema pallidum and Chlamydia trachomatis infections were investigated in four municipalities of the Marajó Archipelago.
Methods: Epidemiological characteristics and detection of specific antibodies were investigated in 1,208 resident persons. Persistence was defined by the presence of HBsAg and viral RNA.
Results: Prevalence of antibodies was 30.9% (C. trachomatis), 8.9% (T. pallidum), 12.4% (anti-HBc/IgG) and 1.3% (anti-hepatitis C virus). Vaccination coverage for hepatitis B virus was 19.8%. C. trachomatis infection was significantly associated among women, 28 to 37 years old. T. pallidum was significantly associated with older age (>68 years), being separated or widowed and illiterate persons. hepatitis B virus was significantly associated with males, 48 to 57 years old and those with low educational level. Consumption of alcoholic beverages and use of illicit drugs were more likely to be infected with C. trachomatis. More than one sexual partner and absence of condoms during sexual intercourse were risk factors for T. pallidum infections.
Discussion: The transmission of infections will only be interrupted by the consistent application of measures including health education, routine examination, immediate treatment and recognition of risk factors associated with infections.
Conclusion: Severe infectious agents circulate in areas far from large urban centers where population have restricted access to healthcare providers.
Keywords: Epidemiology; Sexually transmitted infections; Risk factors; Brazilian amazon
Introduction
Over 200 million new Sexually Transmitted Infections (STI) caused by bacteria, including Chlamydia trachomatis and Treponema pallidum, occur every year, and 350 million people are persistently infected by the Hepatitis B (HBV) and C (HCV) viruses [1]. The treatment of these infections is costly and has a large impact on household income, in addition to compromising the quality of life of infected and sick individuals [2-4].
There are 19 serotypes of C. trachomatis , which present different tropisms and cause several diseases in humans including trachoma, urethritis and venereal lymphogranuloma, in addition to other severe consequences [5]. Infections are treatable with antibiotics, but low adherence to treatment and the high prevalence of the bacterium reinforces the need to implement routine screening [6]. The areas with the highest prevalence of infection include the Western Pacific (37.8 million cases) and the Americas (25.2 million cases) [7]. In the Brazilian Amazon, Chlamydia infection is common in various population groups, both urban and non-urban, and sexual transmission has been reported, including among native Indian communities [8,9].
Syphilis, caused by T. pallidum a disease with a broad spectrum of clinical manifestations, affects reproduction, growth and the sexual and social development of infected individuals [10-15]. The agent is sexually, congenitally and parenterally transmitted [16-18]. There are approximately 36.4 million adults with syphilis worldwide, with the highest prevalence (14.3 million) in Africa and the lowest (0.6 million) in Europe [7].
HBV causes disease that varies from acute to fulminant or chronic hepatitis and may result in cirrhosis and liver cancer [10-21]. Global differences in HBV prevalence by world region were seen because changes in demographic characteristics and hepatites B prevention and control programs [22]. Approximately 240 million people are chronically infected and that 2015 to 2030, over 20 million HBVrelated deaths will occur [23]. The most endemic regions include the African continent and the Western Pacific region [24]. In Brazil, 212,031 cases of hepatitis B were confirmed from 1999 to 201625 as a result of sexual, congenital and parenteral transmission [25-27].
HCV causes mostly asymptomatic infections but also acute and chronic disease that can progress to cirrhosis and hepato-carcinoma [28-31]. There are 130 to 150 million people chronically infected and no vaccine against HCV, but treatment and cure are possible [23]. High prevalences of antibodies against HCV (>3.5%) were found in East Asia, North Africa and the Middle East [32]. In Brazil, 189,389 cases of hepatitis C were reported from 1999 to 2016, 32.5% of the total cases of clinical hepatitis reported in the period [25]. Transmission of HCV occurs mainly by the parenteral route, [33] but sexual intercourse with an infected partner, multiple sexual partners, dental treatment, surgery, tattoos, use of injectable drugs and occupational accidents, are also associated with HCV infection [26,34,35].
The Marajó Archipelago, the largest river-sea island system in the world is located in the North of the Brazilian Amazon. The Archipelago shows a low socioeconomic level, low educational level and poor sanitary conditions that contribute to the spread of sexually transmitted infections. Information on various infections and diseases is scarce, which makes it difficult to develop public health policies aimed at disease prevention and control [36]. The Archipelago is far from large urban centers, resulting in social, political and economic isolation as well as poor access to healthcare providers, which may contribute to the disparities that affect these communities [37].
The present study aimed to describe the frequency, distribution and risk factors of C. trachomatis , T. pallidum , HBV and HCV infection in four communities of the Marajó Archipelago based on a serological study in order to determine the best way to develop policies for the prevention and control of infections that are primarily sexually transmitted.
Methods
Studied population and information gathering
A cross-sectional study was designed to estimate the prevalence and the frequency distribution of C. trachomatis , T. pallidum , HBV and HCV infection according to the demographic, social and behavioral variables of four populations within the Marajó Archipelago: Chaves (northern Marajó Island), Anajás (center of the island), São Sebastião da Boa Vista (SSBV, southeast) and Portel (continental area southwest of Marajó Island) (Figure 1).
Sample size was calculated considering the prevalence of the agents compared to that of Belem as reference considering that the prevalence on the island could be lower (unilateral test) or higher (bilateral test) than that in the municipalities. BioEstat version 5.339 for proportions (one sample), with test power of 0.90 and alpha level of 0.01, was used for sample size estimation, and 1,217 subjects were chosen according to sex, age profile and the population of each municipality. The numbers of subjects per municipality were 225 (Chaves), 230 (Anajás), 250 (SSBV) and 482 (Portel).
The invited participants were briefed of the project’s goals, and those who agreed to participate signed an Informed Consent Form. Demographic, social and behavioral data, as well as a 10 mL blood sample for laboratory tests, were collected. The study was submitted and approved by the Research Ethics Committee of the Center for Hemato-therapy and Hematology Foundation of Para (protocol number 0003.0.324.000-10).
Laboratory tests
Detection of antibodies and antigens: Plasma samples were subjected to ELISA assays for the detection of infection markers. The assays were performed according to the manufacturer’s recommendations. The presence of IgG antibodies against C. trachomatis was detected by the Serion ELISA Classic Chlamydia trachomatis IgG, Würzburg, Germany, and IgG-negative samples were tested for the presence of IgM antibodies using Serion ELISA Classic Chlamydia trachomatis IgM, Würzburg, Germany.
Detection of IgG and IgM antibodies against T. pallidum was performed using the ELISA, Symbiosys Diagnóstica, São Paulo, Brazil. Positive samples were further tested using a qualitative RPR test (RPR BRÁS, Laborclin, Paraná, Brazil) followed by a quantitative assays of positive samples.
Anti-HBs, anti-HBc total and anti-HBc/IgM antibodies were detected using ELISA assays of Symbiosys Diagnóstica, São Paulo, Brazil, and HBsAg used the Autobio Diagnostic, Zhengzhou, China. The prevalence of infection by HCV was assessed by the ELISA, Symbiosys Diagnóstica, São Paulo, Brazil.
The results that were regarded as diagnostic information (anti-C. trachomatis/IgM; RPR; anti-HBc/IgM and HBsAg) were provided to the health care centers of the respective municipalities for patient care and treatment, when applicable.
HCV RNA detection: HCV persistence, was investigated in antibody positive plasma samples and tested for the presence of viral RNA using a multiplex real-time PCR kit for HIV-1 and HCV (NAT HIV/HCV Bio-Manguinhos/Fiocruz, Rio de Janeiro, Brazil) in a fully automated procedure according to the manufacturer’s recommendations.
Statistical analysis
Questionnaire information was stored in a database (Epi Info 7 software). Associations between the presence of antibodies and the participants’ demographic, social and behavioral characteristics were calculated using the chi-square test and the G-test. Multiple logistic regression analysis was used to investigate for variables that could be considered as risk factors for infection. A confidence interval of 95% (CI 95%) was used for all analyses, and differences were considered significant at p ≤ 0.05 [38].
Results
Demographic and social characteristics of the populations investigated are shown in Table 1. All municipalities included more women (77.7% on average) than men. Age ranged from 18 to 99 years, mostly 18 to 47 years old, and most of the subjects reported themselves as married (60.9% on average).
| Demographic/Social characteristics | CHAVES | ANAJÁS | SSBV | PORTEL | TOTAL |
|---|---|---|---|---|---|
| n=255 (%) | n=230 (%) | n=250 (%) | n=482 (%) | n=1217 (%) | |
| Sex | |||||
| Female | 195 (78) | 166 (72.2) | 187 (79.2) | 381 (79.4) | 929 (77.7) |
| Male | 55 (22) | 64 (27.8) | 49 (20.8) | 99 (20.6) | 267 (22.3) |
| *NI | 5 | 0 | 14 | 2 | 21 |
| Age group | |||||
| 18-27 years old | 64 (25.7) | 62 (28.2) | 52 (22.1) | 99 (20.9) | 277 (23.6) |
| 28-37 years old | 57 (22.9) | 42 (19.1) | 59 (25.1) | 141 (29.9) | 299 (25.4) |
| 38-47 years old | 50 (20.1) | 47 (21.4) | 49 (20.9) | 80 (16.9) | 226 (19.2) |
| 48-57 years old | 36 (14.5) | 30 (13.6) | 31 (13.2) | 62 (13.1) | 159 (13.5) |
| 58-67 years old | 23 (9.2) | 19 (8.6) | 23 (9.8) | 54 (11.4) | 119 (10.1) |
| >68 years old | 19 (7.6) | 20 (9.1) | 21 (8.9) | 36 (7.6) | 96 (8.2) |
| *NI | 6 | 10 | 15 | 10 | 41 |
| Marital Status | |||||
| Married | 137 (58.0) | 119 (57.5) | 142 (63.4) | 280 (62.8) | 678 (60.9) |
| Separated | 4 (1.7) | 14 (6.8) | 5 (2.2) | 30 (6.7) | 53 (4.7) |
| Single | 84 (35.6) | 58 (28.0) | 62 (27.7) | 105 (23.5) | 309 (27.8) |
| Widowed | 11 (4.7) | 16 (7.7) | 15 (6.7) | 31 (7.0) | 73 (6.6) |
| *NI | 19 | 23 | 26 | 36 | 104 |
| Educational level | |||||
| Illiterate | 25 (10) | 50 (23.4) | 23 (9.8) | 89 (19.0) | 187 (16.0) |
| Literate | 14 (5.6) | 11 (5.1) | 42 (17.9) | 69 (14.8) | 136 (11.7) |
| Elementary school | 120 (48.1) | 95 (44.4) | 98 (41.7) | 165 (35.4) | 478 (41.1) |
| High school | 67 (26.9) | 44 (20.6) | 48 (20.4) | 99 (21.2) | 258 (22.2) |
| College degree | 23 (9.2) | 14 (6.5) | 24 (10.2) | 44 (9.4) | 105 (9.0) |
| *NI | 6 | 16 | 15 | 16 | 53 |
| Household income (minimum wage) | |||||
| Less than 1 MW | 62 (26.2) | 88 (41.9) | 105 (47.2) | 98 (21.0) | 353 (31.1) |
| More than 1 MW | 175 (73.8) | 122 (58.1) | 117(52,8) | 367 (79.0) | 781 (68.9) |
| *NI | 18 | 20 (0) | 28(0) | 17 | 83 |
| Note: NI: Not informed and not considered in the statistical analysis | |||||
Table 1: Demographic and social characteristics of populations from the municipalities of Chaves, Anajás, SSBV and Portel, Marajó Archipelago, state of Pará, Brazil.
The highest and lowest educational levels were found in the municipalities of Chaves and Portel, in which 15.6% and 33.8% of the persons were considered as illiterate, respectively (“literate” indicating knowing how to write one’s name and having at least a low reading level). The frequency of participants who had completed elementary school (9 years of schooling) was 48.1% in the municipality of Chaves and 35.4% in the municipality of Portel. Completing high school and obtaining a college degree was similar among the four municipalities and ranged from 20.4% to 26.9% and from 6.5% to 10.2%, respectively. Chaves was the only municipality in which the frequency of illiterate persons (15.6%) was lower than the frequency of persons who finished high school (26.9%). The household income was less than one minimum wage for 31.1% of the subjects. In Chaves and Portel, it was more than one minimum wage in 73.8% and 79% of the cases, respectively; however, only 58.1% and 52.8% in Anajás and SSBV, respectively, received more than one minimum wage.
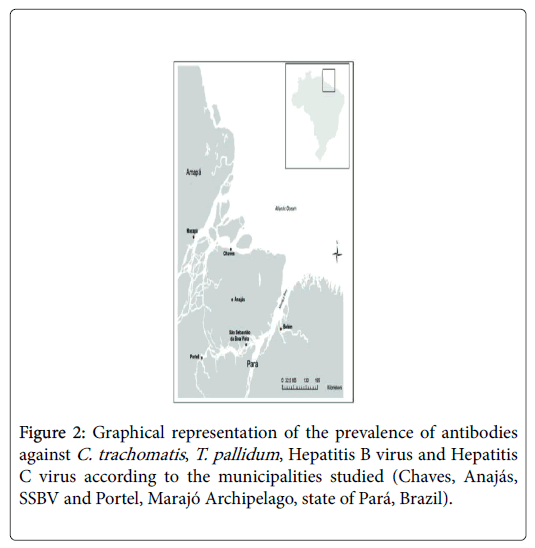
The prevalence of antibodies against C. trachomatis (IgM and IgG) in the municipalities was 30.9% (362/1173), and 6.7% (40/594) of the persons had IgM antibodies. Anajás showed the highest prevalence (38.2%; 86/225) (Figure 2), but similar to the other municipalities. The prevalence of antibodies against T. pallidum was 8.9% (103/1151); the highest and lowest prevalences were observed in Portel (15%; 69/462) and SSBV (3.6%; 8/221). T. pallidum recent infections were detected in 4.8% (5/103) distributed in Chaves (1.3%; 1/12), Anajás (7.1%; 1/14) and Portel (4.3%; 3/69).
The presence of anti-HBs alone, an indicative of vaccine immunity, was found in 19.8% (236/1190). Vaccination coverage by municipality was 22.4% (57/255) in Chaves, 17.8% (41/230) in Anajás, 10.6% (25/235) in SSBV, and 24% (113/470) in Portel. None of the samples were positive for the anti-HBc/IgM marker, but anti-HBc/IgG was present in 12.4% (118/954) of the persons. The highest and lowest prevalences were observed in Portel (18.5%; 66/357) and SSBV (3.3%; 7/210), respectively (Figure 2). Among the 118 positive persons for anti-HBc/IgG, 74 showed the presence of anti-HBs, and among the other 44 persons, nine (7.6%) presented the virus persistence marker, HBsAg, including four (0.42%) from Chaves, three (0.31%) from Anajás and two (0.21%) from Portel.
The prevalence of antibodies against HCV was 1.3% (16/1202); the municipalities with the highest and lowest prevalence were Anajás and Portel, with 2.7% (6/220) and 0.8% (4/482), respectively (Figure 2). HCV viral RNA was found in four subjects (25%; 4/16), two from Chaves and two from Portel.
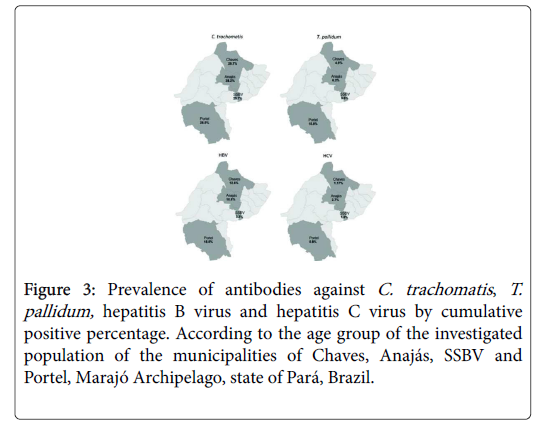
Figure 3 shows the entrance dynamics of the infectious agents according to the cumulative positive percentage and the age groups. C. trachomatis is present in the communities among young adults (18-27 years old), increases its frequency in the following age group, maintains its prevalence levels and then slightly decreases among older persons. T. pallidum is present in the communities among young adults, and its prevalence gradually increases by more than 4-fold (2.2% to 9%) with the age increase. HBV presents similar behavior; however, it shows increases in prevalence from 8.6% to 12.3%, with the peak Anajás showed the highest prevalence (38.2%; 86/225) (Figure 2), but similar to the other municipalities. The prevalence of antibodies against T. pallidum was 8.9% (103/1151); the highest and lowest prevalences were observed in Portel (15%; 69/462) and SSBV (3.6%; 8/221). T. pallidum recent infections were detected in 4.8% (5/103) distributed in Chaves (1.3%; 1/12), Anajás (7.1%; 1/14) and Portel (4.3%; 3/69).
Figure 3: Prevalence of antibodies against C. trachomatis, T.pallidum, hepatitis B virus and hepatitis C virus by cumulative positive percentage. According to the age group of the investigated population of the municipalities of Chaves, Anajás, SSBV and Portel, Marajó Archipelago, state of Pará, Brazil.
The presence of anti-HBs alone, an indicative of vaccine immunity, was found in 19.8% (236/1190). Vaccination coverage by municipality was 22.4% (57/255) in Chaves, 17.8% (41/230) in Anajás, 10.6% (25/235) in SSBV, and 24% (113/470) in Portel. None of the samples were positive for the anti-HBc/IgM marker, but anti-HBc/IgG was present in 12.4% (118/954) of the persons. The highest and lowest prevalences were observed in Portel (18.5%; 66/357) and SSBV (3.3%; 7/210), respectively (Figure 2). Among the 118 positive persons for anti-HBc/IgG, 74 showed the presence of anti-HBs, and among the other 44 persons, nine (7.6%) presented the virus persistence marker, HBsAg, including four (0.42%) from Chaves, three (0.31%) from Anajás and two (0.21%) from Portel.
The prevalence of antibodies against HCV was 1.3% (16/1202); the municipalities with the highest and lowest prevalence were Anajás and Portel, with 2.7% (6/220) and 0.8% (4/482), respectively (Figure 2). HCV viral RNA was found in four subjects (25%; 4/16), two from Chaves and two from Portel.
Figure 3 shows the entrance dynamics of the infectious agents according to the cumulative positive percentage and the age groups. C. trachomatis is present in the communities among young adults (18-27 years old), increases its frequency in the following age group, maintains its prevalence levels and then slightly decreases among older persons. T. pallidum is present in the communities among young adults, and its prevalence gradually increases by more than 4-fold (2.2% to 9%) with the age increase. HBV presents similar behavior; however, it shows increases in prevalence from 8.6% to 12.3%, with the peak occurring in the 48-to-57-year-old group. The prevalence of HCV does not change as a function of age.
The association between the presence of antibodies and the demographic and social characteristics of the subjects is shown in Table 2. Residual analysis showed that C. trachomatis infection was significantly more frequent among women (p<0.0281) and among subjects 28 to 37 years old (p<0.0317). T. pallidum was significantly associated with the presence of antibodies and older age (>68 years old; p<0.0001), being separated or widowed (p<0.0001) and being illiterate (p<0.0078). Antibodies against HBV were associated with males (p=0.0029), with 48 to 57-year old (p=0.0206) and with persons with low educational levels (p=0.0157). No association was found with HCV infection.
| Demographic/Social characteristics | C. trachomatis | T. pallidum | HBV | HCV ** |
|---|---|---|---|---|
| Positive/n (%) | Positive/n (%) | Positive/n (%) | Positive/n (%) | |
| Sex | ||||
| Female | 292/897 (32.6) | 78/877 (8.9) | 76/725 (10.5) | 11/919 (1.2) |
| Male | 64/255 (25.1) | 23/255 (9.0) | 40/218 (18.3) | 5/263 (1.9) |
| *NI | 21-Jun | 19-Feb | 11-Feb | 0/20 |
| x2/G | 5.568 | 0.004 | 9.612 | 0.7005 |
| P | 0.0281 | 0.9499 | 0.0029 | 0.5801 |
| Age group | ||||
| 18-27 years old | 87/268 (7.6) | 6/266(0.5) | 14/174 (1.5) | 4/276 (0.3) |
| 28-37 years old | 104/281 (9.3) | 23/289 (2.1) | 24/241 (2.6) | 4/297 (0.3) |
| 38-47 years old | 67/219 (6.0) | 16/217 (1.4) | 19/191 (2.0) | 4/224 (0.3) |
| 48-57 years old | 47/155 (4.2) | 17/151 (1.5) | 24/136 (2.6) | 0/156 (0) |
| 58-67 years old | 27/115 (2.4) | 19/107 (1.7) | 16/104 (1.7) | 2/117 (0.2) |
| >68 years old | 20/94 (1.8) | 19/84 (1.7) | 17/86 (1.8) | 2/92 (0.2) |
| *NI | Oct-41 | Mar-37 | 22-Mar | 0/40 |
| x2/G | 12.235 | 45.946 | 13.318 | 5.0451 |
| P | 0.0317 | < 0.0001 | 0.0206 | 0.4619 |
| Marital Status | ||||
| Married | 194/654 (18.1) | 56/649 (5.3) | 67/548 (7.6) | 10/668 (0.9) |
| Separated | 24/52 (2.2) | 10/52 (0.9) | 5/43 (0.6) | 1/53 (0.1) |
| Single | 98/295 (9.1) | 15/291 (1.4) | 27/225 (3.1) | 3/308 (0.3) |
| Widowed | 22/72 (2.0) | 13/65 (1.2) | 10/65 (1.1) | 0/71 (0) |
| *NI | 24/100 | Sep-94 | Sep-73 | 2/102 |
| x2/G | 6.631 | 21.831 | 0.606 | 2.4485 |
| P | 0.0846 | < 0.0001 | 0.8952 | 0.55 |
| Educational level | ||||
| Illiterate | 50/185 (4.5) | 26/174 (2.4) | 33/170 (3.6) | 2/185 (0.2) |
| Literate | 44/134 (3.9) | 16/130 (1.4) | 16/117 (1.7) | 2/134 (0.2) |
| Elementary school | 138/455 (12.3) | 38/454 (3.4) | 45/393 (4.8) | 8/473 (0.7) |
| High school | 80/245 (7.1) | 15/247 (1.4) | 12/164 (1.3) | 3/255 (0.3) |
| College degree | 34/102 (3.0) | 5/99 (0.4) | 9/82(1.0) | 1/104 (0.1) |
| *NI | 16/52 | Mar-47 | 28-Mar | 0/51 |
| x2/G | 2.24 | 13.844 | 12.233 | 0.6882 |
| P | 0.6916 | 0.0078 | 0.0157 | 0.959 |
| Household income (minimum wage) | ||||
| Less than 1 MW | 119/340 (10.9) | 30/339 (2.8) | 33/300 (3.6) | 5/351 (0.4) |
| More than 1 MW | 220/751 (20.1) | 70/739 (6.5) | 82/605 (9.1) | 9/770 (0.8) |
| *NI | 23/82 | Mar-73 | Feb-49 | Feb-81 |
| x2/G | 3.558 | 0.107 | 1.327 | 0.1249 |
| P | 0.0694 | 0.8304 | 0.2954 | 0.9463 |
| Note: NI: Not informed and not considered in the statistical analysis **G: G test | ||||
Table 2: Association between demographic and social characteristics and the presence of antibodies against C. trachomatis, T. pallidum , HBV and HCV in the Marajó Archipelago, state of Pará, Brazil.
The use of simple logistic regression (Table 3) showed that persons who consume alcoholic beverages are 1.5 times more likely to acquire C. trachomatis infection, and those who use illicit drugs are 2.1 times more likely to be infected. T. pallidum was associated with having more than one sexual partner and never using condoms during sexual intercourse increasing the odds of acquiring the infection by 1.8 and 1.6 fold, respectively. No association was detected for HBV and HCV.
| Risk behavior | C. trachomatis | T. pallidum | HBV | HCV |
|---|---|---|---|---|
| Positive/n (%) | Positive/n (%) | Positive/n (%) | Positive/n (%) | |
| Consumption of alcoholic beverages | ||||
| No | 134/517 (25.9) | 40/505 (7.9) | 46/428 (10.7) | 6/528 (1.1) |
| Yes | 210/594 (35.3) | 59/592 (9.9) | 69/489 (14.1) | 9/613 (1.4) |
| P | 0.001 | 0.237 | 0.127 | 0.625 |
| OR (CI) | 1.56 (1.20-2.02) | 1.28 (0.84-1.95) | 1.36 (0.91-2.03) | 1.29 (0.45-3.66) |
| Use of illicit drugs | ||||
| No | 298/955 (31.2) | 84/940 (8.9) | 96/784 (12.2) | 12/981 (1.2) |
| Yes | 19/39 (48.7) | 5/39 (12.8) | 5/35 (14.3) | 1/40 (2.5) |
| P | 0.026 | 0.436 | 0.725 | 0.53 |
| OR ( CI ) | 2.09 (1.01-3.98) | 1.49 (0.57-3.93) | 1.19 (0.45-3.15) | 2.07 (0.26-16.32) |
| Sexual choice | ||||
| Heterosexual | 315/1015 (31) | 89/1000 (8.9) | 102/834 (12.2) | 16/1042 (1.5) |
| Homosexual/Bisexual | 19/54 (35.1) | 5/52 (9.6) | 5/48 (10.4) | 0/56 |
| P | 0.526 | 0.861 | 0.703 | 0 |
| OR ( CI ) | 1.20 (0.67-2.14) | 1.08 (0.42-2.80) | 0.83 (0.32-2.15) | 0(0)* |
| One sexual partner | ||||
| No | 80/234 (34.1) | 31/229 (13.5) | 28/195 (14.3) | 5/243 (2.0) |
| Yes | 251/835 (30.0) | 66/823 (8.0) | 81/687 (11.8) | 11/855 (1.28) |
| P | 0.217 | 0.014 | 0.344 | 0.392 |
| OR ( CI ) | 1.20 (0.88-1.64) | 1.80 (1.14-2.84) | 1.26 (0.79-2.00) | 1.61 (0.55-4.70) |
| Sexual relations with sex workers | ||||
| No | 294/959 (30.6) | 80/943 (8.5) | 96/782 (12.3) | 14/988 (1.4) |
| Yes | 22/68 (32.3) | 10/70 (14.2) | 10/58 (17.2) | 2/70 (2.8) |
| P | 0.778 | 0.124 | 0.294 | 0.391 |
| OR ( CI ) | 1.08 (0.63-1.83) | 1.80 (0.88-3.65) | 1.48 (0.72-3.04) | 2.04 (0.45-9.17) |
| Condom Use | ||||
| Never | 141/487 (28.9) | 52/471 (11) | 57/424 (13.4) | 7/493(1.4) |
| Always/sometimes | 195/580 (33.6) | 40/583 (6.9) | 51/457 (11.1) | 8/605(1.3) |
| P | 0.101 | 0.017 | 0.302 | 0.89 |
| OR ( CI ) | 0.80 (0.61-1.04) | 1.68 (1.09-2.59) | 1.23 (0.82-1.85) | 1.07 (0.38-2.98) |
| Previous case of STD | ||||
| No | 283/886 (31.9) | 75/869 (8.6) | 86/720 (11.9) | 8/911 (0.9) |
| Yes | 29/110 (26.3) | 15/114 (13.1) | 15/96 (15.6) | 3/116 (2.6) |
| P | 0.225 | 0.127 | 0.318 | 0.143 |
| OR ( CI ) | 0.76 (0.48-1.19) | 1.60 (0.88-2.90) | 1.36 (0.75-2.47) | 2.99 (0.78-11.45) |
| *Statistical analysis not performed. | ||||
Table 3: Association between behavioral characteristics and the presence of antibodies against C. trachomatis, T. pallidum, HBV and HCV in the Marajó Archipelago, state of Pará, Brazil.
Table 4 shows the results of multiple logistic regression analysis for the association between the presence of antibodies against the two bacteria and behavioral variables. It confirmed that consumption of alcoholic beverages increases the likelihood of being infected with C.trachomatis by 1.5-fold and that never using condoms and having more than one sexual partner are risk factors for acquiring T. pallidum infection.
| Bacterium/Risk behavior | OR | 95% CI | P |
|---|---|---|---|
| C. trachomatis | |||
| Consumption of alcoholic beverage | 1.5631 | 1.2066-2.0248 | <0.001 |
| T. pallidum | |||
| More than one sexual partner | 1.8507 | 1.1554-2.9643 | 0.013 |
| No use of condom | 1.7596 | 1.1373-2.7222 | 0.011 |
Table 4: Multiple logistic regression of behavioral characteristics and the presence of antibodies against C. trachomatis and T. pallidum in the Marajó Archipelago, state of Pará, Brazil.
Discussion
Epidemiological surveillance studies are useful to understand the impact of diseases on urban and non-urban communities to assess prevalence of infectious agents and their diseases and the changes occurring according to risk factors influenced by socioeconomic characteristics and educational level [39,40] The present study is the first joint approach intended to describe the epidemiology of C. trachomatis, T. pallidum , HBV and HCV infection within four municipalities of the Marajó Archipelago, North of the Brazilian Amazon.
It is relevant to observe cultural factors associated with the population such as the presence of more women than men in the study. It reinforces the low demand for health services by males. The official census indicates the presence of more males than females in Marajo, [41] but behavioral and cultural barriers still prevent men from adopting self-care practices [42,43].
There was a large age group of sexually active persons who were initially tought to be more likely to have had sexual relations with more than one partner and more susceptible to be involved with STI. However, the study included more married subjects than single ones, a factor that could possibly influence the number of sexual partners as well as the degree of exposure to risk factors associated with sexual behavior.
One of the most striking characteristics was their low educational level, a main barrier to the progress and development of a community. Few of the study participants reported having formal education of at least nine years, a qualification that would enable them to better jobs which are more complex or allow them to understand basic reading instructions and to learn infection prevention measures. There are few high schools in the Marajó Archipelago and fewer college-level institutions [41]. School facilities are precarious, and few are close to rural areas, which makes attending school unattractive with a high rate of student dropout [44,45]. The combination of low educational level and weak primary economic structure results in a population with a low income level; the Marajó Archipelago has one of the lowest income levels of the state of Pará and it is the region with the lowest level of access to agricultural loans in the state [46]. The critical nature of the economic situation in the region is that almost half of the households in SSBV receive less than the country’s minimum wage.
The municipalities of Chaves, Anajás and SSBV are close to each other, which favours the movement of persons among them. Portel is more distant from the other three and although the population has low purchasing power there are few regular transportation facilities. This geographical situation apparently influenced the prevalence rates of C. trachomatis , T. pallidum and HCV; they were higher in Anajás, a possible epicenter of the spread of infections to the other municipalities. The pattern appears to be different for HBV, and Chaves seems to be the center for the spreading. Chaves is close to a large urban area, the municipality of Macapá, capital of the state of Amapá, which may explain the greater frequency of HBV. Portel showed the highest frequency of C. trachomatis , T. pallidum and HBV infections, but not for HCV. It is the largest municipality and this may explain the highest prevalence of STI, but it does not explain the lowest HCV prevalence among the four municipalities.
The general prevalence of antibodies against C. trachomatis was similar to the previously reported for the Amazon region, both in urban and non-urban areas [8,9] The presence of IgM antibodies shows the bacterium actively spreading within the communities.
The use of cumulative positive percentage of the prevalences according to the age allowed the understanding of the dynamics of transmission of the agents. C. trachomatis is already present at high levels in the first age group; its prevalence slightly increases in the older age groups but then decreases among the old population. The bacterium causes several clinical manifestations, including trachoma, a disease that is widespread in the Archipelago [47,48].
The association between C. trachomatis and women is relevant for the development of control measures regarding reproductive and gynecological complications as infection is commonly asymptomatic with bacterial persistence [49,50]. The presence of IgM antibodies in adults stresses the evidence of active infection and the possibility of transmission, mainly sexually, considering the frequency of IgM positive among sexually active adults. The majority of persons with IgM against C. trachomatis were married females (23/40; 57.5%). Prevention and control policies are in urgent need because reporting of C. trachomatis infection is not mandatory; as a result, there are no prevalence and/or incidence estimates for the screening and treatment of infected patients.
Among the behavioral risk factors, C. trachomatis infection was associated with the use of illicit drugs and with the consumption of alcoholic beverages. Although the main route of transmission of C. trachomatis in adults is the sexual route, risk factors related to sexual behavior and blood exposure were not associated with the presence of antibodies. Among men living in developed countries, alcohol and drug abuse are not associated with Chlamydia infection [51]. However, excessive alcohol consumption and the use of illicit drugs may affect individuals’ perception, making them more likely to engage in sexual intercourse without using appropriate precautions and thereby increasing the chances of bacterial transmission. In 2016, 1.6 million new cases of C. trachomatis infection were reported in the USA, [52] demonstrating its widespread distribution and placing it as the most frequent STI. In Brazil, the perception is that a similar situation also exists but it needs to be fully assessed.
The prevalence of antibodies against T. pallidum was similar in three of the municipalities. The prevalence increased more than fourfold from the first age group to the last. The increase in syphilis among young adults and adults in Brazil [53] has been a major concern, and a similar situation apparently occurs in the Archipelago. The finding of acute infection and possibly of ongoing disease is of special concern because it demonstrates the failure of prevention measures. It is possible that clinical manifestations of primary syphilis do not stimulate the search for treatment, contributing to the spread of the bacterium. T. pallidum is currently transmitted among the population and brings an unfavorable outcome for sexually active women of reproductive age because an undiagnosed and untreated infection may result in pregnancies with disastrous consequences for the fetus. Syphilis control has flaws that are evidenced by the high number of cases in pregnant women. Failures in primary care are identified only in referral services, and the fragility in diagnosis, treatment and monitoring of infection compromises the epidemiological control of the disease [4,54].
T. pallidum was associated with older age, low educational level and separated/widowed marital status. There was no association with sex or household income, although infection is associated with less favored population groups [55]. Low educational levels may explain why some individuals have poor understanding of or little access to prevention methods as they would increase the risk of exposure to behavioral risks. The association with older age may be a consequence of longer periods of exposure to the bacterium as well as of neglect by public health agencies whose education campaigns do not focus on individuals who are older than 30 years of age [55]. The association between the presence of T. pallidum antibodies and separated/ widowed marital status is possibly related to subjects who often have more than one sexual partner and may not use condoms during sexual intercourse, two risk factors for T. pallidum infection in the present study as well as in other populations [56,57]. Although no other association between infection markers and other risk factors was found in the present study, it has been associated with a previous history of Sexually Transmitted Diseases (STD), alcohol consumption and use of illicit drugs [57,58].
HBV showed endemicity for both past and chronic infections, and no acute infections were detected. The percentage of persistent infections is a matter of concern due to the potential clinical outcomes of the infection [59,60] and the paucity of healthcare providers in the Archipelago. Although HBV infection is prevented by vaccine, our results showed that vaccination coverage was very low, leaving the community in a state of great vulnerability. Additional vaccination campaigns must be conducted, and their target population coverage must be improved.
HBV transmission included a gradual increase in its prevalence and it was sharp among individuals 50 years of age and older. The pattern of HBV occurrence was not uniform, and there were areas in which its prevalence showed high variation, such as Portel and SSBV, similar to what has been observed in villages in rural areas of the Amazonas state and in other South American and Asian countries [60-64]. Flaws in the vaccination strategy may explain some of the variations in prevalence among rural populations in the Amazonas state considering that the socioeconomic conditions of these rural populations are very similar [62].
It is important to reassess the established HBV vaccination strategies and consider the need of each area, taking into account the individual geographical characteristics and population. The vaccination strategy to focus mainly among younger age groups resulted in a large susceptible older population and has recently led to an increase in the age range priority for vaccination groups [65]. In the population of the Marajó Archipelago, the infection was associated with older illiterate males which is similar to previously reported information by the Brazilian Ministry of Health [25].
Prevalence of HCV in the Marajó Archipelago was similar to that found in other areas of the Amazon region and in the country as a whole [25,66,67] but it was higher than that found in another region of the state of Pará [68]. The prevalence was low in the Marajó Archipelago, unlike South America in general, which presents a moderate prevalence [67] No association was seen between the presence of antibodies and the social and demographic characteristics, a different situation observed for other Brazilian populations with low per capita income and low educational levels [25]. HCV infection in urban populations is associated with older people, females, persons with low educational level and persons with low income [65,68]. Sexualbehavior and exposure to blood were not associated with HCV infection, possibly due to the low efficiency of sexual transmission of HCV [62].
The present study described the circulation of infectious agents of medical importance that are sexually, congenitally and parenterally transmitted. Apart from T. pallidum , the infectious agents studied were not found to be associated with risk factors related to sexual behavior. The Marajó Archipelago is located far from developed urban centers and has one of the lowest human development indexes, and it needs health policies, that are specifically designed for the region [9]. The transmission of infections will only be interrupted by the consistent application of measures including health education, routine examination, immediate treatment (including treatment of infected partners) and recognition of risk factors associated with infections, in order to contribute to the successful planning and implementation of prevention and control strategies. It is crucial that health education campaigns provide information that are easily understood and accordingly to the daily lives of the population for immediate assimilation.
Acknowledgments
To all participants who contributed to this study and Universidade Federal do Pará -UFPA (Programa PAPQ/2018) for the financial contribution in the preparation of the article.
Funding
Supported by grant from the Fundação Amazônia Paraense de Amparo a Pesquisa (FAPESPA–ICAAF 014/2012) and Ministério da Saúde do Brasil (MS). Funding source(s) had no involvement for the conduct of the research and/or preparation of the article.
The study was submitted and approved by the Research Ethics Committee of the Center for Hematotherapy and Hematology Foundation of Para (protocol number 0003.0.324.000-10).
Author Contributions
RONF, ACRV, MOGI and RI designed the study. GRONF, FBF performed the experiments. GRONF, MAFQ, SSL and RI analyzed and interpreted the date. GRONF, MAFQ and RI wrote the manuscript. RI and ACRV oversaw the experiments and edited the manuscript. GRONF, FBF, MAFQ, SSL, ACRV, MOGI and RI reviewed the manuscript.
References
- World Health Organization (2017) Global health sector strategy on sexually transmitted infections, 2016-2021, Geneva.
- Baud D, Goy G, Jaton K, Osterheld MC, Blumer S, et al. (2011) Role of chlamydia trachomatis in miscarriage. Emerg Infect Dis 17: 1630-1635.
- Wusu-Edusei K, Chesson HW, Gift TL, Tao G, Mahajan R, et al. (2013) The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 40: 197-201.
- Fernandes HD, Araújo EC, Neves DCO, Ribeiro KTS (2014) Prevalence of HIV and syphilis in parturients attended at a reference maternity hospital in the city of Marabá-Pará. Revista Paraense de Medicina 28: 55-62.
- Morré SA, Ossewaarde JM, Lan J, van Doornum GJ, Walboomers JM, et al. (1998) Serotyping and genotyping of genital chlamydia trachomatis isolates reveal variants of serovars Ba, G, and J as confirmed by omp1 nucleotide sequence analysis. J Clin Microbiol 36: 345-351.
- Silveira MF, Sclowitz IK, Entiauspe LG, Mesenburg MA, Stauffert D, et al. (2017) Chlamydia trachomatis infection in young pregnant women in Southern Brazil: A cross-sectional study. Cad Saúde Pública 33: e00067415.
- World Health Organization (2008) Global incidence and prevalence of selected curable sexually transmitted infections-2008, Geneva.
- Ishak, MOG, Ishak R (2001) The impact of chlamydia infection on indigenous populations of the Brazilian. Cad de Saúde Pública 17: 385-396.
- Ishak M de O, Costa MM, Almeida NC, Santiago AM, De Brito WB, et al. (2015) Chlamydia trachomatis serotype A infections in the Amazon region of Brazil: Prevalence, entry and dissemination. Rev Soc Bras Med Trop 48: 170-174.
- French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, et al. (2009) IUSTI: 2008 European guidelines on the management of syphilis. Int J STD AIDS 20: 300-309.
- Cruz AR, Pillay A, Zuluaga AV, Ramirez LG, Duque JE, et al. (2010) Secondary syphilis in cali, colombia: New concepts in disease pathogenesis. PLoS Negl Trop Dis 4: e690.
- Forrest CE, Ward A (2016) Clinical diagnosis of syphilis: A ten-year retrospective analysis in a South Australian urban sexual health clinic. Int J STD AIDS 27: 1334-1337.
- Moradi A, Salek S, Daniel E, Gangaputra S, Ostheimer TA, et al. (2015) Clinical features and incidence rates of ocular complications in patients with ocular syphilis. Am J Ophthalmol 159: 334-343.
- Piraccini BM, Broccoli A, Starace M, Gaspari V, D'Antuono A, et al. (2015) Hair and scalp manifestations in secondary syphilis: epidemiology, clinical features and trichoscopy. Dermatology 231: 171-176.
- Wijesooriya NS, Rochat RW, Kamb ML, Turlapati P, Temmerman M, et al. (2005) Global burden of maternal and congenital syphilis in 2008 and 2012: A health systems modelling study. Lancet Glob Health 4: e525-533.
- Solomon MM, Mayer KH, Glidden DV, Liu AY, McMahan VM, et al. (2014) Syphilis predicts hiv incidence among men and transgender women who have sex with men in a preexposure prophylaxis trial. Clin Infect Dis 59: 1020-1026.
- Chen YC, Chu CM, Liaw YF (2010) Age-specific prognosis following spontaneous hepatitis be antigen seroconversion in chronic Hepatitis B. Hepatology 51: 435-444.
- Coppola N, Sagnelli C, Pisaturo M, Minichini C, Messina V, et al. (2014) Clinical and virological characteristics associated with severe acute hepatitis B. Clin Microbiol Infect 20: O991-997.
- Zeng LY, Lian JS, Chen JY, Jia HY, Zhang YM, et al. (2014) Hepatitis B surface antigen levels during natural history of chronic hepatitis B: A Chinese perspective study. World J Gastroenterol 20: 9178-9184.
- Ott JJ, Stevens GA, Groeger J, Wiersma ST (2012) Global epidemiology of hepatitis B virus infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30: 2212-2219.
- World Health Organization (2016) Global health sector strategy on viral hepatitis 2016-2021 towards ending viral hepatitis. Geneva.
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ (2015) Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 386: 1546-1555.
- Brasil (2017) Boletim epidemiológico das hepatites virais, 2017-Ministério da Saúde.
- Baha W, Foullous A, Dersi N, They-they TP, El alaoui K, et al. (2013) Prevalence and risk factors of hepatitis B and C virus infections among the general population and blood donors in Morocco. BMC Public Health 13: 50.
- Li Z, Xie Z, Ni H, Zhang Q, Lu W, et al. (2014) Mother-to-child transmission of hepatitis B virus: Evolution of hepatocellular carcinoma-related viral mutations in the post-immunization era. J Clin Virol 61: 47-54.
- Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, et al. (2003) Acute hepatitis C: High rate of both spontaneous and treatment-induced viral clearance. Gastroenterology 125: 80-88.
- Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, et al. (2010) Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 52: 518-527.
- Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, et al. (2003) Progression of fibrosis in chronic Hepatitis C. Gastroenterology 124: 97-104.
- Bunchorntavakul C, Jones LM, Kikuchi M, Valiga ME, Kaplan DE, et al. (2015) Distinct features in natural history and outcomes of acute Hepatitis C. J Clin Gastroenterol 49: e31-40
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatolo 57: 1333-1342.
- Dienstag JL, Purcell HR, Alter HJ, Feinstone SM, Wong DC, et al. (1977) Non-a, non-b post-transfusion Hepatitis. Lancet 1: 560-562.
- Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, et al. (1992) The natural history of community-acquired hepatitis C in the United States. N Engl J Med 327: 1899-1905.
- Oliveira-Filho AB, Sawada L, Pinto LC, Locks D, Bahia SL, et al. (2014) Epidemiological aspects of HCV infection in non-injecting drug users in the Brazilian state of Pará, eastern Amazon. Virol J 11: 38.
- Rotermann M, Langlois K, Andonov A, Trubnikov M (2013) Seroprevalence of hepatitis B and C virus infections: Results from the 2007 to 2009 and 2009 to 2011 canadian health measures survey. Health Rep 24: 3-13.
- Kaufman CE, Shelby L, Mosure DJ, Marrazzo J, Wong D, et al. (2007) Within the hidden epidemic: Sexually transmitted diseases and HIV/AIDS among american indians and alaska natives. Sex Transm Dis 34: 767-777.
- Ayres M, Ayres JR, Ayres DL, Santos AS (2011) BioEstat 5.3: Statistical applications in the areas of biological and medical sciences. Belém: Editora Sociedade Civil Mamirauá.
- Vriend HJ, Van Veen MG, Prins M, Urbanus AT, Boot HJ, et al. (2013) Hepatitis C virus seroprevalence in the Netherlands. Epidemiol Infect 141: 819-821.
- Daw MA, Shabash A, El-Bouzedi A, Dau AA (2014) Seroprevalence of HBV, HCV & HIV co-infection and risk factors analysis in tripoli-libya. PLoS ONE 9: e98793.
- IBGE (2017) National continuous household sample survey-continuous PNAD.
- Gomes R, Nascimento EF, Araújo FC (2007) Why do men seek health services less than women? The explanations of men with low schooling and men with higher education. Cad Saude Publica 23: 565-574.
- Moreira MC, Gomes R, Ribeiro CR (2016) And now the man comes ?! Men's Health Care Strategies. Cad Saúde Pública 32: e00060015.
- Araújo SMS (2010) One photograph, multiple images: Rural education in Northern Brazil. Hist Cienc Saude Manguinhos 17:1009-1022.
- Miranda Costa E (2017) Degree in Field Education: Intentionalities in the formation of educators of the field in the Marajó. Educación 26: 88-103.
- Santos MAS, Santana AC, Rebello FK (2013) Rural credit policy in the Marajá archipelago, State of Pará: an analysis of the period 2000-2010. Society and Rural Development on line.
- Reilly LA, Favacho J, Garcez LM, Courtenay O (2007) Preliminary evidence that synanthropic flies contribute to the transmission of trachoma causing Chlamydia trachomatis in Latin America. Cad Saúde Pública 23: 1682-1688.
- Favacho J, Alves da Cunha AJL, Gomes STM, Freitas FB, Queiroz MAF, et al. (2018) Prevalence of trachoma in school children in the Marajó Archipelago, Brazilian Amazon, and the impact of the introduction of educational and preventive measures on the disease over eight years. PLoS Negl Trop Dis 12: e0006282.
- Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, et al. (2005) The Natural Course of chlamydia trachomatis infection in asymptomatic colombian women: A 5-year follow-up study. J Infect Dis 191: 907-916.
- Agrawal T, Gupta R, Dutta R, Srivastava P, Bhengraj AR, et al. (2009) Protective or pathogenic immune response to genital chlamydial infection in women-A possible role of cytokine secretion profile of cervical mucosal cells. Clin Immunol 130: 347-354.
- Baud D, Jaton K, Bertelli C, Kulling JP, Greub G (2008) Low prevalence of Chlamydia trachomatis infection in asymptomatic young Swiss men. BMC Infect Dis 8: 45.
- Centers for Disease Control and Prevention (2016) Sexually transmitted diseases surveillance. National profile overview. Chlamydia.
- Brasil (2016) Ministry of health. Department of surveillance, prevention and control of STIs, HIV/AIDS and viral hepatitis. Epidemiological Bulletin of Syphilis-2016.
- Casal CA, Silva MO, Costa IB, Araújo Eda C, Corvelo TC (2011) Molecular detection of treponema pallidum sp. pallidum in blood samples of VDRL-seroreactive women with letal pregnancy outcomes: A restrospective observational study in Nothern Brazil. Rev Soc Bras Med Trop 244: 451-456.
- Matos SB, Jesus AL, Pedroza KC, Sodre HR, Ferreira TL, et al. (2013) Prevalence of serological markers and risk factors for bloodborne pathogens in Salvador, Bahia state, Brazil. Epidemiol Infect 141: 181-187.
- Vallinoto ACR, Santos DAS, Rosal, EC, Pontes GS, Rodrigues AMC, et al. (2003) Serological evaluation and risk factors associated with syphilis. Rev Para Med 17: 29-33.
- Miranda AE, Figueiredo NC, Pinto VM, Page K, Talhari S (2012) Risk factors for Syphilis in Young women attending a Family health program in Vitória, Brazil. An Bras Dermatol 87: 76-83.
- Asiki G, Mpendo J, Abaasa A, Agaba C, Nanvubya A, et al. (2011) HIV and syphilis prevalence and associated risk factors among fishing communities of Lake Victoria, Uganda. Sex Transm Infect 87: 511-515.
- Carvalho JR, Portugal FB, Flor LS, Campos MR, Schramm JMA (2014) Method for estimation of prevalence of chronic hepatitis B and C and liver cirrhosis - Brazil, 2008. Epidemiol Health Services 23: 691-700.
- Passos TR, Santos FS, Martins MC, Pinto VB, Carrilho FJ, et al. (2017) Clinical pharmacology profile of care in Hepatology clinic. Rev Assoc Med Bras 63: 401-406.
- Alvarado-Mora MV, Fernandez MF, Gomes-Gouvêa MS, de Azevedo Neto RS, Carrilho FJ, et al. (2011) Hepatitis B (HBV), Hepatitis C (HCV) and Hepatitis Delta (HDV) viruses in the Colombian population-how is the epidemiological situation? PLoS ONE 6: e18888.
- Braga WSM, Castilho MC, Borges FG, Martinho AC, Rodrigues IS, et al. (2012) Prevalence os hepatites B vÃrus infection and carriage after nineteen years of vacination program in the western Brazilian amazon. Rev Soc Bras Med Trop 45: 13-17.
- Lee BS, Cho YK, Jeong SH, Lee JH, Lee D, et al. (2013) Nationwide seroepidemiology of hepatitis B virus infection in South Korea in 2009 emphasizes the coexistence of HBsAg and anti-HBs. J Med Virol 58: 1327-1333.
- Flichman DM, Blejer JL, Livellara BI, Re VE, Bartoli S, et al. (2014) Prevalence and trends of markers of hepatitis B virus, hepatitis C virus and human Immunodeficiency virus in Argentine blood donors. BMC Infectious Diseases 14: 218.
- Brasil (2013) Ministry of health. Department of surveillance, prevention and control of STIs, HIV/AIDS and viral hepatitis. Joint technical note 02/2013. Increase in the supply of hepatitis B vaccine for the age group from 30 to 49 years in 2013.
- Fagundes GD, Bonazza V, Ceretta LB, Back AJ, Bettiol J (2008) Detection of the hepatitis C virus in a population of adults. Rev Lat Am Enfermagem 16: 396-400.
- Menegol D, Spilki FR (2014) Seroprevalence of hepatitis B and C markers at the population level in the municipality of Caxias do Sul, southern Brazil. Braz J Microbiol 44: 1237-1240.
- Nunes HM, Malheiros A, Sarmento, VP, Soares MCP (2017) Viral hepatitis: Epidemiological, clinical and prevention aspects in municipalities in the Parauapebas Microregion, southeastern Pará state, Brazil. Rev Pan-Amaz Saude 8: 29-35.
- El Maerrawi I, Carvalho HB (2015) Prevalence and risk factors associated with HIV infection, hepatitis and syphilis in a state prison of São Paulo. Int J STD AIDS 26: 120-127.
Citation: Ferreira GRON, Freitas FB, Queiroz MAF, Lima SS, Vallinoto ACR, et al. (2019) Epidemiology and Risk Factors for Chlamydia trachomatis, Treponema pallidum, Hepatitis B Virus and Hepatitis C Virus in the Marajó Archipelago, Brazilian Amazon. J Community Med Health Educ 9:643. DOI: 10.4172/2161-0711.1000643
Copyright: Ferreira GRON, Freitas FB, Queiroz MAF, Lima SS, Vallinoto ACR, et al. (2019) Epidemiology and Risk Factors for Chlamydia trachomatis, Treponema pallidum, Hepatitis B Virus and Hepatitis C Virus in the Marajó Archipelago, Brazilian Amazon. J Community Med Health Educ 9:643.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4396
- [From(publication date): 0-2019 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 3555
- PDF downloads: 841