Case Report Open Access
Ependymoma as a Secondary Malignancy after Treatment of Non- Hodgkin Lymphoma
Mehran K1, Zareifar S1, Mandana T2, Babak A3*, Afsaneh H3, Karmella K1 and Maral M11Shiraz University of Medical Science, Shiraz, Iran
2Paediatric Haematologist Oncologist, Hematology Oncology, Mahak Hospital-Tehran, Iran
3Hematology Oncology, Lorestan University of Medical Science, Khoramabad, Iran
- *Corresponding Author:
- Babak Abdolkarimi
Hematology Oncology
Lorestan University of Medical Science
Khoramabad, Iran
Tel: +989183605274
E-mail: b.abdolkarimi@yahoo.com
Received Date: July 13, 2016; Accepted Date: January 21, 2017; Published Date: January 27, 2017
Citation: Mehran K, Zareifar S, Mandana T, Babak A, Afsaneh H, et al. (2017) Ependymoma as a Secondary Malignancy after Treatment of Non- Hodgkin Lymphoma. OMICS J Radiol 6:247. doi: 10.4172/2167-7964.1000247
Copyright: © 2017 Mehran K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Radiology
Abstract
Therapeutic advances in the treatment of paediatric neoplasms have improved the prognosis but have also increased the risk of developing rare second malignant neoplasms (SMNs) in late period after chemotherapy or radiation course. We are going to report a patient therapy-related solid tumors (brain ependymoma after non Hodgkin lymphoma) short latency period after primary tumor which is a rare case among secondary tumors. After 4 years from lymphoma involvement a mediastinal mass revealed in CXR in favor of relapse in follow up period. The key point of this case is that secondary solid malignancy followed infiltrative malignancy despite of similar cases that infiltrative malignancy followed solid tumor and without radiation therapy.
Keywords
Ependymoma; Radiation-induced malignancy; Secondary malignancy; Radiation-induced chemotherapy
Introduction
A secondary malignancy or Subsequent Malignant Neoplasms (SMNs) is a new cancer that occurs in an individual because of previous treatment with radiation or chemotherapy in cancer survivors.
Unique clinical and pathologic characteristics have resulted in a conventional classification of SMNs into 2 distinct groups: Therapy-related myelodysplasia/acute myeloid leukaemia (t-MDS/AML) and therapy-related solid tumors. Characteristics of t-MDS/AML include a short latency (typically less than 3 years from primary cancer diagnosis) and exposure to alkylating agents (nitrogen mustard, cyclophosphamide, procarbazine) and/or topoisomerase II inhibitors (etoposide) and radiation therapy for some lymphomas and childhood leukaemia [1].
Therapy-related solid tumors, on the other hand, have a strong and well-defined association with radiation, and are characterized by latencies that exceed 10 years. They are unrelated to the first cancer that was treated, and may occur months or even years after initial treatment [2]. These agents are all capable of causing secondary cancers, but not to the same degree. Secondary cancers after non- Hodgkin’s lymphoma (NHL) due to chemotherapic agents as combination of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) were seen [3].
Case Report
Our patient is a 14-year-old boy. The patient was in his usual state of health until Feb 2010 when he developed upper respiratory infection symptoms. He had no other significant complaints, and no loss of weight or fever. Examination did not reveal any peripheral nodes were palpable and organomegaly. Chest radiography and CT scan revealed an inhomogen anterior mediastinal mass about 8 cm with inter tumoral necrosis with adhesion to chest wall and without pleural effusion. Bone marrow aspiration and biopsy had no evidence of malignancy with normocellular marrow. Trucut needle biopsy of mass showed high-grade T-cell non-Hodgkin lymphoma and open mediastinal mass biopsy revealed T-cell lymphoblasticlymphoma with flowcytometry markers:
CD3(+), CD7(+), CD10(-), CD30(-), CD20(-), CD3(+), pax-5(-), TdT(+)
Multidetectal abdominopelvic CT scan was normal. CSF cytology was normal. The patient received Lymphoblastic lymphoma protocol.
Lymphoblasic lymphoma in primary involvement chemotherapy was initiated for him according to this protocol
Induction protocol
1. Prednisone (PO) 60 mg/m2;
2. Vincristine (IV) 1.5 mg/m2;
3. Daunorubicin30 mg/m2;
4. L-Asparaginase 10,000 units/m2;
5. Cyclophosphamide 1,000 mg/m2;
6. Mesna 1,000 mg/m2;
7. Cytarabine (IV) 75 mg/m2;
8. 6-Mercaptopurine (PO) 60 mg/m2;
9. MTX (IT)#.
Consolidation protocol Mf
1. Mercaptopurine (PO) 25 mg/m2;
2. MTX (24-h infusion) g5 g/m2;
3. Leukovorinafter ending of MTX 15 mg/m2 × 15 dose;
4. MTX (IT).
Reinduction protocol II
1. Dexamethasone (PO) 10 mg/m2;
2. Vincristine (IV) 1.5 mg/m2;
3. Doxorubicin (IV) 30 mg/m2;
4. L-Asparaginase (IV) 10,000 units/m2;
5. Cyclophosphamide1000 mg/m2;
6. Mesna1000 mg/m2;
7. Cytarabine (IV) 75 mg/m2;
8. 6-Thioguanine (PO) 60 mg/m2;
9. MTX (IT) (age-adjusted dose).
The response to chemotherapy was favourable and he completed cycles of chemotherapy. He was accordingly asked to attend our institute for regular follow-up.
After 15 months, cervical ultrasonography showed multiple enlarged lymph nodes at right lateral neck growing into 10 mm.
After 2.5 months, he started experiencing generalized morning headaches. Brain MRI with and without contrast revealed:
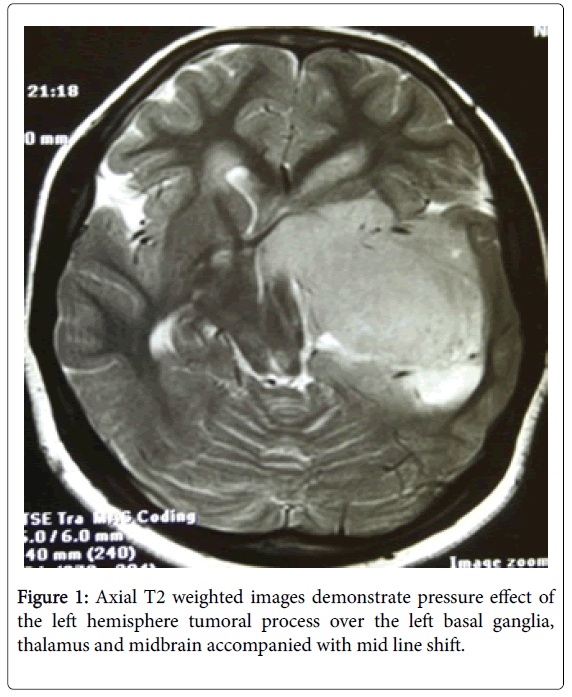
A left temporal lobe mass which was hypo signal in T1 and hyper signal in T2 weighted images measuring 7 cm in greater diameter. This lesion showed areas of increased signal intensity in T1 weighted images in the posterior superior aspect in favour of intra tumoral haemorrhage. It was enhancing with gadolinium in inhomogeneous pattern. The lesion was intraaxial and encased the left. Internal carotid artery branched and caused shift of the midline to the right side. It was highly in favor of a high-grade malignant tumor like metastasis or high-grade glioma. Because it was intraaxial in position, direct lymphomatous involvement of the brain was unlikely so metastasis from another tumoral process or a primary left temporal lobe malignancy needed to be taken into consideration. There was no hydrocephalus. 7th and 8th nerve root complexes were normal bilaterally. Pituitary gland was normal, too (Figures 1 and 2).
Pathologic investigations including immunohistochemistry staining on brain tumor confirmed anaplastic ependymoma.
Surgical resection was done and pathological findings showed:
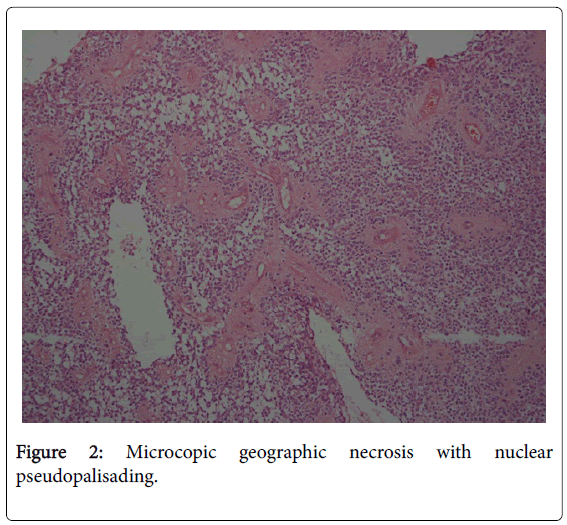
The extirpated tumor was well circumscribed from the surrounding brain dense proliferation of atypical, short spindle or polygonal cells. Extensive geographic necrosis with nuclear pseudopalisading was seen. Perivascular pseudorosettes were observed in many areas, true ependymal rosettes were absent. Immunohistochemistry for glial fibrillary acidic protein and epithelial membrane antigen revealed: GFAP(+), Ki67>10%, EMA(+), Scant(+), LCA(-), Synaptophysin (-) Suggestive of anaplastic ependymoma grade 3.
Radiotherapy for ependymoma was not done. After 4 years from lymphoma involvement a mediastinal mass was revealed in CXR in favor of relaps in follow up period. Unfortunately, the patient died due to CMV pneumonia induced by severe neutropenia after new chemotherapy course.
Discussion
Second malignancies represent an important iatrogenic complication of the treatment of neoplasms. Although, it is hard to identify the exact cause of anyone's cancer, here we will try to focus on the risk of second cancers that may be linked to past cancer treatment. Cyclophosphamide-based chemotherapy radiation can be one of the causes triggering second malignancies.
Lopes Cardozo and Martens, reported Secondary tumors after high-dose cyclophosphamide and total-body irradiation followed by bone marrow transplantation in a rat model for human acute myelocytic leukemia (BNML) [4].
Majhail et al. reported Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning [5].
Mostly, it has been indicated that solid tumors take much longer to develop at least 10-15 years after radiation therapy.
Pfeiffer et al. reported a case of metastatic induced leiomyosarcoma thirty-year therapy for Wilms’ tumor [6].
Tumor Survivors of NHL also are at risk of several cancers such as Melanoma [7].
Radiation-induced brain tumor represents an increasingly, Lung cancer, Kidney cancer, Kaposi sarcoma, Cancers of the head/neck area, Colon cancer, Thyroid cancer, Bone and soft tissue cancer, Bladder cancer, Leukemia and myelodysplastic syndrome [8].
Brain ependymoma followed NHL haven't been reported especially in children but Omidvari and Ahmadloo in 2006 reported a 50-year-old man developing intramedullary ependymoma extending from C4 to C6 levels of the cervical spinal cord of the cervical spinal cord 1.5 years following chemoradiation for Waldeyer's ring lymphoma [9].
We didn't find any similar reports from CNS (brain or spinal cord) ependymoma followed NHL either in children group or adult. Early-onset cancers are associated with the inheritance of predisposing genes of low penetrance. Genetic predisposition also plays a major role in the formation of SMNs, and clinicians should individualize treatment for patients.
Exposure to radiotherapy shortens the lag time from diagnosis of first to the second malignancy in this group. Patients with the Li- Fraumeni syndrome characterized by the presence of an abnormal p53 gene are also at increased risk. This gene limits a cell's ability to stop proliferation in the face of chromosomal damage [10].
The most important aspect of treatment of secondary cancer is early diagnosis and risk group determination. It is important to understand that, malignancy patients are not only at risk of a recurrence of their primary cancer but also at risk of a secondary cancer. For this reason, determination of risk group before initiation treatment of primary tumor is lifesaving.
On the other hand risk groups is that less treatment can be given to those who have a low risk of recurrence of cancer with standard treatments and more treatment can be given to those at high risk.
Treatment protocols should be modified (for example by radiation in Hodgkin disease) to reduce the risk for SMNs without compromising the effectiveness of the initial therapy.
The interesting points in our patient are:
• Secondary solid malignancy followed infilterative malignancy despite of similar cases that infilterative malignancy followed solid tumor;
• Short term latency period after primary malignancy (<10 ears);
• The development of the intramedullary ependymoma following treating lymphoma just one patient reported in PUBMED, EMBASE has not been reported and we did not find cerebral ependymoma following treating infilterative malignancy especially in children;
• The development of the ependymoma without radiation therapy.
Conclusion
Decreasing dose of chemotherapy in low risk groups is probably the most important way to prevent secondary cancers. Another way to decrease the incidence of second cancers is to avoid radiation therapy.
The follow-up of cancer survivors are important because they give us clues to better understand the long-term effects of cancer treatments even lifelong. Follow-up with imaging modalities such as ultrasonography or Magnetic Resonance Imaging (MRI) without ionizing radiation is indispensable for all childhood cancer survivors.
References
- Kollmannsberger C, Hartmann JT, Kanz L, Bokemeyer C (1999) Therapy-related malignancies following treatment of germ cell cancer. Int J Cancer 83: 860-863.
- van Leeuwen FE, Klokman WJ, Veer MBVT, Hagenbeek A, Krol AD, et al. (2000) Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol 18: 487-487.
- Kalinka-Warzocha E, Wajs J, Lech-Maranda E, Ceglarek B, Holowiecki J, et al. (2008) Randomized comparison of cladribine alone or in combination with cyclophosphamide, and cyclophosphamide, vincristine and prednisone in previously untreated low-grade B-cell non-Hodgkin lymphoma patients. Cancer 113: 367-375.
- Cardozo BL, Martens AC, Zurcher C, Hagenbeek A (1984) Secondary tumors after high-dose cyclophosphamide and total-body irradiation followed by bone marrow transplantation in a rat model for human acute myelocytic leukemia (BNML). Eur J Cancer Clin Oncol 20: 695-698.
- Majhail NS, Brazauskas R, Rizzo JD, Sobecks RM, Wang Z, et al. (2011) Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood 117: 316-322.
- Pfeiffer J, Boedeker CC, Ridder GJ, Maier W, Kayser G (2006) Radiation-induced leiomyosarcoma of the oropharynx. Diagn Pathol 1: 22.
- Tuohimaa P, Pukkala E, Scélo G, Olsen JH, Brewster DH, et al. (2007) Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: vitamin D as a possible explanation. Eur J Cancer 43: 1701-1712.
- Schou G, Storm HH, Jensen OM (1985) Second cancer following cancers of the buccal cavity and pharynx in Denmark, 1943-80. National Cancer Institute Monograph 68: 253-276.
- Mohammadianpanah M, Vasei M, Mosalaei A, Omidvari S, Ahmadloo N (2006) Malignant spinal cord compression in cancer patients may be mimicked by a primary spinal cord tumour. European journal of cancer care 15: 497-500.
- Hisada M, Garber JE, Li FP, Fung CY, Fraumeni JF (1998) Multiple primary cancers in families with Li-Fraumeni syndrome. Journal of the National Cancer Institute 90: 606-611.
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 3304
- [From(publication date):
February-2017 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 2423
- PDF downloads : 881