Eosinophilic Gastroenteritis with Ascites during Pregnancy: A Case Report
DOI: 10.4172/2161-069X.1000654
Abstract
Background: Eosinophilic gastroenteritis (EGE), a rare disorder of unknown etiology, is characterized by
eosinophilic infiltration of the gastrointestinal tract in the absence of secondary causes for eosinophilia. EGE has
nonspecific symptoms, but abdominal pain, nausea, and vomiting are the most common presenting symptoms.
Eosinophilic ascites is a very unusual presentation.
Case presentation: A 38 year old women at 11 weeks of gestation presented with nausea, diarrhea, and
abdominal pain. Magnetic resonance imaging revealed diffuse bowel wall thickening and a small amount of ascites.
Despite antibiotic treatment, her symptoms were persistent and she developed marked abdominal distension and
weight gain despite antibiotic treatment. Her blood eosinophil count was high, and ultrasonography showed large
ascites. Diagnostic paracentesis revealed significant eosinophilia. She completely recovered with steroid treatment
and delivered a healthy baby without any complications. She was newly diagnosed bronchial asthma after 3 months
of delivery.
Conclusion: To our knowledge, this is the first case of EGE presenting with ascites during pregnancy. The
serosal EGE is very rare type of EGE, but it can occur during pregnancy. Additionally, she was diagnosed with
asthma after delivery. The augmented Th2 immunity during pregnancy may be related with EGE and newly onset
asthma.
Keywords: Ascites; Eosinophilic gastroenteritis; Pregnancy; Case report
Abbreviations
(EGE) Eosinophilic Gastroenteritis; (IL-4) Interleukin 4; (IL-5) Interleukin 5; (IL-13) Interleukin 13
Background
Eosinophilic gastroenteritis (EGE) is an uncommon disorder characterized by eosinophilic infiltration of the gastrointestinal tract. EGE presents with a variety of clinical manifestations according to the site affected and depth of eosinophilic infiltration of the gut wall. It is classified into three types mucosal, muscular, and serosal [1,2]. Serosal EGE is the most unusual and least common type of EGE, and eosinophilic ascites is considered a special feature of serosal EGE [3,4]. Furthermore, limited cases of EGE have been reported in women during the antepartum or postpartum period [5,6].
However, to our knowledge, there have been no reports on the development of EGE during pregnancy and newly development of asthma after EGE. Here, we have reported a case of EGE presenting with ascites during pregnancy and newly onset of asthma after delivery.
Case Presentation
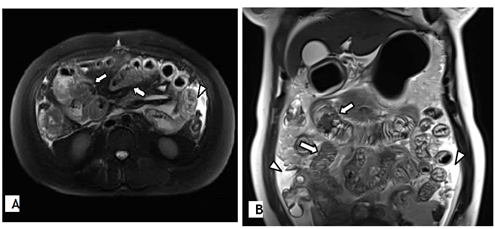
A 38 year old otherwise healthy woman (gravida 4, para 2) was referred to our hospital with a 10 day history of nausea, diarrhea, and abdominal pain at 12 weeks of gestation. Magnetic resonance imaging revealed diffuse bowel wall thickening and small volume ascites, suggesting enterocolitis (Figure 1). She was administered ceftriaxone intravenously for 6 days. However, her symptoms persisted, and she developed marked abdominal distension and weight gain. Hence, she was transferred to our clinic.
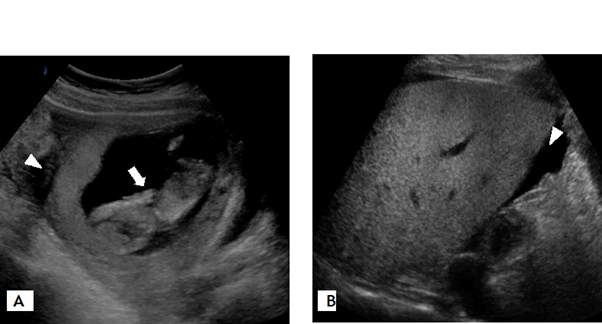
Her initial blood eosinophil count was high (14,600/mm3, 48%). In addition, her total serum immunoglobulin E level (3215 kU/L) and troponin I level (2.3964 ng/mL) were increased. There was no evidence of parasite infection in the stool and blood tests. Skin prick tests with a standard panel of 80 allergen extracts, including foods and inhalant allergens, showed positive results only for house dust mites. The fetal status was stable and large volume ascites was identified on ultrasonography (Figure 2). Echocardiography showed normal findings. Diagnostic paracentesis revealed significant eosinophilia (white blood cell count, 6,800/mm3; eosinophil count, 71%). Bacterial culture and the tuberculosis test using a sample of the ascitic fluid revealed negative results and no cytological signs of malignancy.
The patient was treated with intravenous methylprednisolone (1 mg/kg/day) for 5 days. Two days later, her gastrointestinal symptoms improved, and her body weight and blood eosinophil count decreased. Subsequently, ultrasonography showed a moderate reduction in the ascites. The patient did not consent for esophagogastroduodenoscopy and colonoscopy for EGE diagnosis. She was administered oral prednisone 50 mg; the dose was gradually tapered for 45 days. After 2 weeks of therapy, her symptoms completely resolved, and her blood eosinophil count and troponin I level normalized. She delivered a healthy baby with normal birth weight at 40 weeks of gestation. She presented with intermittent cough from 28 weeks of gestation, and was diagnosed with bronchial asthma after 3 months of delivery based on shortness of breath, bronchodilator responsiveness (post bronchodilator forced expiratory volume in one second increased by 32% and 460 ml), and increased value of sputum eosinophil (49%) and fractional exhaled nitric oxide (124 ppb). She started combination treatment of inhaled corticosteroids/long acting beta-2 adrenergic bronchodilator, and asthma has been under control.
Discussion
EGE is a rare, heterogeneous disorder of unknown etiology. There have been no large studies, but hundreds of cases and small case series have been reported. The epidemiology of EGE is unclear. It affects all age groups, from infants to adults, but it is the most common in patients in their 30’s and 40’s had has a slight male predominance [2,7,8].
The symptoms of EGE are nonspecific and varied; however, the most common symptoms are abdominal pain, nausea, and vomiting [9,10]. EGE is classified into three types depending on the clinical manifestations and depth of eosinophilic infiltration of the gastrointestinal tract wall mucosal, muscular, and serosal [1]. Mucosal EGE is the most common type of EGE. It manifests with abdominal pain, nausea, vomiting, diarrhea, bleeding, anemia, and protein losing enteropathy, which may lead to malabsorption, and weight loss. Muscularis involvement results in gut wall thickening and may lead to obstruction. Serosal EGE is the rarest type of EGE. In serosal EGE, eosinophil rich inflammatory infiltrate permeates all layers of the gastrointestinal wall and lead to eosinophilic ascites; this type of EGE responds well to steroid treatment [2,11]. Serosal EGE presenting with eosinophilic ascites is more common in females than in males [12,13]. Occasionally occurring during pregnancy or delivery [3,5,6,14,15]. A French study reported the natural history and long term outcomes of 43 adult patients with EGE during a median follow up of 13 years [9]. Most cases of serosal EGE presented as single flare ups and did not follow a continuous chronic course, whereas most cases of mucosal EGE followed a continuous course.
There are no strict or standardized diagnostic criteria for EGE. Definitive diagnosis is based on typical gastrointestinal symptoms along with histological evidence of eosinophilic infiltration of the gastrointestinal tract wall. In addition, other causes of hypereosinophilia, such as drug reactions, malignancy, parasites, infection, or systemic disease, should be excluded. The endoscopic appearance of EGE is nonspecific. However, diffuse or local mucosal hyperemia and thickened gastric folds are common manifestations [7]. Moreover, mucosal biopsies may show no eosinophils in serosal EGE because the serosal layer is predominantly involved [3]. On computed tomography, the hallmark of EGE is nodular and irregular thickening of the folds in the distal stomach and proximal small bowel [8,16]. Although mesenteric inflammation and ascites are not uncommon findings, they are usually nonspecific.
Laparoscopic serosal biopsies may be required for a definite diagnosis. However, our patient was in early pregnancy, and similar to computed tomography findings, magnetic resonance imaging findings were suggestive of EGE, and also responded well to steroid therapy, as observed in other previous cases [3,17,18]. There was no evidence of other causes of blood eosinophilia. Hence, EGE was diagnosed without a biopsy.
Eosinophils are normally present in the lamina propria of the mucosa, except esophagus, along the gastrointestinal tract. It is involved in the mucosal immune system, and the number of eosinophils increases in numerous inflammatory processes, including parasitic infections and allergic diseases [19]. Activated eosinophils produce and release inflammatory mediators that lead to tissue damage through their proinflammatory functions.
The Th2 immune response may be involved in EGE [20-23]. Th2 cytokines, such as interleukin (IL)-4, IL-5, and IL-13, and chemokines, such as eotaxin, play a central role in the recruitment of eosinophils from the circulating blood into the tissues related to the pathogenesis of EGE [24]. The expression of these Th2 cytokines and eotaxin tend to be upregulated in the tissues of patients with EGE compared to that in tissues of patients without EGE. Moreover, the blood eosinophil count correlated with tissue pathology [23]. EGE is defined as a systemic Th2 associated disorder with profound blood and gastrointestinal track eosinophilia.
There have been no reports on the prevalence of EGE during pregnancy, but only a few cases have reported pregnancies in patients with EGE. A switch from Th1 immunity to Th2 immunity has been reported during pregnancy; it contributes to the maintenance of pregnancy [25-27]. Both Th2 cell migration and Th2 cell differentiation induce Th2 immunity at the feto maternal interface. This may be associated with EGE in pregnant women [6]. described that EGE may be connected with late preterm delivery after reviewing a total of 12 cases of EGE in pregnant women; 5 cases were antepartum EGE, and 5 were postpartum EGE. The other 2 cases occurred in one patient [28]. A 27 year old woman developed hypereosinophilia with pericardial effusion, pleural effusion, and ascites at 10 weeks of gestation. However, intrauterine fetal death occurred, and there was no eosinophil infiltration in the tissues obtained by uterine curettage. The patients may be associated with hypereosinophlia with hyperpermeability symptoms rather than EGE. Moreover, they described that it is unclear the relationship between the fetal death and the eosinophilia.
Dietary treatment such as elimination or elemental diet has been used for the treatment of EGE. Our patient had no history of food allergy. Furthermore, skin prick tests for food allergens revealed negative results. Therefore, dietary treatment was not administered to our patient. Corticosteroids are mainly used for the treatment of EGE as they have shown good therapeutic efficacy. However, prenatal exposure to immunosuppressive medications is associated with potentially adverse effects on the fetus. No complications have been reported in newborns of mothers treated with corticosteroids for EGE, including in our case [6].
The serosal EGE is very rare type of EG. However, it can occur during pregnancy. Further studies are needed to clarify the relationship between EGE and pregnancy.
It has been reported that about 45%-63% of patients with EGE had an allergic disease such as asthma, rhinitis, eczema, and drug or food allergy [29]. It can be thought to be a phenomenon caused by the similarity of the pathogenesis of eosinophil mediated diseases. There have been some reports of the occurrence of EGE in asthmatics, however no reports of the newly development of asthma in patient with EGE [30,31].
Conclusion
To our knowledge, this is the first reported case of EGE with eosinophilia ascites that developed during pregnancy and newly developed asthma after delivery.
Ethics Approval and Consent to Participate
Written informed consent was obtained from the patient.
Competing Interest
No potential conflict of interest relevant to this article was reported.
Funding
Not applicable.
Availability of Data and Material
The datasets used during this study are available from the corresponding author on reasonable request.
Consent for Publication
Written informed consent was obtained from the patient.
Acknowledgments
This work was supported by the Dong-A University research fund.
References
- Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH (1970) Eosinophilic gastroenteritis. Medicine 49:299-319.
- Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR (1990) Eosinophilic gastroenteritis: A clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut 31:54-58.
- Hepburn IS, Sridhar S, Schade RR (2010) Eosinophilic ascites, an unusual presentation of eosinophilic gastroenteritis: A case report and review. World J Gastro Path phy 1:166-170.
- Zhang M, Li Y (2017) Eosinophilic gastroenteritis: A state of the art review. J Gastroenterol Hepatol 32:64-72.
- Park SJ, Kenny PR, Palekar NA (2012) Labor-associated eosinophilic gastroenteritis. Mil Med 177:99-100.
- Amadori R, Stampini V, Rapetti R, Pirisi M, Vigone A, et al (2020) Eosinophilic gastroenteritis in pregnancy: A review of the literature. Eur J Obstet Gynecol Reprod Biol 248:102-105.
- Chang JY, Choung RS, Lee RM, Locke III GR, Schleck CD, et al (2010) A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastro Hepatol 8:669-675.
- Chen MJ, Chu CH, Lin SC, Shih SC, Wang TE (2003) Eosinophilic gastroenteritis: Clinical experience with 15 patients. World J Gastroenterol 9:2813-2816.
- Pineton de Chambrun G, Gonzalez F, Canva JY, Gonzalez S, Houssin L, et al. (2011) Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 9:950-956.
- Zhang L, Duan L, Ding S, Lu J, Jin Z, et al. (2011) Eosinophilic gastroenteritis: Clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol 46:1074-1080.
- Baek MS, Mok YM, Han WC, Kim YS (2014) A patient with eosinophilic gastroenteritis presenting with acute pancreatitis and ascites. Gut Liver 8:224-227.
- Alhmoud T, Hanson JA, Parasher G (2016) Eosinophilic gastroenteritis: An underdiagnosed condition. Dig Dis Sci 61:2585-2592.
- Durieu I, Nove-Josserand R, Cathebras P, Durand DV, Rousset H, et al. (1992) Eosinophilic ascites. 2 new case reports. Rev Med Interne 13:446-448.
- Lang R, Jutrin I, Ravid M (1981) Re current post-partum gastroenteritis with eosinophilia. Hepato gastro 28:118-119.
- Milic S, Poropat G, Malic D, Stimac D (2012) A case of postpartum eosinophilic gastroenteritis and review of the literature. Dig Dis 30:232-235.
- Horton KM, Corl FM, Fishman EK (1999) CT of nonneoplastic diseases of the small bowel: spectrum of disease. J Comput Assist Tomog 23:417-428.
- To Y, Ogawa C, Otomo M, Arai Y, Sano Y, et al. (1999) A case of eosinophilic gastroenteritis complicated with ileus and ascites collection. Arerugi 48:50-55.
- Feng W, Zheng K, Shen H (2020) Eosinophilic ascites: An unusual manifestation of eosinophilic gastroenteritis. Int J Colorectal Dis 35:765-767.
- Lucendo AJ (2008) Immunopathological mechanisms of eosinophilic oesophagitis. Allergol Immunopathol 36:215-227.
- Khan S, Orenstein SR (2008) Eosinophilic gastroenteritis. Gastroenterol Clin North Am 37:333-348.
- Kelly KJ (2000) Eosinophilic gastroenteritis. J Pediatr Gastroenterol Nutr 30:28-35.
- Méndez-Sánchez N, Chávez-Tapia NC, Vazquez-Elizondo G, Uribe M (2007) Eosinophilic gastroenteritis: A review. Dig Dis Sci 52:2904-2911.
- Caldwell JM, Collins MH, Stucke EM, Putman PE, Franciosi JP, et al (2014) Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity and a unique gastric transcriptome. J Allergy Clin Immunol 134:1114-1124.
- Daneshjoo R (2002) Eosinophilic gastroenteritis. Curr Gastroenterol Rep 4:366-372.
- Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol Today 14:353-356.
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, et al. (1998) Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med 4:1020-1024.
- Saito S (2000) Cytokine network at the feto-maternal interface. J Reprod Immunol 47:87-103.
- Ogasa M, Nakamura Y, Sanai H, Ueda K (2006) A case of pregnancy associated hypereosinophilia with hyperpermeability symptoms. Gynecol Obstet Invest 62:14-16.
- Sunkara T, Rawla P, Yalagadda KS, Gaduputi V (2019) Eosinophilic gastroenteritis: Diagnosis and clinical perspectives. Clin Exp Gastroenterol 12:239-253.
- Bassiony MAA (2018) Bronchial asthma and eosinophilic gastroenteritis. G J Dig Dis 4:2.
- Kim YJ, Lee HB, Lee H, Hong EK, Rhim HC (1995) A case of non-IgE-mediated serosal eosinophilic gastroenteritis in a child with chronic asthma. J Korean Pediatr Soc 38:1694-1700.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2042
- [From(publication date): 0-2021 - Jan 30, 2025]
- Breakdown by view type
- HTML page views: 1537
- PDF downloads: 505