Environmentally Friendly Approach to Bimetallic Copper and Silver CucoreAgshell Nanoparticles Synthesis on Fibrous Materials
Received: 14-Mar-2022 / Manuscript No. ico-22-57017 / Editor assigned: 16-Mar-2022 / PreQC No. ico-22-57017 (PQ) / Reviewed: 20-May-2022 / QC No. ico-22-57017 / Revised: 23-May-2022 / Manuscript No. ico-22-57017 (R) / Published Date: 30-May-2022 DOI: 10.4172/2469-9764.1000190
Abstract
Fibrous materials of natural origin are easily subjected to microbiological destruction. Hence, their modification is necessary to increase their stability. Moreover, imparting antibacterial properties to textile materials is relevant nowadays as the diversity of resistant strains of microorganisms increases. The current research suggests an environmentally friendly method of modifying fibrous materials of different origins using bimetallic copper-silver nanoparticles without applying hazardous chemicals. The study examines the antibacterial and antifungal properties and provides photomicrographs of the obtained samples.
Keywords
Bimetallic nanoparticles; Copper; Silver; Modified polymers; Antimicrobial; Antifungal.
Introduction
In recent decades, the application of metal nanoparticles as antimicrobial agents has been of intense scientific interest. Due to their large specific surface area, metal nanoparticles have unique chemical, magnetic, electrical, optical, mechanical, antibacterial, antifungal, and antiviral properties making them applicable in various industrial sectors. Silver is known for its antimicrobial activity against more than 650 types of pathogens. Silver is beneficial for the human immune system in concentrations of up to 80 μg per day [1,2]. There is evidence that silver prevents the development of cancer and can also inhibit its growth [3]. In addition, research confirms the antiviral activity of silver, including against COVID-19 and SARS-COV-2 [4]. Copper also shows antimicrobial activity [5]. Moreover, copper is an enzyme cofactor involved in biochemical reactions in a mammalian organism. In the textile industry, copper compounds are used for antibacterial and antifungal treatment as substitutes for the more expensive noble metals.
Methods of metal nanoparticles synthesis can be classified into three main categories:
1) Physical Methods that include mechanical milling, plasma method, electrical arc discharge, electro-explosion of wire technique, laser ablation and other methods of heat or force treatment.
2) Chemical Methods that represent obtaining nanoparticles by chemical reduction, decomposition, or synthesis from metal salts.
3) Biological Methods based on applying biological objects such as plant extracts, bacteria, fungi [6].
Despite a large amount of information on the methods of producing noble metals nanoparticles, the information on the synthesis of bimetallic nanoparticles is quite limited [6-10]. This research area is still emerging, though promising in terms of practical application. Bimetallic nanoparticles, such as copper-silver, have an antibacterial effect and can be used to impart bactericidal properties to fibrous materials.Over the past few years, serious attention of researchers was attracted to the fibrous materials of different origins and their modification with silver nanoparticles to impart antibacterial and antifungal properties [11-14]. Predominantly, these methods involve the dispersion of silver nanoparticles obtained in advance and contain many various excipients and reducing agents that contaminate a nanoparticle shell. Previous research investigated into the methods of silver nanoparticles synthesis directly on fibrous materials with the minimum use of addition agents and without any chemical reducing agents, because the functional groups of the substrate itself play their role [15-19]. At the same time, bicomponent copper-silver nanoparticles allow obtaining antibacterial effect and economic benefit by reducing the cost of raw materials. Therefore, research into the methods of bicomponent nanoparticles synthesis is a relevant task. The present paper aims at exploring an environmentally safe and high-tech method of bicomponent coppersilver nanoparticles synthesis on fibrous materials for imparting antibacterial and antifungal properties, increasing resistance to microbiological destruction, and ensuring the properties retention during usage by means of stronger fixation of bimetallic nanoparticles in the porous structure and on the material surface.
Materials and Methods
Materials
Silver nitrate (AgNO3), copper sulphate pentahydrate (CuSO4⸱5H2O), cotton fabric, viscose fabric, linen fabric, hemp fiber, wool, silk, and fabric made of polyamide and acetate fibers were used in the current research.
Nanoparticles Synthesis on Fibrous Materials
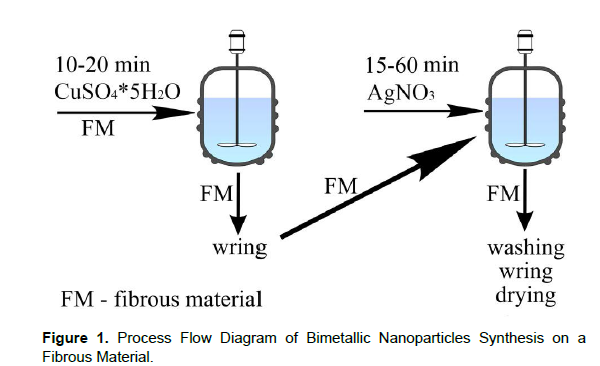
The chemical substances used in the current research were measured using the analytical scale with an accuracy of 10-4 g before processing, the materials were preliminarily decontaminated from mechanical and natural impurities in an aqueous solution containing a surfactant (soap or synthetic detergent) - 5.0 g/l, sodium carbonate - 0.4 g/l. The following precursors were used for bimetallic copper-silver nanoparticles synthesis: copper sulphate pentahydrate (CuSO4⸱5H2O,0.00005-0.001 M), silver nitrate (AgNO3, 0.0001–0.0006 M). The synthesis of bimetallic nanoparticles is shown in (Figure 1).
Research Methods
The size of the synthesized nanoparticles and their distribution on the surface were determined using a scanning electron microscope produced by the Japanese brand JEOL, model JSM-639 OLA. The obtained materials gained a particular coloristic effect characterized by the optical reflection spectra identified using the Gretag Macbeth laboratory spectrophotometer specified for high-precision color measurement. Changes in the structure of the fibrous material after the deposition of nanoparticles were analyzed using Shimadzu FTIR- 8400S (Japan) spectrometer. The color fastness of the modified samples of fibrous materials to washing was tested in accordance with GOST R ISO 105-C06:2011 ‘Textiles - Tests for color fastness - Part C06: Color fastness to domestic and commercial laundering’. Microbiological destruction stability tests were carried out in accordance with GOST 9.060-75 ‘Unified system of corrosion and ageing protection. Fabrics. Method of laboratory tests for microbiological destruction stability’. The method is based on the exposure of fabrics under certain conditions to a natural complex of soil microflora by adding a special mixture on the fabric's surface and then determining the microbiological destruction stability by varying the breaking strength. The test period was 10 days. The breaking strength was determined on the tensile testing machine PT-250M. The maximum load was 2.5 k N. The accuracy is ±1% of the measured load. The laboratory testing of polymer materials for mold fungi resistance were carried out in accordance with GOST 9.048-89 ‘Unified system of corrosion and ageing protection. Technical items. Methods of laboratory tests for mold resistance’. A sample of the studied fibrous material with an area of 1 сm2 was placed in the center of the Petri dish on the nutrient medium prepared in advance. Penicillium chrysogenum spore suspension was sprayed on the sample. The Petri dish was then placed in a desiccator filled with water. Tests were conducted at a temperature of (29±2)°C and relative humidity of more than 90% for 28 days. It is crucially important to be sure that the obtained material is not toxic for consumers because its production supposes the usage of heavy metals. Literature review showed that Saccharomyces cerevisiae yeast is a suitable biological material for toxicity determination of various pollutants, including heavy metals [20]. Shredded fibrous material weighing 0.2-0.3 g was placed in glass test tubes, then 5 сm3 of Ringer's solution (6.5 g sodium chloride, 0.42 g potassium chloride and 0.25 g calcium chloride in 1 dm3 of double distilled water) and 2 сm3 of S. cerevisiae yeast culture suspension was added. A test tube containing 1 сm3 of water is used as a control. The experimental and control tubes were kept in an incubator at room temperature for 72 hrs. Yeast growth was stopped by immersing the test tubes in boiling water. After cooling, the extinction value of the test tubes was measured using a spectrophotometer KFK-3 ‘ZOMZ’ specified for the analysis of liquid solutions. The antibacterial activity of the modified samples was examined using the test object method in accordance with ISO 20645:2004 ‘Textile fabrics - Determination of antibacterial activity - Agar diffusion plate test’ by placing the test objects into the broth with test cultures. Test objects with a surface area of 2 сm2 were placed into a broth culture containing gram-positive S. aureus and gram-negative E.coli bacteria counting 1·108 CFU/ml (CFU = colony-forming unit). Test tubes with bacteria but without the test objects were used as a control. The test tubes rack was placed on a shaker platform (250 rpm) for 24 hours. Then 1 ml of suspension was taken from each tube, diluted 10, 100, 1000 and 10 000 times and inoculated on the upper layer of Petri dishes with a meat peptone agar. The cultures were kept in the incubator for 24 hours at 37°C. The next day, the extent of bacterial growth was counted and multiplied by the dilution ratio. The data was further recorded and expressed in CFU/ml.
Results and Discussion
The current research employs the method of reduction of copper and silver cations from solutions of their salts in order to impart antibacterial properties to fibrous materials of different origins. The process involves ion diffusion into the porous structure of fibers, interaction with the functional groups of the substrate, which act as a reducing agent for metal nanoparticles resulting in stronger bond fixation. The size of bicomponent copper-silver nanoparticles in the fiber volume is limited to the pore size. Still, it is worth mentioning that larger agglomerates are also formed on the surface. Natural fibers of plant origin, such as cotton, flax, hemp, have hydroxy –ОН and carboxy –COOH groups in their structure and natural impurities, which act as reducing agents in the metal nanoparticles synthesis directly on the surface of the fibrous substrate. Natural fibers of animal origin, wool, and silk, have amino acids, including carboxy–COOH group and amino group –NH2, which can act as reducing agents in the process of metal nanoparticles synthesis. Viscose fiber is a fiber of artificial origin. It is polyatomic alcohol and contains hydroxy groups, which are also reducing agents for metal cations. Capron (nylon 6) refers to polyamide fibers, a large group of fibers, which main chains contain repeating –CO–NH– amide groups (like peptide groups in wool and silk) and –NH2 amino end groups capable of reducing metal cations to nanoparticles. These fibers have low hygroscopicity and small pore sizes, thus allowing the size of the synthesized nanoparticles to be controlled within a narrow range. According to the literature review results, the significant difference of oxidation potential (Е0) of Ag+/ Ag0 (+0,7994 V) and Cu2+/Cu0 (+0,3450 V) pairs indicate the lower activity of copper cations in reactions with reducing agents and ability of metallic copper and Cu+ cations to reduce silver cations [7]. Hence, the synthesized bimetallic nanoparticles contain a copper core and a silver shell. Polymeric materials treated using this method have an antibacterial effect that lasts for the total service life of the product. By removing excipients, reducing agents and stabilizers at the preparation stage of the treatment solutions, the obtained bimetallic nanoparticles are free of harmful impurities. At the same time, this treatment gives the material a coloristic effect varying from golden yellow to dark brown. It should be mentioned that shades of the colors may be controlled by changing the metal salts concentrations and the reaction conditions. The effect of adding to the reaction bath ammonium hydroxide NH4OH to pH = 8-10 and sodium hydroxide NaOH to pH =11 to intensify the process of the bimetallic nanoparticles synthesis was studied. Except for being an intensifier, ammonium hydroxide also has a stabilizing effect and prevents the development of aggregates. When NH4OH is added to the silver nitrate solution, Ag+ presumably forms the complex with NH3:
AgNO3 + 2(NH4OH) = [Ag(NH3)2]NO3 + 2H2O
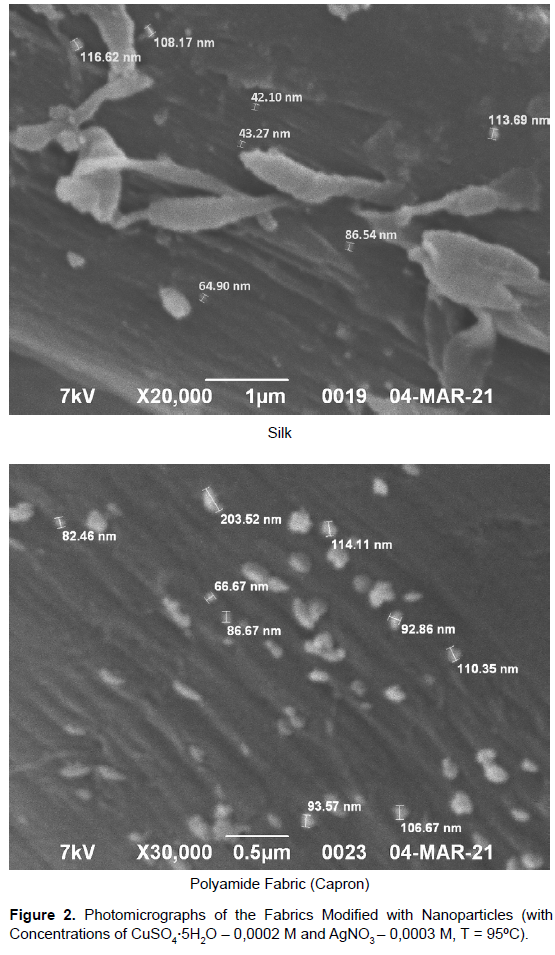
Then, [Ag(NH3)2]+ interacts with copper cations evenly distributed in the porous structure of the polymeric material and is reduced to bimetallic nanoparticles by means of the oxidation potential difference. The obtained polymeric materials get deeper shades that are more uniform. The best results were achieved with salts concentrations CuSO4⸱5H2O from 0.0003 to 0.0006 M, and AgNO3 from 0.0001 to 0.0006 M. These conditions allow even distribution of copper-silver nanoparticles in the porous structure and on the surface of the substrate. It was observed that at concentrations higher than was mentioned above, the obtained color of modified samples was not uniform, indicating the formation of bimetallic particles aggregates. The determined optimum treatment temperature is 80–100ºC with intermittent mixing and the water-to-material ratio of 50 ml/70g. These conditions are required for the complete diffusion of ions into the porous structure of the fibrous material and for the reduction process initiation.The microscopic investigation using JEOL JSM-639 OLA scanning electron microscope showed the formation of bicomponent copper-silver nanoparticles with sizes ranging from 30 to 100 nm (Table 1). Photomicrographs of the obtained materials are shown in (Figure 2). The reflection maxima recorded with Gretag Macbeth laboratory spectrophotometer are in the 410-430 nm wavelength range for all obtained samples. This indicates that the surface of the formed nanoparticles consists of silver. The obtained colors of textile materials were tested for fastness to washing in accordance with GOST R ISO 105-C06:2011. High color fastness values (5/5/5 for most polymeric materials) were achieved even under the harshest washing conditions (240 min, 95ºС), confirming the strong fixation of bimetallic nanoparticles in the porous structure and on the substrate surface (Table 2).
| Material | Color Fastness Indices According to GOST R ISO 105-C06:2011 | ||||
|---|---|---|---|---|---|
| Washing 1 | Washing 2 | Washing 3 | Washing 4 | Washing 5 | |
| Crude Cotton Fabric | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/4-5/5 | 5/4-5/5 |
| Crude Wool Fabric | 5/5/2005 | 5/5/2005 | 5/5/2005 | ||
| Silk Fabric | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/5/2005 | |
| Crude Linen Fabric | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/5/2005 |
| Viscose Staple | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/5/2005 |
| Polyamide Fabric | 5/5/2005 | 5/5/2005 | 5/5/2005 | 5/4/2005 | 5/4/2005 |
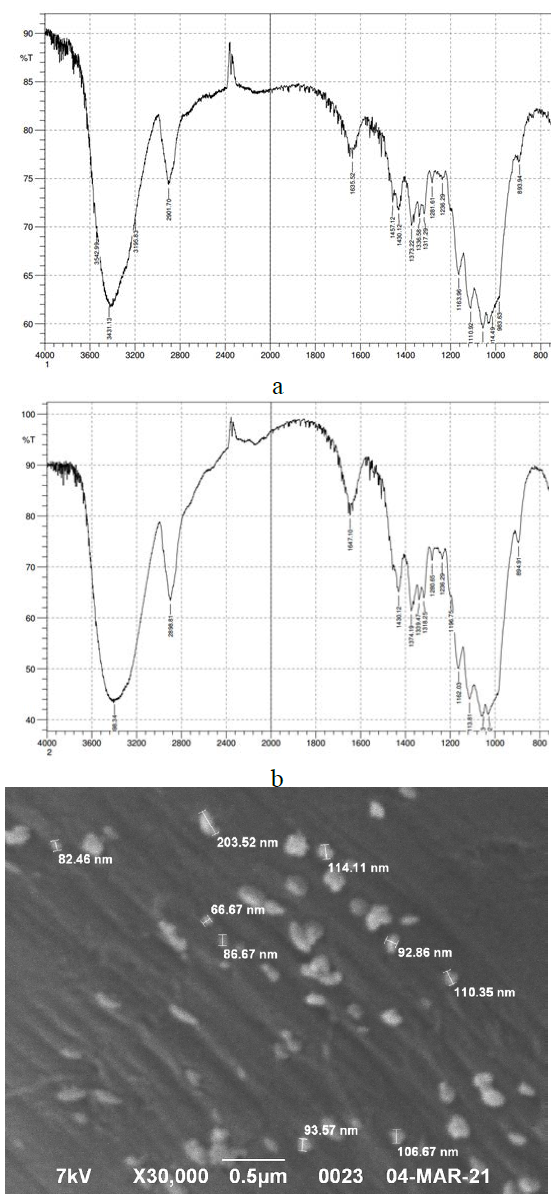
Figure 2 shows the IR spectra explored using Shimadzu FTIR- 8400S spectrometer for cotton fabric samples: a – without treatment, b – modified with bimetallic copper-silver nanoparticles (with concentrations of CuSO4⸱5H2O – 0,0003 M and AgNO3 – 0,0003 M).The spectra of cotton cellulose feature the following frequencies: 3543–3196 сm-1 – stretching vibrations (SV) of –OH groups involved in intermolecular and intramolecular hydrogen bonds; 2899-2902 сm-1 – SV of bonds in groups =CH– H– and –СН2–; 1650–1635 сm-1 – bending vibrations (BV) of bonds H-O-H are due to the presence of bound water; 1457– 1236 сm-1 - BV of –OH group; 1430 сm-1, 1370 сm-1 – BV of groups СН2; 1340 cm-1 – BV of O-H in CH2OH; 1160 сm- 1, 1110 сm-1, 1060 сm-1 – SV of bonds C-O. The intense blurred band at 3600–3200 сm-1 and the less intensive band around 3000–2800 сm-1 are due to the stretching vibrations of hydroxy groups presented in the hydrogen bond and CН, СН2 groups [21,22] (Figure 2, a). The tests carried out according to GOST 9.060-75 confirm the microbiological destruction resistance of the obtained materials. The materials are resistant if the destruction stability coefficient P is ≥ (80±5)%. The test results are presented in (Table 3).The fabric samples modified with monometallic copper nanoparticles (0.0003 M) and silver nanoparticles (0.0002 M) were tested. It was found that for the viscose staple fabric with monometallic copper and silver nanoparticles, the microbiological destruction stability coefficient was 12.65% and 8.37%, respectively. However, in the case of bimetallic copper-silver nanoparticles, a synergistic effect is observed, and the obtained antimicrobial properties are enhanced, P = 94.04%. The resistance of the obtained materials to mold fungi Penicillium chrysogenum was determined according to GOST 9.048-89. The samples having bicomponent nanoparticles in the structure of the material showed more significant antifungal activity than the samples with monometallic nanoparticles. This means that a synergistic effect is observed. Moreover, in vitro antibacterial effect according to ISO 20645:2004 was determined (Table 4). The number of gram-positive and gram-negative bacteria decreased by several orders of magnitude in 24 hours compared to the control sample. The antibacterial effect was evident during the test: the number of gramnegative bacteria (CFU/ml) E. coli grown on the modified cotton fabric (8·107) is substantially lower compared to the control sample (2·109); the number of gram-positive S. aureus bacteria is also lower (5·106) compared to the control sample (4·108). The experiments and the obtained materials testing revealed the significant antibacterial, antifungal effects, microbiological destruction stability, and fastness of the resulting colors to washing. The toxicity of the materials with bimetallic nanoparticles was evaluated using the biological method of Saccharomyces cerevisiae yeast growth inhibition [20]. It was found that the obtained materials are not toxic, which means they do not cause harm to people when used.
| Material | Number of Microorganisms Grown (КОЕ/мл) | |
|---|---|---|
| Control | Experiment | |
| E. coli | ||
| Crude Cotton Fabric | 2·109 | 8·107 |
| Viscose Staple | 2·109 | 6·107 |
| Silk Fabric | 2·109 | 7·108 |
| S. aureus | ||
| Crude Cotton Fabric | 4·108 | 5·106 |
| Viscose Staple | 4·108 | 7·107 |
| Silk Fabric | 4·108 | 2·106 |
| Material | Crude Cotton Fabric | Crude Wool Fabric | Silk Fabric | Crude Linen Fabric | Viscose Staple | Polyamide Fabric |
|---|---|---|---|---|---|---|
| Size of the Nanoparticles, nm | 38,13– 92,81 | 33,71–83,72 | 42,1–86,54 | 59,66–81,22 | 50,28–75,42 | 32,73–93,57 |
Conclusion
The method of bimetallic copper-silver nanoparticles synthesis on fibrous materials presented in the current research is environmentally friendly. Based on the titration method results, it was found that the suggested technology provides a 100% salt utilization rate for copper sulphate pentahydrate and 99% for silver nitrate.The advantage of this method is the increase in technological efficiency by means of removing excipients at the stage of treatment solutions preparation, along with the rise in the antibacterial properties retention during usage by means of stronger fixation of bimetallic nanoparticles in the porous structure and on the surface of the material. The antibacterial activity of the obtained bicomponent copper-silver nanoparticles against gram-positive and gram-negative bacteria and the antifungal activity was confirmed. It is worth mentioning that due to a particular coloristic effect that material gains after treatment, it’s possible to save a significant number of dyes used in fibrous materials processing. The proposed method allows obtaining antimicrobial fabrics to produce underwear, hosiery, outerwear; antimicrobial materials for hygiene and medical purposes: personal protective equipment with high antibacterial, antifungal, and antiviral effects (e.g. masks) hospital bed linen, medical clothing; grain sacks and even Capron filters. The treatment solution tested for fabrics can also be used for the antibacterial treatment of wood. Due to the gained coloristic effect featuring beige and brown colors with a golden hue, the materials can be used in industrial, fashion and furniture design.
Acknowledgements
This work was supported by Saint Petersburg State University of Industrial Technologies and Design, Russian Federation.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The method of bimetallic copper-silver nanoparticles synthesis on fibrous materials presented in the current research is environmentally friendly. The advantage of this method is the increase in technological efficiency by means of removing excipients at the stage of treatment solutions preparation, along with the rise in the antibacterial properties retention during usage by means of stronger fixation of bimetallic nanoparticles into the structure and on the surface of the material.
References
- Rodriguez NS, Bright K R, Uhlmann DR, Gerba CP (2007) Inactivation of Pseudomonas aeruginosa and Aeromonas hydrophila by silver in tap water. Environmental Science and health 42: 1579-1584.
- O. V. Mosin (2008) Physiological effects of silver nanoparticles on the human body. NanoWeek 2008: 34-37.
- Khan MS, Alomari A., Tabrez S, Hassan I, Wahab R ,et.al (2021) Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study Pharmaceutics 13: 707-712.
- O Zachar (2020) Formulations for COVID-19 Treatment via Silver Nanoparticles Inhalation Delivery at Home and Hospital. ScienceOpen Preprints 2020: 1-14.
- Mahmoodi S, Elmi A, Hallaj-Nezhadi S (2018) Copper Nanoparticles as Antibacterial Agents. J Mol Pharm Org Process Res 6: 140-147.
- Anju A, Khushbu G, Tejpal SC, Dipti V (2018) Biogenic Synthesis of Copper and Silver Nanoparticles Using Green Alga Botryococcus braunii and Its Antimicrobial Activity. Bioinorg Chem and Appl Hindawi 2018: 1-9.
- Erohina EV (2017) The peculiarities of copper/silver bimetallic nanoparticles synthesis for antimicrobial protection of cellulose materials. Kosygin Russian State University337-342.
- Wang Y, Wang D, Dares CJ, Marquard SL, Sheridan MV, et al. (2018) CO2 reduction to acetate in mixtures of ultrasmall (Cu)n(Ag)m bimetallic nanoparticles. PNAS 115: 278-283.
- Zain NM, Stapley AGF, Shama G (2014) Green synthesis of silver and copper nanoparticles using ascorbic acid and chitosan for antimicrobial applications. Carbohydrate Polym 112: 195-202.
- Burinskaya AA, Kudriavtseva EV (2021) Eco-friendly way of synthesis of bimetallic copper and silver nanoparticles. The scientific heritage 58: 39-45.
- Kim J, Kwon S, Ostler E (2009) Antimicrobial effect of silver-impregnated cellulose: potential for antimicrobial therapy. J of Biolog Eng 1-9.
- Botelho CM, Fernandes MM, Souza JM, Dias N, Sousa AM, et al.(2021) New Textile for Personal Protective Equipment-Plasma Chitosan/Silver Nanoparticles Nylon Fabric. Fibers 9: 1-13.
- Xu Y, Li S, Yue XW (2018) Review of silver nanoparticles (AgNPs)-cellulose antibacterial composites. BioRes 13: 2150-2170.
- Vilamová Z, Konvičková Z, Mikeš P, Holišová V, Mančík P, et al.(2019) Ag-AgCl Nanoparticles Fixation on Electrospun PVA Fibres: Technological Concept and Progress. Scientific Reports 9: 1-10.
- Burinskaya AA, Izmerova ЕP, Basok МО, Сhekreneva GМ (2013) Production of silver nanoparticles on cellulosic material. St.Petersburg Design Materials Technology ,Russia 45: 21-25.
- Burinskaya АА, Polyansky AV (2014) Production of nano-sized silver particles on polymer materials for medical purposes. St.Petersburg Design Materials Technology5: 108-112.
- Burinskaya AA, Gazizullina АР, Aitova АН, Erohina OА, Akim EL (2019) Getting silver nanoparticles on heated fiber. Bulletin Spgutd 2:54-59
- Burinskaya АА, Gazizullina АР, Kudriavtseva EV (2020) Obtaining silver nanoparticles on the polymer materials without reducing agents. The News of higher educational institutions. Technology of Light Industry 47: 83-87.
- Vyatchina OF, Zhdanova GO, Stom DI (2017) Comparative evaluation of sensitivity of different test functions of saccharomyces cerevisiae to salts of heavy metals. RUDN J of Ecology and Life Safety 25: 206-216.
- Gismatulina YA, Budaeva VV (2014) Comparison of celluloses isolated from miscanthus with cotton cellulose by FTIR spectroscopy. Polzunovsky Bulletin 3: 177-181.
- Tarasevich BN (2012) IR spectra of the main classes of organic compounds: Reference materials.Lomonosov Moscow State University 1-55.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Viktorovna KE, Alexandrovna BA (2022) Environmentally Friendly Approach to Bimetallic Copper and Silver Cucore-Agshell Nanoparticles Synthesis on Fibrous Materials. Ind Chem, 8: 190 DOI: 10.4172/2469-9764.1000190
Copyright: © 2022 Viktorovna KE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2165
- [From(publication date): 0-2022 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 1708
- PDF downloads: 457