Enhancement of Cisplatin-Induced Apoptosis of Human Oral Squamous Cell Carcinoma by FBXW7
Received: 03-Jun-2022 / Manuscript No. AOT-22-55652 / Editor assigned: 07-Jun-2022 / PreQC No. AOT-22-55652 / Reviewed: 28-Jun-2022 / QC No. AOT-22-55652 / Revised: 05-Jul-2022 / Manuscript No. AOT-22-55652 / Published Date: 15-Jul-2022 DOI: 10.4172/aot-1000185
Abstract
Background: About one-third of Oral Squamous Cell Carcinoma (OSCC) patients have a risk of occurrence and chemo resistance, making the survival abysmal. We aim to evaluate the role of F-Box/WD repeat-containing protein 7 (FBXW7) to further develop efficient treatment of chemo resistant OSCC.
Methods: FBXW7 overexpression was induced by a lent viral vector, Lv-FBXW7 or lv-NC (non-coding control) and overexpression efficiency was assessed using quantitative real-time PCR (qRT-PCR) and western blot of FBXW7. Cell viability was measured using MTT assay. The effects of FBXW7 overexpression on cell migration and invasion was evaluated by the colony formation assay and Matrigel assay. Apoptosis of cells with lv-FBXW7 transfection was measured by qRT-PCR and western blot analyses of BAX, BAK, MCL1 and BCL2 expression. Growth rate and Cisplatin sensitivity of CAL27 xenografts with or without FBXW7 overexpression was monitored. Ki-67 and PCMA levels, which are biomarkers of intra-tumoral apoptosis, BAX, MCL1, Beclin1, LC3I and II, which are autophagy biomarkers, were assessed.
Results: Transfection of lv-FBXW7 in SCC9 and CAL27 cells resulted in increased sensitivity to Cisplatin treatment, as evidenced by slower cell proliferation, lower colony formation and invasion, higher apoptosis and autophagy, compared to those transfected with lv-NC. Mice with CAL27 xenografts overexpressing FBXW7 also demonstrated slower tumor growth and up regulation in Ki067 and PCNA. Tumors also showed higher apoptosis and autophagy activities.
Conclusion: FBXW7 overexpression was herein shown to effectively sensitize OSCC cells to Cisplatin treatment in vitro and in vivo, potentiating FBXW7 transduction as a new treatment strategy for OSCC.
Keywords: Oral squamous cell carcinoma; FBXW7; Chemo resistance; Cisplatin
Introduction
Oral Squamous Cell Carcinoma (OSCC) constitutes 90% cases of oral cancer, and is among the most prevalent cancers worldwide [1]. It is more common in males than females, but the number of female patients has gradually increased in recent years [2]. Surgery remains the main intervention for OSCC, but chemotherapy and radiotherapy are mostly used for those with advanced malignancy [3]. Due to the inability to detect OSCC early, one-third of OSCC patients are in advanced stage and have the risk of developing chemo resistant recurrent OSCC, resulting in a poor five-year survival rate of <60% [4]. Strategies to delay or block metastasis, and sensitize OSCC to chemotherapies, such as the front-line drug Cisplatin, are needed to effectively prolong the survival of patients.
F-Box/WD repeat-containing protein 7 (FBXW7) is 40 amino-acid protein that plays a critical role in regulating cytosolic misfiled proteins and damaged organelles mediates the ubiquitin-dependent proteolysis of several key regulatory proteins involved in cell division and cell fate determination, including c-Myc, cyclin E1, Notch, c-Jun [5-7]. FBXW7 was first identified in budding yeast and the human FBXW7 gene, located at chromosome 4q31q.3, is found to be deleted in 30% of cancers [8,9]. Such frequent deletion of FBXW7 in cancer, which leads to an increase in genetic instability [10], a hallmark of human cancers, has intrigued many researchers because of its potential as a tumor suppressor. It’s reported that FBXW7 expression negatively correlates with the clinical grade of patients, making it a potential predictive marker [11]. By comparing the expression of autophagy biomarkers, including MCL1, BECN1 and ATG7, between OSCC tissues and adjacent normal tissues, it is found that the expression of MCL1 was significantly higher, while the expression of BECN1 and ATG7 mRNA was significantly lower, suggesting a decreased autophagy activity [12]. MCL1 mRNA expression also showed a significant negative correlation with FBXW7 mRNA levels, while BECN1 and ATG7 mRNA expression was significantly positively correlated with FBXW7 levels. In another work, overexpressing FBXW7 in the OSCC cell lines and tumors was shown to inhibit cancer cell proliferation and promote autophagy [13]. Besides, the ability of FBXW7 overexpression in enhancing temozolomide sensitivity in glioma has been demonstrated [14].
Based upon these findings on the anti-tumor role of FBXW7, we herein aimed to investigate the effects of FBXW7 in enhancing the antitumor efficacy of Cisplatin on OSCC cells. We focused on evaluating if FBXW7 overexpression using a lent viral vector could inhibit cancer proliferation, migration, and invasion and enhance OSCC’s sensitivity to Cisplatin by increasing apoptosis and autophagy. The results of the study could potentiate the use of FBXW7 overexpression to improve OSCC patients’ survival.
Materials and Methods
Cell culture and cisplatin treatment
Human OSCC cell line CAL27, SCC9 was acquired from American Type Culture Collection (ATCC, Manassas, VA), and was cultured in RPMI-1640 medium with 10% fatal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin. Cells were cultured in a 37°C humidified incubator with 5% CO2. For Cisplatin treatment, CAL27 cells transfected with Lv-FBXW7 plasmids or control plasmids were treated with vehicle (PBS) or 4 μg/ml Cisplatin based on previously protocols [15].
Ectopic overexpression of FBXW7
Human FBXW7 complementary DNA was reversely-transcribed from the longest transcript NM_013233 containing all three isoform-encoding sequences (GAGGATCCCCGGGTACCGGTCGCCACCATGAATC). The cDNA was inserted into the lent viral vector GV358 (purchased from Genechem, Shanghai, China) to create the complete functional overexpression plasmid named as Lv-FBXW7. Another non-coding control lent viral vector was also constructed as Lv-NC. Conditioned medium containing lent viruses was harvested 48 hours from transfected 293T cells and prepared for further transfection.
Animal studies
BALB/c nude mice of 4-6 weeks were subcutaneously injected with 5 × 106 CAL27 cells that were transfected with Lv-FBXW7 expression plasmids in 100 μL PBS. The cells were divided into three groups, namely, control group, Cisplatin-treated group, and group transfected with FBXW7 and treated with Cisplatin. Cisplatin treatment was started from the day after cell injection and the mice were intra-peritoneal injected with vehicle or 5 mg/kg body weight Cisplatin. The treatment was performed every 3 days from day 1 to day 28. Animal studies were approved by the institutional animal care and use committee of Changzhou Central Hospital.
RT-PCR
DNA was extracted using the TIZOL agent and reversely transcribed. The SYBR Green mix was used in real-time PCR. The following primers were used:FBXW7: forward: 5′-ACTGGGCTTGTACCATGTTCA-3′ and reverse: 5′-TGAGGTCCCCAAAAGTTGTTG-3′; GAPDH: forward: 5′-TGTTGCCATCAATGACCCCTT-3′ and reverse: 5′-CTCCACGACGTACTCAGCG-3′. MCL1: Forward: TGCTTCGGAAACTGGACATCA, Reverse: TAGCCACAAAGGCACCAAAAG; PCNA: Forward: GGCTCTAGCCTGACAAATGC, Reverse: GCCTCCAACACCTTCTTGAG; Ki67: Forward: AAGCCCTCCAGCTCCTAGTC, Reverse: TCCGAAGCACCACTTCTTCT; BAK: Forward: GTTTTCCGCAGCTACGTTTTT, Reverse: GCAGAGGTAAGGTGACCATCTC; BAX: Forward: CCCGAGAGGTCTTTTTCCGAG, Reverse: CCAGCCCATGATGGTTCTGAT; BCL2: Forward: GGTGGGGTCATGTGTGTGG, Reverse: CGGTTCAGGTACTCAGTCATCC.
Statistics
The data were shown as median (interquartile range). Data were analysed with ANOVA analysis (one- or two-way) with a post hoc test. Statistical significance was determined when p-value was less than 0.05.
Results
FBXW7 enhances cisplatin-induced inhibition of cell proliferation in OSCC
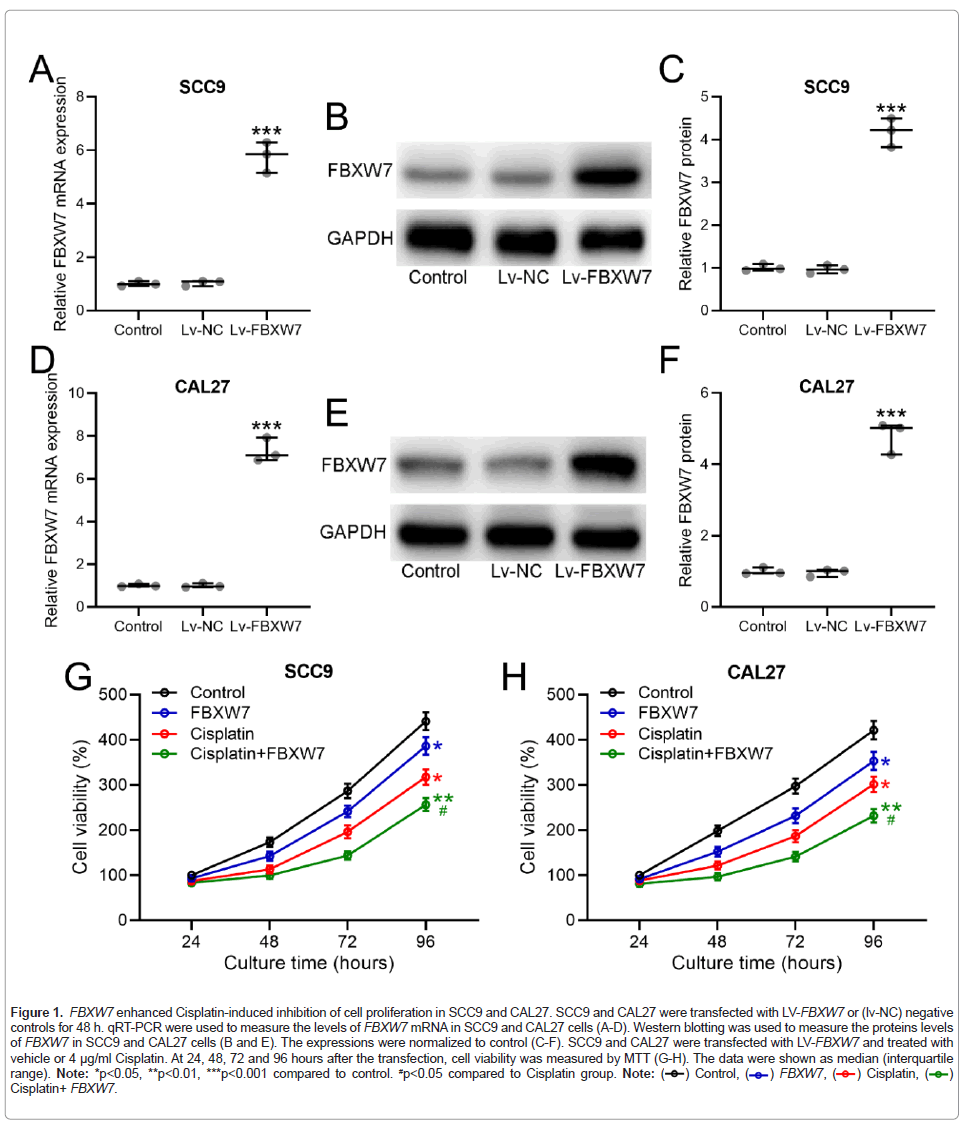
We first validated the overexpression of FBXW7 in OSCC cells including SCC9 in Figures 1A-1C and CAL27 in Figures 1D-1F. The enhancing effects of FBXW7 overexpression on Cisplatin treatment was investigated by MTT assay, which found that FBXW7 was effective in retarding proliferation of OSCC cell lines (Figures 1G and 1H).
Figure 1: FBXW7 enhanced Cisplatin-induced inhibition of cell proliferation in SCC9 and CAL27. SCC9 and CAL27 were transfected with LV-FBXW7 or (lv-NC) negative
controls for 48 h. qRT-PCR were used to measure the levels of FBXW7 mRNA in SCC9 and CAL27 cells (A-D). Western blotting was used to measure the proteins levels
of FBXW7 in SCC9 and CAL27 cells (B and E). The expressions were normalized to control (C-F). SCC9 and CAL27 were transfected with LV-FBXW7 and treated with
vehicle or 4 μg/ml Cisplatin. At 24, 48, 72 and 96 hours after the transfection, cell viability was measured by MTT (G-H). The data were shown as median (interquartile
range). Note: *p<0.05, **p<0.01, ***p<0.001 compared to control. #p<0.05 compared to Cisplatin group. Note: Cisplatin+ FBXW7.
Cisplatin+ FBXW7.
OSCC colony formation and invasion are further reduced in cisplatin-treated cells after FBXW7 expression
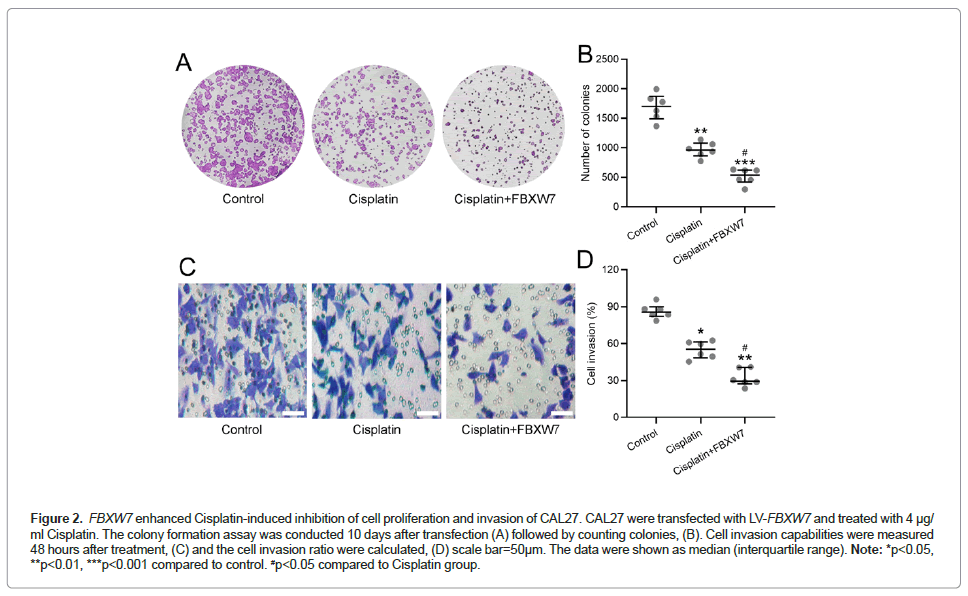
Cell migration and invasion were assessed by the clonogenic assay and Matrigel cell invasion assay. Cells treated with Cisplatin together with FBXW7 overexpression showed lower colony forming abilities (Figures 2A and 2B), and invasion (Figures 2C and 2D), indicating that FBXW7 overexpression enhanced the effects of Cisplatin in inhibiting cell migration and invasion.
Figure 2: FBXW7 enhanced Cisplatin-induced inhibition of cell proliferation and invasion of CAL27. CAL27 were transfected with LV-FBXW7 and treated with 4 μg/ ml Cisplatin. The colony formation assay was conducted 10 days after transfection (A) followed by counting colonies, (B). Cell invasion capabilities were measured 48 hours after treatment, (C) and the cell invasion ratio were calculated, (D) scale bar=50μm. The data were shown as median (interquartile range). Note: *p<0.05, **p<0.01, ***p<0.001 compared to control. #p<0.05 compared to Cisplatin group.
FBXW7 overexpression enhanced cisplatin-induced apoptosis in OSCC
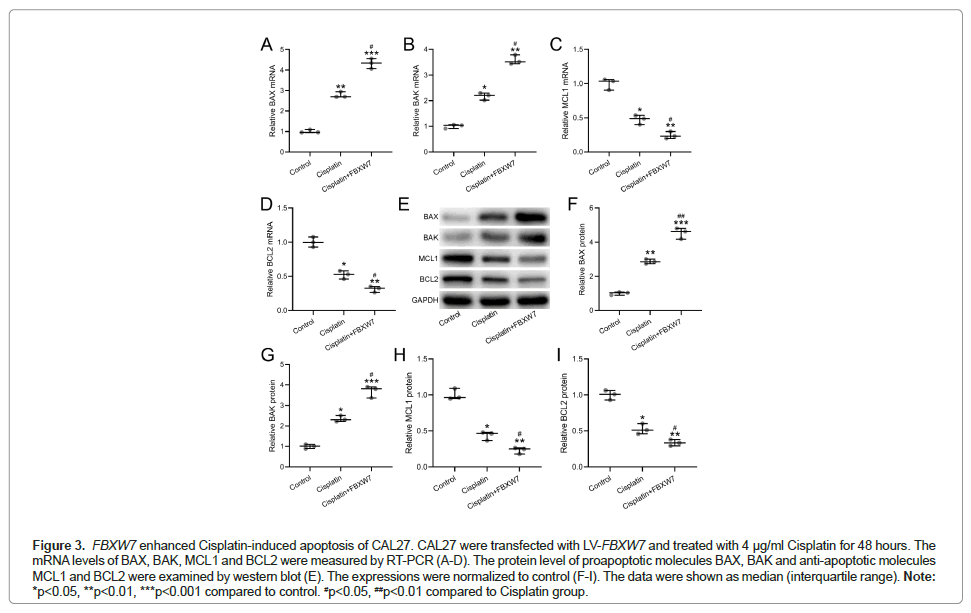
The apoptosis of CAL27 cells was further evaluated in cells with overexpression of FBXW7 and Cisplatin treatment by analyzing mRNA (Figures 3A-3D) and protein (Figures 3E-3I) levels of apoptosis biomarkers including BAX, BAK, MCL1 and BCL2. As shown in Figure 3A, it is evident that FBXW7 overexpression enhanced the Cisplatininduced apoptosis of CAL27 cells, evidenced by up regulation of BAX and BAK, and down regulation of MCL1 and BCL2.
Figure 3: FBXW7 enhanced Cisplatin-induced apoptosis of CAL27. CAL27 were transfected with LV-FBXW7 and treated with 4 μg/ml Cisplatin for 48 hours. The mRNA levels of BAX, BAK, MCL1 and BCL2 were measured by RT-PCR (A-D). The protein level of proapoptotic molecules BAX, BAK and anti-apoptotic molecules MCL1 and BCL2 were examined by western blot (E). The expressions were normalized to control (F-I). The data were shown as median (interquartile range). Note: *p<0.05, **p<0.01, ***p<0.001 compared to control. #p<0.05, ##p<0.01 compared to Cisplatin group.
FBXW7 overexpression sensitizes OSCC tumors to cisplatin treatment
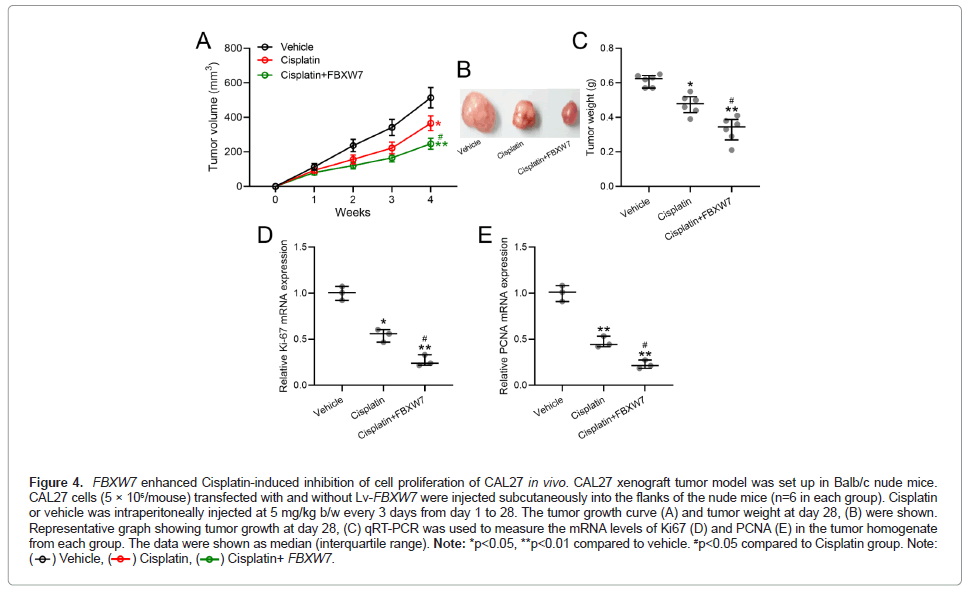
The ability of FBXW7 to enhance antitumor effect of Cisplatin on OSCC was next evaluated in vivo. Tumor volume was monitored for 4 weeks, after which tumor weight was measured after sacrificing the mice. While Cisplatin reduced tumor growth rate in p<0.05, Figure 4A and tumor weight of p<0.05, (Figures 4B and 4C). Compared to vehicle-treated mice, greater reduction was seen in mice with FBXW7- overexpressing tumors (p<0.01, compared to vehicle-treated mice). Our data suggested that FBXW7 overexpression indeed led to lower tumor progression in Cisplatin-treated mice (p<0.05 compared to those without FBXW7 overexpression). The reduced Ki67 Figure 4D and PCNA Figure 4E levels, also confirmed the enhancing effects of FBXW7 overexpression in OSCC (p<0.05 compared to those without FBXW7 overexpression).
Figure 4: FBXW7 enhanced Cisplatin-induced inhibition of cell proliferation of CAL27 in vivo. CAL27 xenograft tumor model was set up in Balb/c nude mice.
CAL27 cells (5 × 106/mouse) transfected with and without Lv-FBXW7 were injected subcutaneously into the flanks of the nude mice (n=6 in each group). Cisplatin
or vehicle was intraperitoneally injected at 5 mg/kg b/w every 3 days from day 1 to 28. The tumor growth curve (A) and tumor weight at day 28, (B) were shown.
Representative graph showing tumor growth at day 28, (C) qRT-PCR was used to measure the mRNA levels of Ki67 (D) and PCNA (E) in the tumor homogenate
from each group. The data were shown as median (interquartile range). Note: *p<0.05, **p<0.01 compared to vehicle. #p<0.05 compared to Cisplatin group. Note:
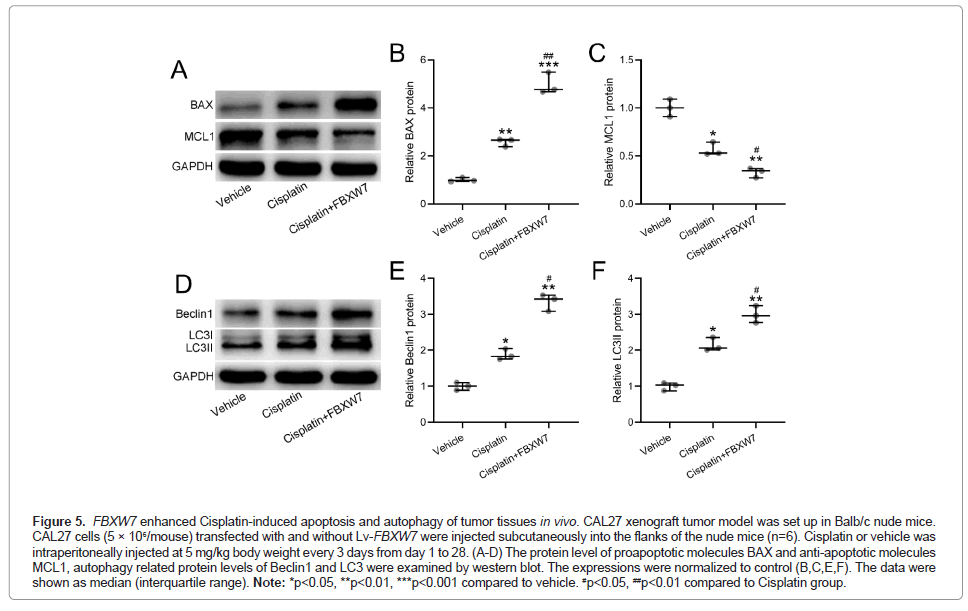
FBXW7 enhanced cisplatin-induced apoptosis and autophagy of tumor tissues in vivo
We next evaluated the apoptosis and autophagy levels in the harvested tumor by measuring expression of BAX, MCL1 apoptosis markers, in Figures 5A-5C, Beclin1 and LC3I and II (Figure 5D-5F). Our data suggested that FBXW7 amplified the Cisplatin-induced apoptosis and autophagy in OSCC tumor tissues.
Figure 5: FBXW7 enhanced Cisplatin-induced apoptosis and autophagy of tumor tissues in vivo. CAL27 xenograft tumor model was set up in Balb/c nude mice. CAL27 cells (5 × 106/mouse) transfected with and without Lv-FBXW7 were injected subcutaneously into the flanks of the nude mice (n=6). Cisplatin or vehicle was intraperitoneally injected at 5 mg/kg body weight every 3 days from day 1 to 28. (A-D) The protein level of proapoptotic molecules BAX and anti-apoptotic molecules MCL1, autophagy related protein levels of Beclin1 and LC3 were examined by western blot. The expressions were normalized to control (B,C,E,F). The data were shown as median (interquartile range). Note: *p<0.05, **p<0.01, ***p<0.001 compared to vehicle. #p<0.05, ##p<0.01 compared to Cisplatin group.
Discussion
To overcome chemo resistance of OSCC, one of the important factors contributing to treatment failure, we explored FBXW7 overexpression as a new strategy for sensitizing OSCC to Cisplatin treatment [16,17]. Our in vitro study showed that FBXW7 overexpression was capable of suppressing cell proliferation, migration, and invasion, in cells with Cisplatin treatment. Such tumor-suppressing and Cisplatin-sensitizing function was further supported by in vivo study, which showed retarded tumor growth and enhanced tumor apoptosis and autophagy. These data suggest the significant clinical implication of FBXW7 in OSCC.
Apoptosis, autophagy, and necrosis are three classic cell-death pathways, which are the mechanisms of cisplatin’s anticancer efficacy. Our results showed that FBXW7 promoted cell death by enhancing Cisplatin-induced cell death. In cancer, autophagy plays opposing roles as autophagy, through degrading organelles and cytoplasmic constituents, on the one hand inhibits tumorigenesis, and on the other hand induces metastasis and chemotherapy resistance. Anticancer drugs, including Cisplatin, are found to induce autophagy, a mechanism cancer cells exploits to acquire chemo resistance. Hence, autophagy has been pursued as a therapeutic target to develop novel anticancer drugs [18-20]. Several studies have investigated FBXW7 as a regulator of autophagy in human diseases including in cancer [15,21-24]. In lung cancer, targeting FBXW7 by miR-223 was shown inhibit Cisplatin-induced autophagy, which suppressed [15]. In studying hepatocarcinoma, FBXW7 overexpression effectively inhibited sorafenib resistance through inhibiting autophagy [24]. Our findings corroborated FBXW7 as not only a tumor suppressor but also an autophagy enhancer in OSCC, which contradicts the aforementioned studies, which could be explained by the complex role of autophagy.
Our study is not without limitation. Despite that FBXW7 overexpression by directly transfecting lent viral vector is proven successful for in vitro study; such approach is not translatable as the delivery of FBXW7 overexpressing vector to tumor cells is crucial for efficient gene transduction. However, with advances in gene delivery technologies, it is feasible to develop a gene vector, either viral or non-viral for targeted delivery of FBXW7 plasmid to cancer. Further, the exact molecular mechanism of how FBXW7 regulates autophagy remains to be elucidated. Exploring the effects of FBXW7 in reducing Cisplatin resistance in animal models of advanced OSCC is also warranted.
Conclusion
In conclusion, by overexpressing FBXW7 in OSCC, we showed that FBXW7 is a tumor suppressor and can sensitize OSCC to Cisplatin treatment. FBXW7 overexpression enhanced the effects of Cisplatin in inhibiting OSCC cell proliferation, migration and invasion in vitro, and also resulted in slower tumor growth and higher apoptosis and autophagy. Our data support further development of FBXW7 overexpression as a potential therapeutic strategy for OSCC.
The control and role of the tumour suppressor FBXW7 in healthy and malignant cells have undergone significant advancement, although many unanswered problems still exist.
Acknowledgement
None
Funding
None
Conflicts of Interest
None declared.
References
- Liu T, Liu J, Chen Q, Jin S, Mi S, et al. (2019) Expression of USP22 and the chromosomal passenger complex is an indicator of malignant progression in oral squamous cell carcinoma. Oncol Lett 17: 2040-2046.
[Crossref] [Google Scholar] [PubMed]
- Capote-Moreno A, Brabyn P, Muñoz-Guerra MF, Sastre-Pérez J, Escorial-Hernandez V, et al. (2020) Oral squamous cell carcinoma: Epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg 49: 1525-1534.
[Crossref] [Google Scholar] [PubMed]
- Liu WC, Liu HE, Kao YW, Qin L, Lin KC, et al. (2020) Definitive radiotherapy or surgery for early oral squamous cell carcinoma in old and very old patients: A Propensity-score-matched, nationwide, population-based cohort study. Radiother Oncol 151: 214-221.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Chen J, Cai X, Yao Z, Huang J (2019) Progress in targeted therapeutic drugs for oral squamous cell carcinoma. Surg Oncol 31: 90-97.
[Crossref] [Google Scholar] [PubMed]
- Minella AC, Clurman BE (2005) Mechanisms of tumor suppression by the SCFFbw7. Cell cycle 4: 1356-1359.
[Crossref] [Google Scholar] [PubMed]
- Kwak EL, Moberg KH, Wahrer DCR, Quinn JE, Gilmore PM, et al. (2005) Infrequent mutations of archipelago (hAGO, hCDC4, Fbw7) in primary ovarian cancer. Gynecol Oncol 98: 124-128.
[Crossref] [Google Scholar] [PubMed]
- Calhoun ES, Jones JB, Ashfaq R, Adsay V, Baker SJ, et al, (2003.) BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: Potential therapeutic targets. Am J Pathol 163: 1255-1260.
[Crossref] [Google Scholar] [PubMed]
- Xie CM, Wei W, Sun Y (2013) Role of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases in skin cancer. J Genet Genomics 40: 97-106.
[Crossref] [Google Scholar] [PubMed]
- Akhoondi S, Lindström L, Widschwendter M, Corcoran M, Bergh J, et al. (2010) Inactivation of FBXW7/hCDC4-β expression by promoter hypermethylation is associated with favorable prognosis in primary breast cancer. Breast Cancer Res 12: 1-13.
[Crossref] [Google Scholar] [PubMed]
- Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, et al. (2004) Inactivation of hCDC4 can cause chromosomal instability. Nature 428: 77-81.
[Crossref] [Google Scholar] [PubMed]
- Ibusuki M, Yamamoto, Shinriki S, Ando Y, Iwase H (2011) Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer science 102: 439-445.
[Crossref] [Google Scholar] [PubMed]
- Meyer G, Czompa A, Reboul C, Csepanyi E, Czegledi A, et al. (2013) The cellular autophagy markers Beclin-1 and LC3B-II are increased during reperfusion in fibrillated mouse hearts. Current pharmaceutical design 19: 6912-6918.
[Crossref] [Google Scholar] [PubMed]
- Li C, Lin XF, Wang JN, Ren XS (2020) FBXW7 inhibited cell proliferation and invasion regulated by miR-27a through PI3K/AKT signaling pathway and epithelial-to-mesenchymal transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci 24: 3701-3709.
[Crossref] [Google Scholar] [PubMed]
- Hagedorn M, Delugin M, Abraldes I, Allain N, Rotureau MAB, et al. (2007) FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell division 2: 1-12.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Chen J, Zhang S, Zheng S, Xie S, et al. (2020) MiR-223 regulates autophagy associated with cisplatin resistance by targeting FBXW7 in human non-small cell lung cancer. Cancer Cell Int 20: 1-14.
[Crossref] [Google Scholar] [PubMed]
- Wang C, Liu XQ, Hou JS, Wang JN, Huang HZ (2016) Molecular mechanisms of chemoresistance in oral cancer. Chin J Dent Res 19: 25-33.
[Crossref] [Google Scholar] [PubMed]
- Vishak S, Rangarajan B, Kekatpure VD (2015) Neoadjuvant chemotherapy in oral cancers: Selecting the right patients. Indian J Med Paediatr Oncol 36: 148-153.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B (2012) Plant natural compounds: Targeting pathways of autophagy as anti‐cancer therapeutic agents. Cell Prolif 45: 466-476.
[Crossref] [Google Scholar] [PubMed]
- Cuomo F, Altucci l, Cobellis G (2019) Autophagy function and dysfunction: Potential drugs as anti-cancer therapy. Cancers 11: 1465.
[Crossref] [Google Scholar] [PubMed]
- Liu YL, Yang PM, Shun CT, Wu MS, Weng JR, et al. (2010) Autophagy potentiates the anti-cancer effects of the histone deacetylase inhibitors in hepatocellular carcinoma. Autophagy 6: 1057-1065.
[Crossref] [Google Scholar] [PubMed]
- Xu Y, Tian C, Sun J, Zhang J, Ren K, et al. (2016) FBXW7-induced MTOR degradation forces autophagy to counteract persistent prion infection. Mol Neurobiol 53: 706-719.
[Crossref] [Google Scholar] [PubMed]
- Zhu T, Liu B, Wu D, Xu G, Fan Y (2021) Autophagy Regulates VDAC3 Ubiquitination by FBXW7 to Promote Erastin-Induced Ferroptosis in Acute Lymphoblastic Leukemia. Front Cell Dev Biol 9: 740884.
[Crossref] [Google Scholar] [PubMed]
- Xie CM, Sun Y (2019) The MTORC1-mediated autophagy is regulated by the FBXW7-SHOC2-RPTOR axis. Autophagy 15: 1470-1472.
[Crossref] [Google Scholar] [PubMed]
- Feng X, Zou B, Nan T, Zheng X, Zheng L, et al. (2022) MiR-25 enhances autophagy and promotes sorafenib resistance of hepatocellular carcinoma via targeting FBXW7. Int J Med Sci 19: 257-266.
[Crossref] [Google Scholar] [PubMed]
Citation: Yang Q, Sun Y, Qiu B, Zhao H (2022) Enhancement of Cisplatin-Induced Apoptosis of Human Oral Squamous Cell Carcinoma by FBXW7. J Oncol Res Treat 3: 185. DOI: 10.4172/aot-1000185
Copyright: © 2022 Yang Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2636
- [From(publication date): 0-2022 - Apr 08, 2025]
- Breakdown by view type
- HTML page views: 2275
- PDF downloads: 361