Research Article Open Access
Endplate Potential Oscillation of Jaw Closing Muscles in Natural Chewing
Toshifumi Kumai*
Oral Science, Matsumoto Dental University, Japan
- *Corresponding Author:
- Toshifumi Kumai
Oral Science, Matsumoto Dental University, Japan
Tel: 81-0263-51-225
E-mail: kumai@po.mdu.ac.jp
Received date: March 22, 2014; Accepted date: June 30, 2014; Published date: July 07, 2014
Citation: Kumai T (2014) Endplate Potential Oscillation of Jaw Closing Muscles in Natural Chewing. J Interdiscipl Med Dent Sci 2:129. doi: 10.4172/2376-032X.1000129
Copyright: © 2014 Kumai T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Surface electromyograms (EMGs) in natural gum chewing were recorded in a monopolar manner from paired anterior temporalis and masseter muscles of four subjects. Electrodes were placed on the temple of the former muscle, and on the inferior portion of the latter muscle. The endplate potential (EPP) component was extracted from raw EMGs of the muscles using a digital filter: the frequency of the high-cut digital filter for eliminating action potentials was set to 12.5 Hz or 45 Hz. The EPP component of the EMG burst of each stroke in the natural chewing showed two phases, early negative slow wave and following oscillation, which was same as observed in the clenching task. From FFT analysis, the frequencies of the ipsilateral EPP oscillations were around 30 Hz for both the temporalis and masseter muscles, which was also the same for the clenching task. It is concluded that the contraction of the jaw closing muscles is regulated in an oscillating manner of the EPP even in natural chewing behaviors. This oscillation phenomenon of the EPP gives a useful hint about the mechanism of muscular movements including chewing.
Keywords
EMG; EPP; Oscillation; Jaw closing muscles
Introduction
The region of the postsynaptic membrane is known to form a sink for the current of the depolarizing synaptic potential [1]. Thus a negative potential field should be produced in the external medium around the endplate with reference to a distant point. In previous papers, I have demonstrated in masticatory, masseter, and temporalis muscles that the endplate potential (EPP) can be extracted from the surface EMG recorded in a monopolar manner as a slow wave by applying an adequate digital filter on the raw EMG signal [2,3]. The deflection of the extracted slow wave exhibited a negative-positive pattern with recording sites, and it was concluded that the site showing the most negative deflection corresponded to the neuromuscular junction. Subsequently, I reported that the negative deflection, an early EPP component, was followed by an oscillation, and its frequency for the temporalis and masseter muscles in jaw clenching was approximately 30Hz [3,4]. As the EPP oscillation was observed in other muscles [5], it appears to be regular phenomenon in muscle contraction. All muscular movements should be controlled in a feedback manner in order to accomplish their tasks. The oscillation of the EPP appears to be another expression of the feedback control.
The EPP oscillation of the masticatory muscles reported in previous papers was demonstrated in the artificial jaw clenching task. Interest arises as to whether the same EPP oscillation as observed in the clenching task would be observed in natural chewing. In the present study, this is examined in natural gum chewing. If we could observe EPP oscillation in natural chewing through simple EMG surface recording, it would be a positive step in research of the chewing mechanism.
Materials and Methods
Surface EMGs were recorded from four healthy subjects, 3 males and 1 female aged 19-28, using traditional disc electrodes (f9 mm, Ag/AgCl). All subjects gave informed consent prior to participating in this examination. They had all previously received some dental treatment, but none had serious problems in chewing.
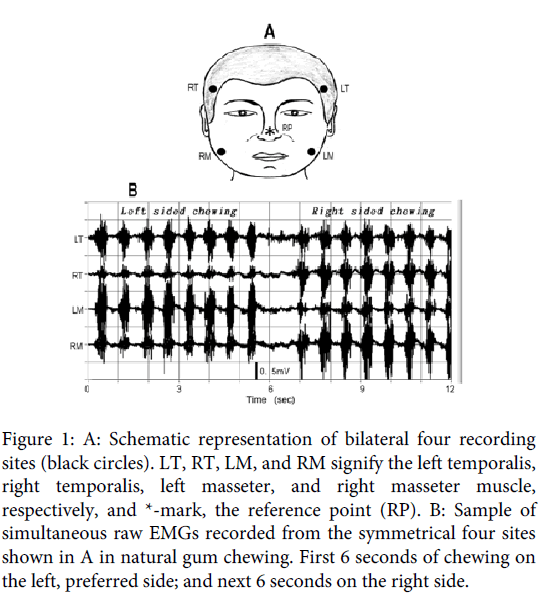
EMGs were recorded simultaneously from four sites located at the temple portion of the anterior part of the bilateral temporalis muscles and the lower portion of the bilateral masseter muscles (Figure 1A). In previous examinations, the positions were taken as the estimated location of the neuromuscular junctions [4,5]. The deflection manner of the slow wave for the temporalis muscle suggested the existence of multiple synaptic sites, but one might have been located near the temple. High viscosity paste was used in attaching electrodes to the skin. For the masseter muscles, electrodes were attached with adhesive tape, in which beard of male subjects had been shaved. For the hairy temple portions, the electrodes were attached simply by pushing them strongly onto the place without tape, which caused no difficulty for recording.
All recordings were carried out in a monopolar manner, where the reference electrode was placed at the tip of the nose (Figure 1A). In this examination, chewing gum (Green Gum, Lotte Inc.), was used as a test food. The subjects were instructed to chew the bolus (3g) naturally for 12 seconds: first 6 seconds on their left side, and next 6 seconds on their right side. EMG recordings were started after the bolus had fully softened. Electrical signals ranging from DC (0Hz) to 10kHz were amplified suitable to the voltage ranges (± 5V) of an A/D converter, the resolution of which was 12 bits. The analogue signals during the 12 seconds were led to a computer system with a sampling rate of 5 kHz. In conducting the series, the recording was repeated 10 times with intervals of about 3 minutes, which was tried 3 times on different days, and the data showing stable chewing were adopted on the analysis of this examination.
Figure 1: A: Schematic representation of bilateral four recording sites (black circles). LT, RT, LM, and RM signify the left temporalis, right temporalis, left masseter, and right masseter muscle, respectively, and *-mark, the reference point (RP). B: Sample of simultaneous raw EMGs recorded from the symmetrical four sites shown in A in natural gum chewing. First 6 seconds of chewing on the left, preferred side; and next 6 seconds on the right side.
The EPP component was extracted from each of the raw EMG recordings by removing the action potential component using a programmable digital filter (Butterworth type). In the previous clenching examination, the EPP component showed two phases [3,4]: early slow wave and following oscillation with relatively high frequency, which are respectively called the “early slow EPP” and the “EPP oscillation” (or simply “oscillation”) in this article. The cut-off frequency of the high-cut digital filter was set to 12.5 Hz for the extraction of the early slow EPP. To extract only the oscillation phase, both the action potential and the early slow EPP were eliminated with the high-cut digital filter at 45 Hz and with the low-cut digital filter at 12.5 Hz, respectively. Frequency of the EPP oscillation in each sample was measured using FFT analysis (Hamming window). It was applied on the filtered signals of the ipsilateral, chewing, side. Means (± standard deviation) of the frequency were calculated, for which the FFT analysis was applied on an adequate partial, 2-seconds, sample exhibiting a single clear spectrum peak for the ipsilateral temporalis and masseter muscle.
Results
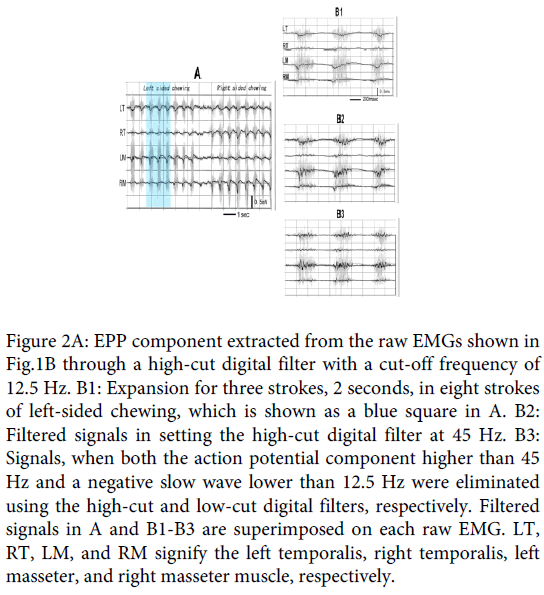
Figure 1B is a typical sample of the paired temporalis and masseter raw EMG recordings for natural gum chewing in a 28-year-old male subject whose preferred chewing side was the left (Subject 1 in Table1). In the traces, the first 8 strokes of discharge burst were for left sided chewing, and the following 8 bursts for right sided chewing. Figure 2A shows the signal with eliminated action potential component from the raw recordings using the high-cut digital filter with 12.5 Hz. The filtered signal is superimposed on each raw EMG. In the figure, the slow wave similar to that observed in the clenching examination is observed, which matches with discharge burst of each chewing stroke. In most strokes, the slow wave, especially for the ipsilateral, chewing side, muscles deflected negative, polar of which shifted to positive in its late phase. Figure 2-B1 is the expansion of three strokes (2 seconds) of the preferred, left-sided, chewing (blue color square in Figure 2A), where the negative deflection matching with the discharge burst can be observed more obviously: the negative deflection must correspond to the EPP, but its later positive overshoot must have originated from a capacitance factor contained in the muscle and skin, because the EMGs were, in this examination, recorded ranging from the DC level.
Figure 2: EPP component extracted from the raw EMGs shown in Fig.1B through a high-cut digital filter with a cut-off frequency of 12.5 Hz. B1: Expansion for three strokes, 2 seconds, in eight strokes of left-sided chewing, which is shown as a blue square in A. B2: Filtered signals in setting the high-cut digital filter at 45 Hz. B3: Signals, when both the action potential component higher than 45 Hz and a negative slow wave lower than 12.5 Hz were eliminated using the high-cut and low-cut digital filters, respectively. Filtered signals in A and B1-B3 are superimposed on each raw EMG. LT, RT, LM, and RM signify the left temporalis, right temporalis, left masseter, and right masseter muscle, respectively.
| Subjects | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Gender, Ages, PCS | m, 28ys, left | m, 19ys, right | f, 23ys, right | m, 23ys, left |
| Frequency | ||||
| Temporalis | 30.5(±6.3) n=25 | 31.4(±5.3) n=25 | 29.7(±7.4) n=22 | 25.4(±4.7) n=24 |
| Masseter | 27.3(±5.6) n=25 | 33.3(±6.7) n=23 | 28.5(±4.9) n=20 | 27.1(±6.0) n=25 |
Table 1: EPP oscillation frequencies for ipsilateral muscles. Subjects 2, 3 and 4 correspond, respectively, to subject A, B and C in Figure 4. The figure within each pair of parentheses is the standard deviation. f, female; m, male; PCS, preferred chewing side; n, sample numbers in the calculation.
Next, the frequency of the high-cut digital filter was changed. When it was increased from 12.5 Hz, the negative slow wave exhibited oscillation. Figure 2-B2 shows the signal when the filter was set at 45Hz. The oscillation is presented more clearly in Figure 2-B3, where the early negative wave in Figure 2-B2 was also eliminated using the low-cut digital filter with 12.5 Hz.
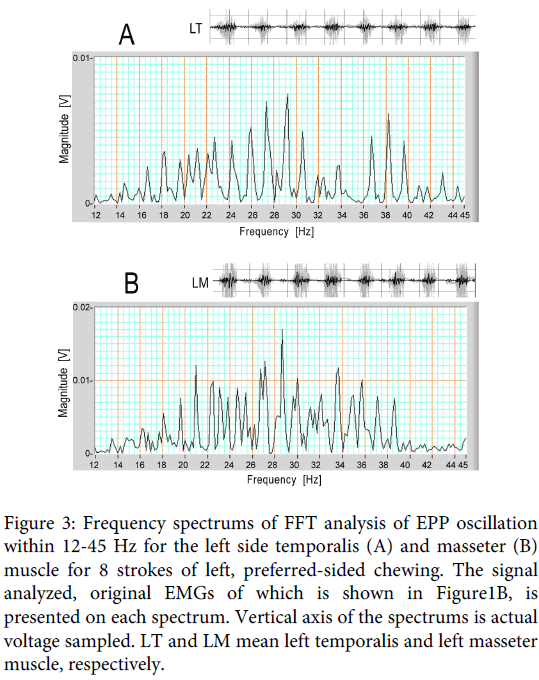
The frequency of the oscillation contained in the signal ranging from 12Hz to 45 Hz was measured using the FFT analysis. Figure 3A and B are the frequency spectrums adopted on the 12.5-45 Hz signals. Figure 3A is the spectrum for the left side temporalis during 8 strokes of left sided chewing, and Figure 3B is the same spectrum for the masseter muscle. Although the frequency spread relatively widely in both spectrums, and the pattern was different between the two spectrums, both of them had a peak frequency near 29 Hz in this chewing trial.
Figure 3: Frequency spectrums of FFT analysis of EPP oscillation within 12-45 Hz for the left side temporalis (A) and masseter (B) muscle for 8 strokes of left, preferred-sided chewing. The signal analyzed, original EMGs of which is shown in Figure1B, is presented on each spectrum. Vertical axis of the spectrums is actual voltage sampled. LT and LM mean left temporalis and left masseter muscle, respectively.
The means of the peak frequency (± standard deviation) were calculated for the temporalis and masseter muscles. FFT analysis was applied on a partial, 2-second sample exhibiting clear oscillation to obtain simple spectrums. For this subject, 38 ipsilateral spectrums were obtained for either muscle from 19 chewing trials showing stable conditions, and 25 of the 38 spectrums for each muscle were adopted in this statistics. The values were 30.5 (± 6.3) Hz for the ipsilateral temporalis muscle, and 27.3 (± 5.6) Hz for the ipsilateral masseter muscle (Subject 1 in Table 1). However, it needs to be kept in mind that these mean frequencies were obtained from spectrums exhibiting a single peak: the spectrum pattern of the filtered signal showed variation with partial samples, and there were spectrums having multiple peaks, which were omitted from the calculation data.
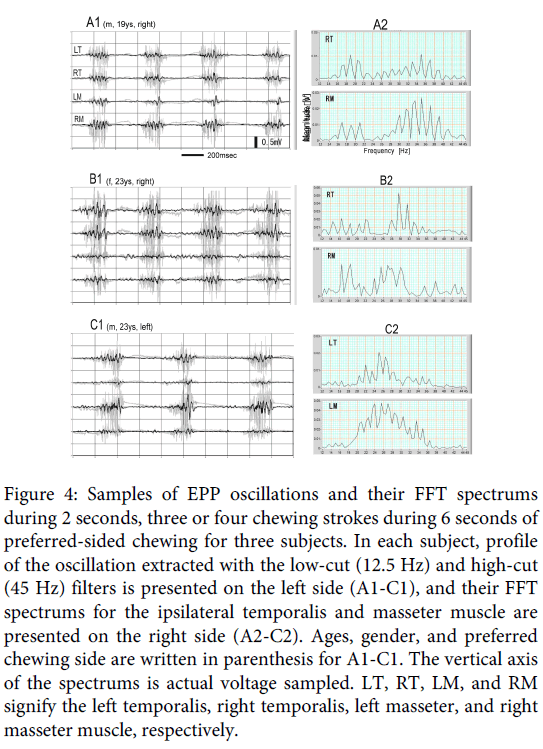
The EPP oscillation in natural gum chewing was also examined in three other subjects. Figure 4 is a partial, 2-second, sample containing three or four strokes in 6 seconds of preferred sided chewing for the subjects. The profile of the oscillation extracted with the low-cut (12.5 Hz) and high-cut (45 Hz) filters is presented on the left side (A1, B1, and C1), and the FFT spectrums for the ipsilateral temporalis and masseter muscles are presented on the right side (A2, B2, and C2); A, B and C are, respectively, for Subjects 2, 3 and 4 in Table 1. In the filtered signals, clear EPP oscillation is observed in all subjects, especially on the ipsilateral signals. The FFT spectrums showed a difference for subjects and muscles, but the patterns for the ipsilateral temporalis and masseter muscles were roughly similar, and the main frequencies can be considered to lie around 30Hz. The similarity of FFT patterns between the two ipsilateral muscles must originate from the synchronization of EPP oscillation between the two ipsilateral muscles [4]. Means (± standard deviation) of the frequency of an adequate partial, 2-seconds, sample exhibiting a single clear spectrum peak in 6-seconds for the ipsilateral temporalis and masseter muscle in the three subjects are shown in Table 1. (The FFT analysis in the present examination was applied on filtered signals, but the same peak frequencies can, of course, be obtained by applying on raw EMGs).
Figure 4: Samples of EPP oscillations and their FFT spectrums during 2 seconds, three or four chewing strokes during 6 seconds of preferred-sided chewing for three subjects. In each subject, profile of the oscillation extracted with the low-cut (12.5 Hz) and high-cut (45 Hz) filters is presented on the left side (A1-C1), and their FFT spectrums for the ipsilateral temporalis and masseter muscle are presented on the right side (A2-C2). Ages, gender, and preferred chewing side are written in parenthesis for A1-C1. The vertical axis of the spectrums is actual voltage sampled. LT, RT, LM, and RM signify the left temporalis, right temporalis, left masseter, and right masseter muscle, respectively.
Discussion
It was reported in a previous paper that the EPP component of the temporalis and masseter muscles showed two phases, an early slow wave and a following oscillation at about 30 Hz in the clenching experiment [3]. The same EPP organization was observed in this examination of natural chewing. The standard deviations obtained in this examination were rather high. Its main reason is supposed to be the difference in chewing manner depending on the state of gum bolus in the mouth; increasing fatigue in the chewing process, or in the trials, might also be related. The oscillating fashion of the EPP means that muscular contraction is not controlled monotonously, which is also suggested from a phenomenon known as the ‘silent period’, a short pause of muscular discharges in muscle contraction. In the dental field, the silent period has been well observed in jaw closing muscles [6,7]. The mechanism of the silent period is thought to be a suppressive effect of sensory signals mainly from mechanical receptors of the periodontal membrane on the corresponding motor neurons [7-9], as the discharge interruption tends to be induced by tooth contact [6-8].
About the mechanism of the EPP oscillation, we consider the possibility of the participation of proprioceptive receptors such as the muscle spindle and tendon organ. A well-known sequence in controlling muscle contraction intermediating the muscle spindle is the following: muscle contraction loose of muscle spindle decrease of Ia-fiber activity decrease of α-motorneuron activity loose of the muscle strain of the muscle spindle increase of the Ia-fiber activity increase of the α-motorneuron activity muscle contraction . The same sequence intermediating Ib-fiber of the tendon organ is possible, although the effect at each step is reversal, as the activity of Ib-fiber has a suppressive effect on the α-motorneuron. However, it is, at the present time, difficult to state how the two proprioceptive systems cooperate and how the periodontal (and temporomandibular joint) receptors participate in building this EPP oscillation.
It has been reported that small involuntary rhythmic movement is measured in many muscular movements, including jaw movement, of humans [10,11]. Such microtremors, known as physiological tremors, are too small to be seen with the naked eye. The dominant frequencies of the physiological tremor of body parts, such as arms, fingers, and the jaw, are reported roughly the same: somewhere around 10 Hz, which is considerably lower than the frequency of the EPP oscillations measured in this examination. (The tremor frequency of the human jaw in isometric condition was measured approximately 6-12 Hz [11-14]) Concerning the origin of the physiological tremor, some investigators have postulated the existence of a central rhythm generator [15-20], while others has posited a main contribution of feedback servo loops [21-23], as in the stretch reflex. At an earlier stage of discussion, the tremors were even ascribed to a mechanical resonance effect in which asynchronous firings of motor units were thought to cause body parts to oscillate at their resonance frequency [24-26].
As for jaw physiological tremors, there are authors who insist that the feedback effect of exteroceptors, or periodontal mechanical receptors, is most strongly involved in tremor generation [13,27-29]. There are, however, certain differences between EPP oscillations and physiological tremors: EPP oscillations represent a phenomenon occurring in the muscular synaptic potential during strong muscular contractions, most of which is under isotonic condition, whereas jaw physiological tremors primarily represent a phenomenon of the jaw during postural or isometric conditions, where sensory feedback signals are relatively weak. However, the possibility remains that the EPP oscillations and physiological tremors may originate from a common servo circuit, such as the stretch reflex, where only the adjusting speeds are different. Another explanation may be possible—that both proprioceptors and exteroceptors participate, but that the feedback effect of proprioceptors would become dominant under isotonic conditions, or jaw motion phase, whereas that of exteroceptors would become dominant under isometric conditions, or occlusion. These could produce different frequencies of rhythmic discharges of the motor neurons with the degree of the respective feedback effect.
Apart from the mechanism of EPP oscillation, it is natural to think that the oscillation of the synaptic potential induces rhythmic muscular discharges. In actuality, EMG investigators occasionally record a rhythmic discharge for muscle contraction. The silent period of jaw closing muscles can be regarded as a part of the rhythmic discharge. Even if we can’t recognize clearly the rhythmic discharges on EMG recordings from a view observation, the frequency of discharges would change, more or less, rhythmically. The EPP oscillation must be expedient in adjusting the strength of muscle contraction through translated discharges, and the silent period that must be a partial reflection of the EPP oscillation functions to protect the chewing apparatus from excessive bite force.
The result obtained in this examination strongly suggested that skeletal muscles might contract intrinsically in an oscillating fashion, which is not detected under regular physiological conditions, as the vibrations, for example 30 Hz, are too fast, although it must be also considered that EPP manner is not necessarily reflected directly in terms of jaw movements as many transmitting steps lie between the two. We are unaware of the vibration of our muscles under normal physiological conditions, but we also often experience strong muscle trembling under fatigue or frigid conditions, after ingesting too much alcohol or caffeine, and under specific psychological stress. These lasting forms of trembling may be the result of magnification of physiological tremors. On the other hand, various types of abnormal tremor in action are also known in humans [30,31]. They, action abnormal tremors, are possible to be the outcome of EPP oscillations, the frequency of which can become lower due to certain causes. Anyway, the fact that the EPP oscillates provides a useful hint on the mechanism of muscular movements, including that for chewing.
Acknowledgments
I would like to offer great thanks to David Carlson, Professor of English at Matsumoto Dental University, for his kind support in the writing of this article. I also thank students at our university for their agreeable compliance as subjects in this investigation.
References
- Eccles JC (1964) The physiology of synapses. (1st edn), Berlin Heidelberg New York: Springer Verlag.
- Kumai T (2005) Location of the neuromuscular junction of the human masseter muscle estimated from the low frequency component of the surface electromyogram. Jpn J Physiol 55: 61-68.
- Kumai T (2009) Extraction of the EPP component from the surface EMG. J Vis Exp .
- Kumai T (2012) Synchronization among endplate potential oscillations in jaw closing muscles. Webmed Central Neurosciences 3.
- Kumai T (2009) Oscillation of the endplate potential. Proceedings of the 36th International Congress of Physiological Sciences.
- Schaerer P, Stallard RE, Zander HA (1967) Occlusal interferences and mastication: an electromyographic study. J Prosthet Dent 17: 438-449.
- Ahlgren J (1969) The silent period in the emg of the jaw muscles during mastication and its relationship to tooth contact. ActaOdontolScand 27: 219-227.
- Brenman HS, Black MA, Coslet JG (1968) Interrelationship between the electromyographic silent period and dental occlusion. J Dent Res 47: 502.
- Erkelens CJ, Bosman F (1985) Influences of periodontal and mandibular-joint receptors on reflex sensitivity of human jaw-closing muscles. Arch Oral Biol 30: 545-550.
- Elbe RJ, Koller WC (1990) Tremore, The John Hopkins University Press.
- Junge D, Rosenberg JR, Halliday DM (1998) Physiological tremor in human jaw-muscle system. Arch Oral Biol 43: 45-54.
- Palla S, Ash MM Jr (1979) Frequency analysis of human jaw tremor at rest. Arch Oral Biol 24: 709-718.
- vanSteenberghe D, De Vries JH (1980) The effect of the suppression of the periodontal neural input on mandibular tremor in man. Arch Oral Biol 25: 471-476.
- Sowman PF, Türker KS (2005) Methods of time and frequency domain examination of physiological tremor in the human jaw. Hum MovSci 24: 657-666.
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R (1997) Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77: 3401-3405.
- Gross J, Tass PA, Salenius S, Hari R, Freund HJ, et al. (2000) Cortico-muscular synchronization during isometric muscle contraction in humans as revealed by magnetoencephalography. J Physiol 527 Pt 3: 623-631.
- Riddle CN, Baker MR, Baker SN (2004) The effect of carbamazepine on human corticomuscular coherence. Neuroimage 22: 333-340.
- Riddle CN, Baker SN (2005) Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol 5 66: 625-639.
- Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6: 285-296.
- Witham CL, Riddle CN, Baker MR, Baker SN (2011) Contributions of descending and ascending pathways to corticomuscular coherence in humans. J Physiol 589: 3789-3800.
- Lippold OC (1970) Oscillation in the stretch reflex arc and the origin of the rhythmical, 8-12 C-S component of physiological tremor. J Physiol 206: 359-382.
- Matthews PB, Muir RB (1980) Comparison of electromyogram spectra with force spectra during human elbow tremor. J Physiol 302: 427-441.
- Durbaba R, Taylor A, Manu CA, Buonajuti M (2005) Stretch reflex instability compared in three different human muscles. Exp Brain Res 163: 295-305.
- Stiles RN, Randall JE (1967) Mechanical factors in human tremor frequency. J ApplPhysiol 23: 324-330.
- Fox JR, Randall JE (1970) Relationship between forearm tremor and the biceps electromyogram. J ApplPhysiol 29: 103-108.
- Allum JH, Dietz V, Freund HJ (1978) Neuronal mechanisms underlying physiological tremor. J Neurophysiol 41: 557-571.
- Sowman PF, Ogston KM, Türker KS (2007) Periodontalanaesthetisation decreases rhythmic synchrony between masseteric motor units at the frequency of jaw tremor. Exp Brain Res 179: 673-682.
- Sowman PF, Türker KS (2007) Mandibular tremor during isometric contractions. Arch Oral Biol 52: 353-356.
- Sowman PF, Brinkworth RS, Türker KS (2008) Mandibular physiological tremor is reduced by increasing-force ramp contractions and periodontal anaesthesia. Exp Brain Res 184: 71-82.
- Findley LJ (1996) Classification of tremors. J ClinNeurophysiol 13: 122-132.
- Deuschl G, Bain P, Brin M (1998) Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. MovDisord 13 Suppl 3: 2-23.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 14290
- [From(publication date):
August-2014 - Jul 14, 2025] - Breakdown by view type
- HTML page views : 9655
- PDF downloads : 4635