Research Article Open Access
Endotoxemia Analysis by the Limulus Amoebocyte Lysate Assay in Different Mammal Species Used in Metabolic Studies
F Laugerette*, G Pineau, C Vors and MC Michalski
Université Lyon 1, INSERM U1060, INSA-Lyon, IMBL, CarMeN laboratory, F-69621 Villeurbanne, France
- *Corresponding Author:
- F Laugerette
INRA, UMR1397, Université Lyon 1
INSERM U1060, INSA-Lyon
IMBL, CarMeN laboratory F-69621 Villeurbanne, France
Tel: 33472438112
Fax: +33472438524
E-mail: fabienne.laugerette@univ-lyon1.fr
Received date: June 08, 2015; Accepted date: June 27, 2015; Published date: July 04, 2015
Citation: F Laugerette, G Pineau, C Vors and MC Michalski (2015) Endotoxemia Analysis by the Limulus Amoebocyte Lysate Assay in Different Mammal Species Used in Metabolic Studies. J Anal Bioanal Tech 6:251. doi: 10.4172/2155-9872.1000251
Copyright: © 2015 F Laugerette, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Introduction: Lipopolysaccharides (LPS) or so-called endotoxins are potent pro-inflammatory compounds. LPS can be present in the bloodstream in case of septic conditions, leading to measure endotoxemia that is the activity of LPS in plasma. Recent research also reveals a low-grade or so-called metabolic endotoxemia associated with metabolic diseases (e.g. obesity, type 2 diabetes, cardiovascular diseases). In this context, research studies use different experimental models, mostly in humans and rodents. Pig is now emerging as a new animal model in nutritional and metabolic studies. However, information is lacking to date about optimal dilution to be used for sample preparation for the Limulus Amebocyte Lysate test, according to species, especially for pig.
Methods: strong>Endotoxemia was measured using the LAL standard reference method. We describe the method of sample preparation and the LAL technique to measure endotoxemia in 4 mammal species: human, mouse, rat and pig.
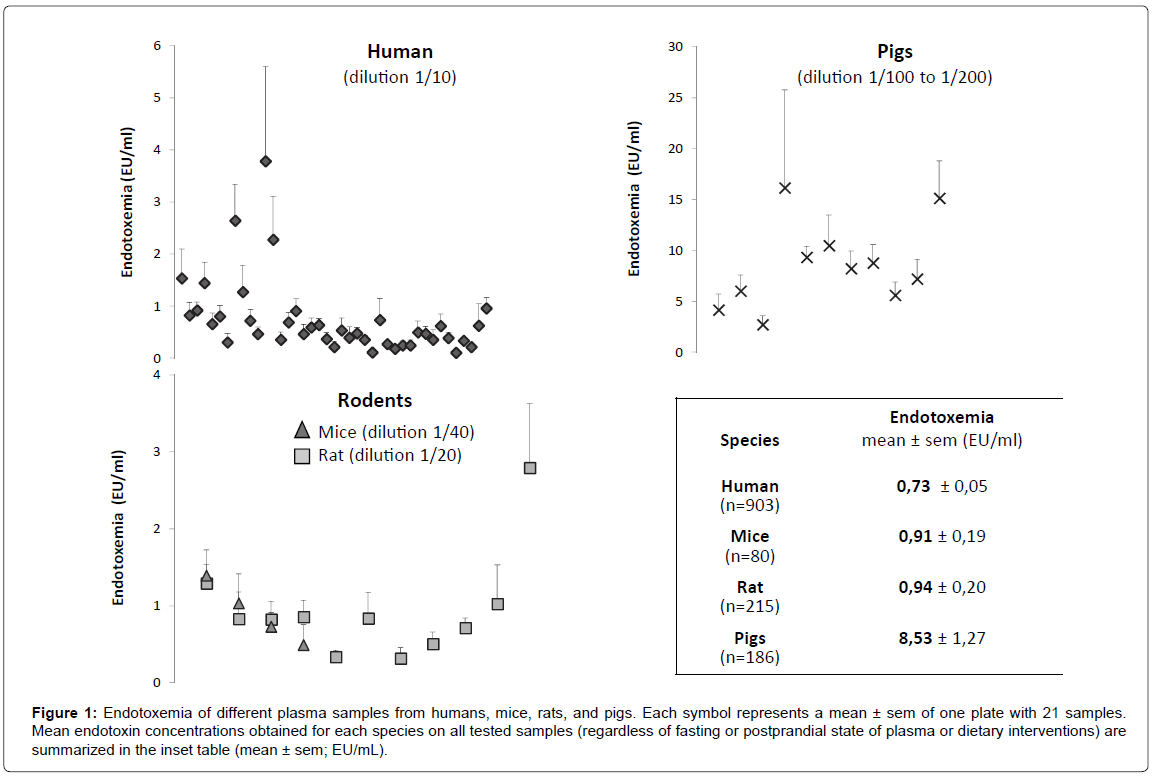
Results: Plasma dilution is necessary to overcome interferences leading to erroneously low or high results. Optimal dilution to avoid interferences in most samples, while maintaining a satisfactory sensitivity, was found to be at least 1/10 for human vs 1/40 for mice, while much higher dilution was mandatory for pigs, namely 1/200. Altogether, mean plasma endotoxemia in all tested samples using the optimal dilution for each species was 0.73 ± 0.05 EU/mL in humans (n=903), 0.9 ± 0.2 EU/mL in rodents (n=295) and 8.5 ±1.3 EU/mL in pigs (n=186), regardless of fasting or postprandial state and/or type of dietary intervention.
Conclusion: We show that the LAL assay can be used to determine endotoxemia in fasting and postprandial blood samples from different mammal species including pig. Because its detection is made difficult by interference from other plasma constituents, an essential parameter to overcome this difficulty is the dilution factor that depends on the studied species.
Keywords
LAL test; Endotoxemia; Mammal species
Abbreviations
CV: Coefficient of Variation; FDA: Food and Drug Administration; LAL: Limulus Amebocyte Lysate; LBP: Lipopolysaccharide Binding Protein; LPS: Lipopolysaccharide; OD: Optical Density; sCD14: Soluble Cluster Differentiation 14
Introduction
In the context of obesity outbreak, recent studies have shown a link between high-fat diet, metabolic inflammation and the occurrence in plasma of pro-inflammatory products from the gut microbiota, namely lipopolysaccharides (LPS) or so-called “endotoxins” [1,2]. Endotoxins of the Gram-negative bacteria, when released into the intestinal lumen after cell lysis, have a high pyrogenic activity and can induce and maintain a low-grade inflammation [3,4]. For a long time the measurement of pyrogenic molecules has been a regulatory requirement for some pharmaceutical supplies including injectables, medical and surgical equipment and dialysis samples. The method for the detection and quantification of endotoxin activity in serum and plasma, the so-called “endotoxemia”, was first described by Levin et al., [5] after pioneering work with the Limulus [6]. The most commonly used test is the Limulus amebocyte lysate. LAL is an extract of the blood cells (amebocytes) of Limulus polyphemus, a horseshoe crab of the North America, containing the blood coagulation mechanism activated by LPS. This test is used by the European pharmacopea and United States Food Drug Administration (FDA). Although, this method appears to be straightforward to execute, but is highly sensitive to interferences. Therefore, the LAL assay requires a well-controlled and validated methodology for its correct implementation. The endotoxin activity level in blood samples can be interfered by constituents in the samples (as proteases) [5,7]. The most commonly used LAL assay is a chromogenic test. Of note, two other methods, the gel-clot LAL and the turbidimetric test can be used to measure endotoxemia. Briefly, the gel-clot method is based on the ability of endotoxin to induce gelation in LAL [8] but this test is semi-quantitative and prone false negatives. The turbidimetric test is similar to the gel-clot test. In this method, the presence of endotoxin with the diluted LAL reagent results in flocculation, which is measured as turbidity and correlated to the endotoxin concentrations.
In the chromogenic LAL test, the presence of endotoxin catalyzes the conversion of a pro-enzyme to enzyme (active form) which leads to the production of p-nitroaniline, and the appearance of a yellow color in the solution [9], which is quantified at 405 nm. The release of p-nitroaniline is proportional to the endotoxin activity in the sample. The endotoxemia levels in the turbidimetric and chromogenic levels can be determined by an endpoint method, usually providing a sensitivity of 0.05 EU/mL, or a kinetic method that allow a higher sensitivity of 0.005 EU/mL. With the kinetic methods, we determine the necessary time to reach an absorbance of 405 nm (reaction time of the absorbance of p-nitroaniline). The higher the endotoxemia is, the shorter the reaction time is. In our experiments we used a chromogenic kinetic assay, known as the most performed method [10] and we used a log-log endotoxin standard curve to calculate endotoxin concentration in our samples.
As previously described [11-13], the detection of endotoxin activity in serum and plasma by the LAL test is tedious due to the possible inhibition or activation of the test by endogenous sample compounds. The presence of an inhibition can be detected when endotoxins added to serum or plasma (positive product control, so-called “spike”) fails to react with the LAL reagent. Several methods have been used to remove inhibition of the LAL test such as dilution, heating [4,14], solvent extraction [11] and acid treatment [14,15]. The combination of dilution and heating processes has been shown to be efficacious, yet we have also added a sonication step as the extraction of LPS is also influenced significantly by physical characteristics that determine its aggregation [4,16]. In fact, Komuro et al. [16], showed sonication may disperse the aggregates of LPS into smaller and more uniform particle which facilitates the measure of biological activities in aqueous media. Serum and plasma must be diluted in LAL pyrogen-free water before heating. The dilution level depends on the species of serum and plasma used. The activation of the LAL test is also easily detected by a fast response in the positive product control well. A contamination of the sample can be the cause or an interaction with endogenous plasma compounds.
The importance of the types of tubes used to collect blood and the effect of sample treatment (e.g., dilution or heating) on the quantification of endotoxins was reported [5,7] and recently gained new interest [17]. As shown by Roth et al. [7], the use of borosilicate tubes leads to a greater activation of the LAL assay than polypropylene. The type of polymer is also of importance because of some LPS loss that can occur due to adhesion of LPS to hydrophobic polymers. For instance, polystyrene tubes are more adequate than polypropylene tubes in this respect [7,18]. However, special polypropylene-tube manufacturing processes exist to induce low molecular adsorption and therefore overcome this analytical pitfall.
The LAL test is also extremely sensitive to glucans, specifically (1→3)-ß-D-glucan. Indeed, a few polymeric forms of glucose have been shown to be LAL reactive when they are present in sufficient quantities [19]. A specific buffer can be used instead of nonpyrogenic water to reconstitute LAL reagent. This renders this reagent insensitive to (1→3)-ß-D-glucan interference by effectively blocking the Factor G pathway of the endotoxin clotting cascade whether glucans are present in the samples. LAL assay can also be affected by the way of collecting blood. In fact, EDTA is known to sequester bivalent cations in the blood and affect the LAL assay [20]. Heparin used to collect blood can impact on the test because it was shown that endotoxemia was detected from plasma collected in commercial heparin vacutainers but not from tubes prepared with extemporaneous heparin addition [21]. Moreover, excess heparin (30 IU/ml blood) can cause test activation [22].
However, to our knowledge, no study specifically compared the LAL test procedure to be used in different species. Yet, there are an increasing number of publications reporting endotoxemia analysis on different species in the context of metabolic disease. In fact, only one study interested in survey for positive LAL test was led in plasma from human and some research animals as dogs [23].
This article discusses the different adjustments used for the detection of endotoxin activity in the plasma of humans, mice, rats and pigs. We highlight that the dilution factor used in the LAL test is an essential parameter for measuring endotoxemia in plasma and is dependent on the species, regardless of the dietary conditions in which plasma was sampled (fasting, postprandial, type of diet).
Methods
Specific nonpyrogenic material
Great care was taken to avoid contamination with exogenous LPS during experiments. Only single-use non pyrogenic material was used: PS tubes (Becton Dickinson), Maxymum Recovery® tubes (Axygen, VWR) and pyrogen-free pipette tips (Eppendorf, Associates of Cape Cod). Pipettes used for this test were autoclaved.
Procedure for collecting blood under pyrogen-free conditions
In human protocols involving normal-weight to obese subjects without diabetes nor dyslipidemia, 2 ml of venous blood was obtained from each subject in nonpyrogenic tubes containing 4 μl of injectable heparin (Choay 5000 IU/ml, i.e. 2 μL heparin/mL blood), thereby ensuring heparin concentration of <10 IU/mL. Plasma was collected by centrifugation (8000 g, 10 min, 4°C). Of note, human plasma samples were collected both in the fasting state (~40% of samples) and during different postprandial periods (~60% of samples). For fasting mice and rats after different diets varying in lipid composition, blood was collected by cardiac puncture under pyrogen-free conditions in heparin-containing tubes (heparin<10 IU/ml plasma, Associate of Cape Code). Plasma was collected by centrifugation (2000 g, 5 min, 4°C). For pigs, samples were collected at fast (15% of samples) and at different postprandial times (85% of samples) after different diets varying in lipid composition. Sitimplant Hubsite (Vygon) was used and the catheter was rinsed with physiological serum and 1% Heparin Choay. Blood was collected in 5 mL non-pyrogen tubes containing 10 μL of heparin before plasma collection by centrifugation. A recent study has shown the importance of the sample storage at -80°C [17,24]. In fact, endotoxemia of samples stored at +4°C was lower than endotoxemia from samples stored at -80°C. In our experiments, all plasma was stored at -80°C until analysis.
Limulus amoebocyte lysate assays
The LAL assay was performed in a room devoted to this test, devoid of air flows due to passageway or air conditioning. Of note, a sterile hood was not used because an air flow causes endotoxin transfer from the air even in the absence of live bacteria (personal communications from LAL test manufacturers, Lonza & ACC).
Plasma endotoxemia (endotoxin unit per milliliter (EU/ml)) was determined by using the LAL assay in kinetic chromogenic conditions (Associate of Cape Cod). In this method, the time at which the optical density (OD) starts to increase is negatively correlated with the sample endotoxemia. The sample was thawed and diluted in pyrogen-free water (Associate of Cape Cod). The dilution with LALwater used for each species is discussed in the results section. To inactivate endotoxin-neutralizing agents that can inhibit the activity of endotoxin in the LAL assay, we heated the diluted sample at 70°C for 10 min. Then the sample was subjected to an ultrasonic bath (37°C, 10 min) and vortexed for 1 min to enhance recovery of measurable LPS activity. LAL reagent (so-called Chromolal) is received in powder form and has to be diluted and very smoothly homogenized (very slow top/down hand shaking) before use. One hundred microliters of the diluted sample was combined with 100 μl of LAL reagent in triplicate in pyrogen-free 96-well plates (Associate of Cape Cod). For each sample, a spike control at 0.45 EU/ml was performed, by adding 10 μL of a 5 EU/mL standard to 100 μL of sample, to check that no significant inhibition or activation occurred. According to the procedure of the US Department of Health and Human Services PHS, Food and Drug Administration (1987), endotoxemia measurement is considered valid if spike recovery is in the range of 50-200%. We must highlight that the spike recovery is a quality control procedure that must not be used for correcting the endotoxin value of a sample (personal communications from LAL test manufacturers, Lonza & ACC). Of note, this means that in principle, only 2-fold differences in endotoxemia between 2 samples may be considered possibly significant. The standard curve was prepared in LAL-water from E. Coli 0111 endotoxin standard. We must highlight that LAL kits are made of a pairwise association of one batch number of LAL reagent with one batch number of E. Coli LPS standard that are delivered with a specific certificate of analysis and should not be dissociated to perform the test. The first time the endotoxin standard was used, the necessary quantity of LAL-water, indicated in the above-mentioned certificate of analysis, was added and the solution was vortexed 1 min every 5 min during 30 min. Of note, this solution can be stored during 1 month at 4°C for other assays; in this case, a continuous vortexing during 5 min is mandatory before each use. The different points of the standard curve (0.005 to 5 EU/ml) were prepared in duplicate in cascade in pyrogen-free water. As the measures in plasma are typically in the low end of the analysis range as far as metabolic endotoxemia is concerned, we used 5 EU/mL as the highest concentration in the standard curve, instead of the 50 EU/mL indicated for general purpose in the instruction kit. In place, we used an intermediate value of 0.25 EU/mL. Importantly, standard curve must present a regression coefficient of >0.985 to validate the plate.
The optical densities (OD) of each sample are determined every 30 seconds at 405 nm for 2 h. According to Cape Cod procedures, endotoxin concentrations are automatically calculated based on the time in seconds that it takes for the initial OD value to increase by 0.1 units. The apyrogenicity of LAL water was systematically checked, with the criterion that time to reach 0.1-OD increase should be greater than 1.05-fold the time reached by the 0.005 EU/mL-standard. Data were analyzed for endotoxemia calculation using the Softmax Pro software. A log (time reaction)-log (endotoxin standard) curve was performed by the software to permit to calculate the endotoxin concentration of the samples.
Results
Validation of the standard curves
The coefficient of variation (CV) of the duplicate of the standard curves of validated plates in this study was between 0.3 to 2.7%. The coefficient of correlation (R2) was between 0.985 and 1, allowing to calculate sample endotoxemia for each plate.
Optimal dilution depends of species
We observed no impact of plasma being taken in the fasting or postprandial phase, nor of the type of lipids in the diet, on optimal plasma dilution for endotoxemia analysis. The recoveries of endotoxin spiked to samples were respectively 126%, 95% and 98% for human, rats and mice with the dilution of 1/10, 1/20, 1/40, respectively. For pigs, the recovery of spiked endotoxin was 118% for dilutions of 1/100 and 1/200. We have also calculated for all samples for which we have measured endotoxemia (human, rodent, pig), the proportion of samples with (i) a within-triplicate variation coefficient >25% and [15] a spike recovery <50% or >200%. As reported Table 1, it is interesting to note that around 10% of the spike recoveries of all species samples are <50% except for mice where the % of samples which have an inhibitory spike recovery (<50%) is 30% (Table 1). Mice had a smaller percentage of samples with a spike recovery >200%. These results indicate that plasma of mice contains more inhibitory factors than the plasma of other species. With a dilution factor of 1/10 for human, 1/20 for rat and 1/40 for mice, most of the samples (>62%) have a spike recovery in the accepted range of 50-200% while keeping the test sensitivity within an acceptable range (0.05 EU/mL for human, 0.1 EU/mL for mice and 0.2 EU/mL for rats).
| Species | Dilution | Sample % | Sample % | Sample % |
| Variation coefficient >25% | Spike recovery<50% | Spike recovery>200% | ||
| Human(n=933) | 1/10 | 39 | 8 | 21 |
| Mice (n=80) | 1/40 | 35 | 30 | 3 |
| Rat (n=221) | 1/20 | 26 | 9 | 11 |
Table 1: Proportion of samples (%) presenting a high variation coefficient (>25%) and/or spike recovery out of the acceptable range (<50% or >200%).
Now a days, many nutrition studies use pigs due to the fact that their lipid metabolism is similar to humans. In fact, the pig is a singlestomached omnivorous mammal and is an important model of human disease and nutrition [25]. Thus, the quantification of endotoxemia now becomes of interest in this model. As shown in Table 2, we had to compare 4 dilution factors, because the pigs presented lots of variation in the LAL assay. Firstly, we used a dilution factor of 1/10 (as human) but unfortunately, 67% of the samples had a spike recovery <50%, and 48% of the samples presented dispersion in the triplicate analysis. Secondly we used the same dilution factor as mice (1/40), resulting in 67% of the samples having a spike recovery >200%, thus triggering an apparently high endotoxemia. Finally we tested dilutions up to 1/200. As shown Table 2, a similar endotoxemia was measured at the higher dilutions: 15.9 ± 6.3 EU/mL (1/100 dilution) and 16.7 ± 3.6 EU/mL (1/200 dilution). Both dilutions thus appear acceptable for use with pigs, with however a lower proportion of samples presenting a withintriplicate variation coefficient >25% with the dilution factor 1/200. Therefore endotoxemia values further presented for pigs were obtained either using a 1/100 dilution if variation coefficient was acceptable, in order to keep a sensitivity of 0.5, or using 1/200 if necessary to lower the variation coeffient thus leading to a sensitivity of 1 EU/mL.
| Pig | Dilution | Endotoxemia* | Sample % | Sample % | Sample % |
| Mean ± SEM (EU/mL) | Variation coefficient >25% | Spike recovery<50% | Spike recovery>200 % | ||
| n=21 | 1/10 | 1.2± 0.4 | 48 | 67 | 0 |
| n=21 | 1/40 | 28.6± 11.6 | 14 | 24 | 67 |
| n=63 | 1/100 | 16.0± 6.3 | 27 | 22 | 14 |
| n=211 | 1/200 | 16.7± 3.6 | 15 | 10 | 17 |
*Mean value obtained for all tested samples including those for which quality criteria were not met, illustrating the impact of interferences on obtaining erroneously low or high endotoxemia values.
Table 2: Endotoxemia (EU/mL) measured in pigs at different dilutions and proportion of samples (%) with variation coefficient >25%, recovery <50% and >200%.
After excluding samples with a spike recovery <50% or >200%, we can measure the mean endotoxemia in the different species (Figure 1). For all plates of LAL assay with human plasma, the dilution factor of 1/10 led to satisfactory measurements with many obtained values laying within a narrow range (Figure 1). Of note, more than 95% of all tested human samples were validated at dilution 1/10 regardless of the fasting or postprandial condition. The dilution factor of 1/20 and 1/40 for rodent plasma could also be validated (Figure 1). For pigs, dilutions of 1/100 or 1/200 were used. A large discrepancy appeared in different sample endotoxemia results, which was due to the different dietary interventions among animals. However, the latter did not impact on optimal sample dilution.
Figure 1: Endotoxemia of different plasma samples from humans, mice, rats, and pigs. Each symbol represents a mean ± sem of one plate with 21 samples. Mean endotoxin concentrations obtained for each species on all tested samples (regardless of fasting or postprandial state of plasma or dietary interventions) are summarized in the inset table (mean ± sem; EU/mL).
Discussion
The LAL assay used to determine endotoxemia in blood samples is changeling analytically due to possible interferences with blood constituents [5,7]. In this study we have shown the importance of the dilution factor in human, mice, rat and pigs; and compared 4 different dilution factors in pigs to explain our rationale. The methodology used in our case to measure endotoxemia combined dilution, heat and sonication to increase the dispersion of LPS in the sample [4]. Moreover great care was taken to avoid contaminations during the experiment.
The first important step of the LAL assay is the standard curve. In fact the samples of a plate will be validated only if the negative control and the standard curve respect the pronounced norms in the materials and methods. Other studies have compared the preparation of the standard curve in LAL water and in plasma [15,26]. In our case and using Cape Cod reagent we performed the standard curve in LAL water. In fact, most of our analyses are performed after nutritional interventions in mice, rats, pigs and human. In the case where we would want to realize standard curve in plasma, a pool of plasma from each species for each nutritional experiment would be necessary. Moreover, this would lead to multiple aliquots to avoid steps of freeze-thawing.
Another important aspect for successful LAL assays is the choice of sample dilution. The dilution factor must permit a recovery (%) of endotoxin activity in spiked sample of >50% and <200%. In our experiments the accepted dilutions were 1/40, 1/20, 1/10 and 1/100 or 1/200 for respectively mice, rats, human and pigs. In the literature, many data can be found on endotoxemia in mice and human but less in rats and pigs. Different methods to measure endotoxemia are reported and only scarce information is given sample preparation procedure. Moreover, the dilution factor is very different according to the manufacturer protocol. For example in mice, endotoxemia is measured in samples diluted at 1/40 to 1/80 using an end-point test (Cambrex, test sensitivity of 0.05 EU/mL) and the valued are between 6 to 10 EU/mL (3), which is higher than our data (0.95 EU/mL). In another study endotoxemia is similar to our data (0.6 to 0.9 EU/mL) but the samples were only diluted 1/5 to 1/10 and heated 10 min at 70°C (Lonza endpoint kits) and no other information on samples preparation was provided [27]. In another study mice plasma were not diluted, endotoxemia was around 0.8 EU/mL (Lonza endpoint kit) but no information on the preparation was provided [28]. The same is observed in humans: only some authors described their procedure to prepare sample, and a study led by Clemente-Postigo et al. [29] did not dilute human plasma with a Lonza kit contrary to Erridge et al. [30] which diluted the samples at 1/10 (as us) with a Cambrex kit. However in these 2 studies endotoxemia values were around 0.8 EU/mL like in our experiments using a Cape Cod kit. These data reinforce the fact that in human there are fewer factors that interfere with the LAL test than in other mammalian species tested. Only in one study with healthy humans, a particularly high dilution factor of 1/2000 was used, which increased the detection limit to 9 EU/mL [31].
It was interesting to compare several dilution factors in pigs because only few data were found in the literature and to our knowledge endotoxemia was not measured with a kinetic chromogenic LAL assay. We observed the necessity for a high dilution factor in pigs. However it is not advised to use a dilution factor >1/200 because it would multiply the error factor by sample and increase the sensitivity threshold to greater than 1 EU/mL. Moreover our data were in accordance with one of the only paper found in pigs where endotoxemia were around 8 EU/ mL, the LAL test used was an end-point method from Lonza and the samples were diluted at 1/1000 [32].
Altogether, the LAL test is a very sensitive assay. This was recently confirmed by Gnauck et al. (17) showing that the LAL test may be sometimes unsuitable to measure human endotoxemia if no method to overcome sequestration by plasma components is found. As proposed by Gnauck et al. and our previous studies [33,34], endotoxemia analysis can be completed by measuring the concentrations of plasma LPS receptor and transporter, namely sCD14 and LBP. The endotoxin metabolic pathway then includes the binding of LPS to LPS-binding protein (LBP) and its transfer to the receptor CD-14. Two forms of CD14 have been characterized, a membrane-bound glycoprotein (mCD14) and a circulating soluble form (sCD14) [35]. LBP and sCD14 can be considered as relevant markers of endotoxins in plasma [36]. In this respect, given the long half-lives of sCD14 and LBP (24-48 h) compared with endotoxin (from <8 min in mice to a maximum of 3 hours in humans), sCD14 and plasma LBP seem to reflect long-term exposure to endotoxin rather than the measurement of endotoxemia itself [37,38], which is more reliable to measure transient kinetics of endotoxin absorption [4,30]. We thus advise to measure others markers as LBP and sCD14 to complete endotoxemia [33,39,40]. Moreover, recently developed alternative assays based on ELISA or recombinant technology for measuring endotoxin concentration (rather that activity) in plasma were developed. Indeed, some recent studies used ELISA or EndoZyme® recombinant Factor C (rFC) Assay (Hyglos, Germany) to measure endotoxemia. However, these tests were used up to date to detect endotoxin in water samples or in HIVpatients [41] but no information was provided about the sensitivity of the technique for a metabolic study where expected LPS levels are very low. These assay are innovative and open new perspectives but should now be tested in nutritional studies before being used routinely.
Finally, we must highlight that endotoxemia analysis reflects the activity of LPS in a sample. However, numerous LPS species exist with various activities according to their molecular structure, the latter being related to the structure of their Lipid A moiety and polysaccharide chain [42,43]. Consequently, an increased endotoxemia in plasma can be due either to a higher number of LPS molecules, or to a similar number of LPS with higher activity due to a different structure. Therefore, the biochemical analysis of LPS molecular composition in plasma samples should now be more used, complementary to the LAL test with optimal dilution, in order to decipher in more details the role of LPS in metabolic inflammation. This approach has proved efficiency in infectious conditions where septic LPS concentrations are found in plasma [44], but its consistency in the field of low-grade metabolic endotoxemia still deserves to be studied.
Conclusion
Altogether, our work shows that the dilution factor is an essential parameter for measuring endotoxemia in plasma and is dependent on the mammalian species. This test permits to compare endotoxemia within a same species using a single optimal dilution independently of the nutritional state, i.e. fasting, postprandial and/or after different diets.
Acknowledgements
We gratefully thank for the sample preparations: (i) in human, Jocelyne Peyrat, Christine Maitrepierre, Naura Torche (CRNH Rhône-Alpes), [15] in pigs, Jean-Paul Lallès (INRA UR1341 ADNC), (iii) in rodents, Bérengère Benoit, Manar Awada and Alain Géloën (CarMeN laboratory). We thank Christine Curat (Associates of Cape Cod) for her advices on the LAL assay procedure. Marie-Françoise Becle, Marie-Claire Troncy-Bresse, Dominique Goullet and Jean Doucet (Hospices Civils de Lyon) are greatly acknowledged for providing our initiation to endotoxemia analysis. Robert Ward (Utah State University) is acknowledged for scientific comments and editing the English language. We thank Françoise Hullin-Mastuda and Noël Peretti (CarMeN laboratory) for fruitful discussions and useful comments on the manuscript.
References
- Fritsche KL (2015) The science of Fatty acids and inflammation. AdvNutr 6: 293S-301S.
- Herieka M, Erridge C (2014) High-fat meal induced postprandial inflammation. MolNutr Food Res 58: 136-146.
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, et al. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761-1772.
- Laugerette F, Vors C, Géloën A, Chauvin MA, Soulage C, et al. (2011) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J NutrBiochem 22: 53-59.
- Levin J, Tomasulo PA, Oser RS (1970) Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med 75: 903-911.
- Levin J, Bang Fb (1964) A description of cellular coagulation in the limulus. Bull Johns Hopkins Hosp 115: 337-345.
- Roth RI, Levin FC, Levin J (1990) Optimization of detection of bacterial endotoxin in plasma with the Limulus test. J Lab Clin Med 116: 153-161.
- Levin J, Bang FB (1968) Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. ThrombDiathHaemorrh 19: 186-197.
- Iwanaga S, Morita T, Harada T, Nakamura S, Niwa M, et al. (1978) Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis 7: 183-188.
- Nachum R, Berzofsky RN (1985) Chromogenic Limulus amoebocyte lysate assay for rapid detection of gram-negative bacteriuria. J ClinMicrobiol 21: 759-763.
- Bos NA, Meeuwsen CG, Visser H, Benner R (1988) Clonal analysis of the synergistic mitogenic effect of lipopolysaccharide and dextran sulphate on B cell activation, growth, and differentiation into Ig-secreting cells. Immunobiology 176: 301-312.
- Czop JK, Puglisi AV, Miorandi DZ, Austen KF (1988) Perturbation of beta-glucan receptors on human neutrophils initiates phagocytosis and leukotriene B4 production. J Immunol 141: 3170-3176.
- Netto EM, Marsden PD, Llanos-Cuentas EA, Costa JM, Cuba CC, et al. (1990) Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with Glucantime. Trans R Soc Trop Med Hyg 84: 367-370.
- Moore JN, Cook JA, Morris DD, Halushka PV, Wise WC, et al. (1990) Endotoxin-induced procoagulant activity, eicosanoid synthesis, and tumor necrosis factor production by rat peritoneal macrophages: effect of endotoxin tolerance and glucan. Circ Shock 3:281-295.
- Yajima Y, Fukuda I, Otsuki M, Suzuki H, Ota S, et al. (1985) Endotoxemia in liver diseases: detection by a quantitative assay using chromogenic substrate with perchloric acid pretreatment. Tohoku J Exp Med 147: 411-419.
- KomuroT, Murai T, Kawasaki H (1987) Effect of sonication on the dispersion state of lipopolysaccharide and its pyrogenicity in rabbits. Chem Pharm Bull (Tokyo) 35: 4946-4952.
- Gnauck A, Lentle RG, Kruger MC (2015) The Limulus Amebocyte Lysate assay may be unsuitable for detecting endotoxin in blood of healthy female subjects. J Immunol Methods 416: 146-156.
- Roslansky PF, Dawson ME, Novitsky TJ (1991) Plastics, endotoxins, and the Limulus amebocyte lysate test. J ParenterSciTechnol 45: 83-87.
- Roslansky PF, Novitsky TJ (1991) Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans. J ClinMicrobiol 29: 2477-2483.
- Sullivan JD Jr, Watson SW (1974) Factors affecting the sensitivity of Limulus lysate. ApplMicrobiol 28: 1023-1026.
- Redl H, Bahrami S, Leichtfried G, Schlag G (1992) Special collection and storage tubes for blood endotoxin and cytokine measurements. ClinChem 38: 764-765.
- Sturk A, Joop K, ten Cate JW, Thomas LL (1985) Optimalization of a chromogenic assay for endotoxin in blood. ProgClinBiol Res 189: 117-137.
- DuBose D, Lemaire M, Brown J, Wolfe D, Hamlet M (1978) Survey for positive Limulus amoebocyte lysate test in plasma from humans and common research animals. J ClinMicrobiol 7: 139-141.
- Newhall KJ, Diemer GS, Leshinsky N, Kerkof K, Chute HT, et al. (2010) Evidence for endotoxin contamination in plastic Na+-heparin blood collection tube lots. ClinChem 56: 1483-1491.
- Merrifield CA, Lewis M, Claus SP, Beckonert OP, Dumas ME, et al. (2011) A metabolic system-wide characterisation of the pig: a model for human physiology. MolBiosyst 7: 2577-2588.
- Tachiyama G, Sakon M, Kambayashi J, Ohshiro T, Mori T (1986) Chromogenic assay of endotoxin in platelet poor or rich plasma. Thromb Res 41: 309-317.
- Gu Y, Yu S, Park JY, Harvatine K, Lambert JD (2014) Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J NutrBiochem 25: 439-445.
- Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E (2009) Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 50: 90-97.
- Clemente-Postigo M, Queipo-Ortuno MI, Murri M, Boto-Ordonez M, Perez-Martinez P, et al. (2012) Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res 53:973-978.
- Erridge C, Attina T, Spickett CM, Webb DJ (2007) A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J ClinNutr 86: 1286-1292.
- Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, et al. (2008) Energy intake is associated with endotoxemia in apparently healthy men. Am J ClinNutr 87: 1219-1223.
- Mani V, Harris AJ, Keating AF, Weber TE, Dekkers JC, et al. (2013) Intestinal integrity, endotoxin transport and detoxification in pigs divergently selected for residual feed intake. J AnimSci 91: 2141-2150.
- Laugerette F, Furet JP, Debard C, Daira P, Loizon E, et al. (2012) Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am J PhysiolEndocrinolMetab 302: E374-386.
- Laugerette F, Alligier M, Bastard JP, Drai J, Chanséaume E, et al. (2014) Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. MolNutr Food Res 58: 1513-1518.
- Hailman E, Lichenstein HS, Wurfel MM, Miller DS, Johnson DA, et al. (1994) Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 179: 269-277.
- Hiki N, Berger D, Dentener MA, Mimura Y, Buurman WA, Prigl C, et al. (1999) Changes in endotoxin-binding proteins during major elective surgery: important role for soluble CD14 in regulation of biological activity of systemic endotoxin. ClinDiagn Lab Immunol6:844-850.
- Matsushita H, Ohta S, Shiraishi H, Suzuki S, Arima K, et al. (2010) Endotoxin tolerance attenuates airway allergic inflammation in model mice by suppression of the T-cell stimulatory effect of dendritic cells. Intimmunol22:739-747.
- Rojo OP, Roman ALS, Arbizu EA, Martinez AH, Sevillano ER, et al. (2007) Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm Bowel Dis 13:269-277.
- Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, et al. (2013) Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One 8: e54600.
- Sun L, Yu Z, Ye X, Zou S, Li H, et al. (2010) A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care 33: 1925-1932.
- Jenabian MA, El-Far M, Vyboh K, Kema I, Costiniuk CT, et al. (2015) Immunosuppressive Tryptophan Catabolism and Gut Mucosal Dysfunction Following Early HIV Infection. J Infect Dis (in press).
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, et al. (1994) Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J 8: 217-225.
- Caroff M, Karibian D (2003) Structure of bacterial lipopolysaccharides. Carbohydr Res 338: 2431-2447.
- Barros JPP, Gautier T, Sali W, Adrie C, Choubley H, et al. (2015) Quantitative lipopolysaccharide analysis using liquid chromatography-tandem mass spectrometry and its combination with the limulus amebocyte lysate assay. J Lipid Res (in press).
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15496
- [From(publication date):
August-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 10860
- PDF downloads : 4636