Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Editorial Open Access
Endometrial Signaling Pathways: A Redundant System for Continuous Nest Rebuilding
| Fernando M. Reis1,3*, Débora M. Morsch2,3, Márcia M. Carneiro1, Carolina P. Rezende1, Sheila Lecke2 and Poli M. Spritzer2,3 | |
| 1Department of Obstetrics and Gynecology, University of Minas Gerais, Belo Horizonte, Brazil | |
| 2Laboratory of Molecular Endocrinology, Department of Physiology, and Gynecological Endocrinology Unit, University of Rio Grande do Sul, Porto Alegre, Brazil | |
| 3National Institute of Hormones and Women’s Health, Porto Alegre, Brazil | |
| *Corresponding Author : | Fernando M. Reis Department of Obstetrics and Gynecology University of Minas Gerais, Belo Horizonte, Brazil E-mail: reis@medicina.ufmg.br |
| Received October 26, 2012; Accepted October 26, 2012; Published October 29, 2012 | |
| Citation: Reis FM, Morsch DM, Carneiro MM, Rezende CP, Lecke S, et al. (2012) Endometrial Signaling Pathways: A Redundant System for Continuous Nest Rebuilding. Biochem Physiol 1:e112. doi:10.4172/2168-9652.1000e112 | |
| Copyright: © 2012 Reis FM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
| The endometrium is the uterine mucosa, a dynamic tissue that undergoes cyclic growth, differentiation and regression under the action of the ovarian steroids, namely estrogen and progesterone. This unique process of tissue plasticity serves essentially to prepare the uterus for embryo implantation and pregnancy. |
| During the reproductive years, the endometrium cycles through two phases: the preovulatory/proliferative phase, controlled by estrogen, and the postovulatory/secretory phase, regulated both by estrogen and progesterone. At the end of each cycle, the withdrawal of progesterone from an estrogen-progesterone primed endometrium initiates the cascade of molecular and cellular interactions that result in endometrial shedding (menstruation). |
| During the proliferative phase, the endometrium grows in response to the increasing levels of estradiol, in a process that involves not only direct stimulation of mitosis but also inhibition of apoptosis [1], along with increased vascular permeability, giving more access to circulating growth factors [2]. The glands and the stroma proliferate, the endometrial lining thickens and the estrogen receptor content also increases. After ovulation, secretory events ensue in the estrogenprimed endometrium. The glands become tortuous with distended lumina, the stroma augments its edema and the spiral vessels become intensely coiled, indicating the beginning of decidualization. The process of decidualization continues with the stromal cells near the spiral arteries becoming eosinophilic, enlarged and round shaped, creating a dense cellular matrix that favors embryo attachment and restricts trophoblast invasion [3]. |
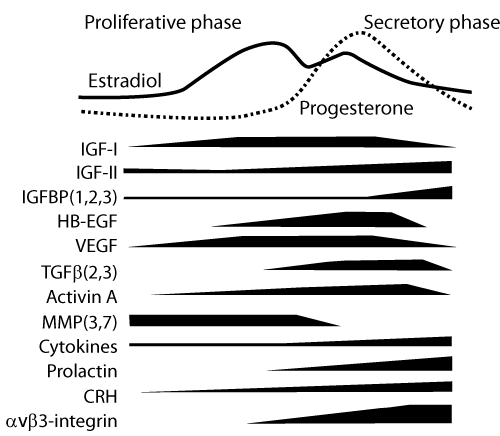
| While it seems clear that both estrogen and progesterone are major elements in the endometrial cycle, their biological effects are constantly modulated by the availability of their specific nuclear receptors and also mediated by locally produced regulatory proteins, such as growth factors, cytokines and enzymes (Figure 1). The existence of so many local effectors indicates that the net result of estradiol and progesterone stimulation depends on the fine-tuning played by endometrial products. |
| Among the paracrine mediators of progesterone action preparing the endometrium for embryo implantation, some growth factors (EGF, HB-EGF, VEGF, TGFÃ?Â?), cytokines (IL-1, LIF) and the cell surface receptor avÃ?Â?3-integrin appear to play critical roles [2,4]. However, synergic and possibly superimposed effects of many paracrine factors driven by progesterone ultimately converge to promote endometrial decidualization. In vitro experiments with isolated endometrial stromal cells have shown that HB-EGF [5], activin A [6] and corticotropinreleasing hormone [7] are able to induce and accelerate the process of cell decidualization. |
| The last phase of menstrual cycle, characterized by progesterone withdrawal, is not simply a passive interruption of endometrial quiescence leading to tissue breakdown. It is rather an active process of tissue remodeling, i.e., breakdown followed by regeneration, and involves local signaling through prostaglandins, proteases, cytokines, chemokines and angiogenic factors [8]. |
| Much about endometrial physiology has been discovered in the past few decades, but the exact mechanism through which local hormones and other regulatory substances orchestrate endometrial growth and differentiation and prepare the nest to embryo implantation has not been unraveled yet. It seems likely that molecular derangements that disrupt endometrial cycle are involved in endometrial pathology. As we gain more knowledge on how these substances interact, new therapies for endometrial dysfunction associated with infertility, recurrent pregnancy loss, endometriosis and endometrial cancer may be developed. |
References
|
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13780
- [From(publication date):
December-2012 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 9414
- PDF downloads : 4366
