Research Article Open Access
Emulsification Properties of Bioemulsifiers Produced by Wild-Type and Mutant Bradyrhizobium elkanii Strains
| Erica Mendes Lopes1, Tereza Cristina Luque Castellane1, Cristiane Moretto1, Eliana Gertrudes de Macedo Lemos1 and Jackson A Marcondes de Souza2* | |
| 1Department of Technology, Faculty of Agricultural and Veterinary Sciences, UNESP - Univ Estadual Paulista, Brazil | |
| 2Department of Applied Biology for Agriculture, College of Agriculture and Veterinary Sciences, UNESP - Univ Estadual Paulista, Brazil | |
| Corresponding Author : | Jackson A. Marcondes de Souza Department of Applied Biology for Agriculture College of Agriculture and Veterinary Sciences UNESP - Univ Estadual Paulista, Brazil Tel: +55-16-32092675 (extension 217) Fax: +55-16-32092675 E-mail: jackson@fcav.unesp.br |
| Received August 07, 2014; Accepted September 20, 2014; Published September 22, 2014 | |
| Citation: Lopes EM, Castellane TCL, Moretto C, Lemos EGM, Souza JAM (2014) Emulsification Properties of Bioemulsifiers Produced by Wild-Type and Mutant Bradyrhizobium elkanii Strains. J Bioremed Biodeg 5: 245. doi:10.4172/2155-6199.1000245 | |
| Copyright: © 2014 Lopes EM, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The use of biosurfactant is a promising alternative over the chemical surfactant as they are better biodegradable and do not pollute the environment. To date, there is little information on the biosurfactant producing microorganisms belonging to different Rhizobium and Bradyrhizobium species. In this study, the emulsifying properties of both culture media and a substantially cell-free medium from bacterial cell cultures in which B. elkanii SEMIA 587 or mutant strains cultivated in defined media supplemented with sucrose were determined by measuring the emulsifying activity, which determined the ability of biosurfactant in forming oil-water emulsion, and emulsifying index (E24), which determined the capacity of surfactant in forming emulsions on different hydrophobic substrates (soybean oil, sunflower oil or diesel oil). The performance of strains as bio emulsifier were very contrasting as shown by their specific emulsification indexes with different substrates, beginning at 24 hours. Regarding emulsifying index for cells, best result of E24 was obtained by using sunflower oil for wild type and mutants with exception for 587::TnphoA31 that witch got better results in soybean oil. However, analysing using cell-free medium have shown higher E24 values mostly in soybean oil except for 587::TnphoA31 and 587::TnphoA50 which have performed better in diesel oil. This finding indicates a probable production of bioemulsificants that adhere to the cell wall of this bacterium and are extracellular. The same E24 value (79.17%) was observed in the case of soybean oil with culture medium from both the wild-type and the mutant strain 587::TnphoA50. The emulsification capacity was very sensitive to the temperature, pH and NaCl concentration changes. These results demonstrate that the Bradyrhizobium strains could be attractive for use in biosurfactants applications, these products is great due to growing demand for biodegradable and environmentally friendly analogues for synthetic chemicals.
| Keywords |
| Bioemulsifier; Bradyrhizobium elkanii; Emulsifying activity; Hydrophobic substrate |
| Abbreviations |
| Exopolysaccharide; E24: Emulsification Index; TY: Tryptone Yeast; YMA: Yeast Mannitol Agar; RDM: Defined Medium for Rhizobia |
| Introduction |
| Biosurfactants were first discovered as extracellular amphiphilic compounds of fermentation bacteria [1]. Initial interest in these compounds was due to their ability to increase the solubility of insoluble or poorly soluble hydrocarbons. However, the increased popularity of using renewable resources in industry (especially in food and pharmaceutical industries) has led to increased interest in identifying and applying the use of natural surfactants, mainly biosurfactants [2]. |
| During the last decade, biosurfactants have been investigated as potential replacements for synthetic surfactants and are expected to have many potential industrial and environmental applications related to emulsification, foaming, detergency, wetting, dispersion and solubilization of hydrophobic compounds [3,4]. Among the various potential replacements for synthetic surfactants, biosurfactants, bioemulsifiers and exopolysaccharides are attracting interest and attention due to their structural and functional diversity [5]. Some investigations showed that surface activity of biosurfactants is comparable with surface activity of synthetic surfactants. Due to their physicochemical properties, low toxicity, excellent surface activity, high specificity, effectiveness under extreme conditions and biodegradability, bioemulsifiers and biosurfactants are widely applied in environmental protection techniques, e.g., water and soil remediation, oil spill removal, etc. [6,7]. These properties of bioemulsifiers and biosurfactants reflect their potential for commercial applications [8]. |
| Bioemulsifiers are microbiological in origin and appear to be made either by the cells themselves or by the production of extracellular emulsifying agents [9]. The outer structure of many bacteria is surrounded by extracellular polysaccharides (EPSs) that occur either as discrete capsules or a thick slime layer. A wide variety of microorganisms present this structure, such as Aeribacillus pallidus [10] species of Candida [11], Pseudomonas and Bacillus [12]. |
| Microorganisms producing biosurfactants can be effectively used for the removal of hydrocarbons as well as heavy metals [13]. As biosurfactants are known to enhance bioavailability and carry out biodegradation of hydrophobic compounds, different technologies, such as soil washing technology and clean up combined technology, employ biosurfactants for the effective removal of hydrocarbons and metals, respectively [14,15]. These biosurfactants can be widely exploited in agricultural areas for the enhancement of biodegradation of pollutants to improve the quality of agriculture soil, for indirect plant growth promotion through antimicrobial activity and to increase the plant-microbe interaction that is beneficial for plants [16]. |
| To date, more than 225 patents are available describing these microbial amphiphilic agents, e.g., Emulsan [17], have been extensively characterized and reached commercial applications, chiefly in the bioremediation sector. To our knowledge, there are few reports in the literature on the production of biosurfactants, bioemulsifiers, the physicochemical properties of purified EPSs or exopolysaccharides and removal of heavy metals from effluents by Bradyrhizobium spp. in industrial applications. Because Brazil’s soybean fields lacked native bacteria able to nodulate legumes, the production of inoculants in this country started in the 1960s with North American strains [18]. Presently, the B. elkanii strain SEMIA 587 [19] combines the advantages found in other strains and is currently recommended (authorized) for the production of commercial rhizobial inoculant for soybean [Glycine max (L.) Merr.] production in Brazil, and this has resulted in an established population in most soils cropped with soybean. Due to the ecological and environmental role of this species, the economy and efficiency of inoculants and their massive usage in soybean crops, B. elkanii has been characterized for its emulsifying properties. Strains with intensive extracellular polysaccharide production tend to have the ability of free-living N2-fixation just like facultative anaerobes [20]. The EPS has been precipitated from cell-free supernatants using alcohol [21,22] and was found to contain carbohydrate, protein, uronic acid and sulfate. Reports published on the production of exopolymers show that the hydroaromatic acids shikimate and quinate, which may be available as carbon sources in the soil, supported production of only low levels of acidic exopolysaccharide by B. elkanii and B. japonicum [23]. Strains BR 29 and SEMIA 587 of B. elkanii produce greater amounts of EPS when grown in acidic conditions than when cultivated at a neutral pH [24]. However, previous results in our laboratory demonstrated that the culture medium of B. elkanii SEMIA 587 and mutant strains was unable to precipitate EPS using alcohol, although we observed biopolymers in the supernatant. As these exopolymers are important for microbial interaction and emulsification of various hydrophobic substrates, this study specifically addresses the capacity of culture media and a substantially cell-free medium from a bacterial cell culture to stabilize emulsions with several hydrophobic compounds. |
| Materials and Methods |
| Bacterial strains and growth conditions |
| The wild-type strain of B. elkanii SEMIA 587 and mutant (587::TnphoA24, 587::TnphoA31, 587::TnphoA33 and 587::TnphoA50) strains were used in the present study. In previous work with our collaboration laboratory, mutagenesis were performed according Herrero et al. [25] through TnphoA transposon in TY medium (5 g L-1 tryptone, 3 g L-1 yeast extract, and 10 mM CaCl2), supplemented with the antibiotics streptomycin (SEMIA587 marker) and kanamycin (TnphoA marker). These plates were kept at 28°C for sevfen days. All transconjugants colonies were stored in 20% glycerol at -80°C. |
| For routine Bradyrhizobium growth, tryptone yeast (TY) medium [26] was used. When required, the medium was supplemented with the antibiotic kanamycin. The mutants were cultivated in YMA medium (0.4 g L-1 yeast extract, 10 g L-1 mannitol, 0.5 g L-1 K2HPO4, 0.2 g L-1 MgSO4, and 0.1 g L-1 NaCl, 9 g L-1 agar, pH 7.0) supplemented with Congo Red (25 μg mL-1) to verify the purity of each mutant culture. The cultures were incubated at 30°C for 24 h. |
| For comparative analyses of the emulsifying properties between the Bradyrhizobium strains, pre-inocula and batch experiments were performed using a defined liquid medium (0.23 g L-1 K2HPO4, 0.1 g L-1 MgSO4, and 1.1 g L-1 C5H8NNaO4, pH 6.8) [27] with modification, containing sucrose (10 g L-1), as a carbon source. The cultures were incubated at 30°C in a gyratory shaker at 150 rpm for 10 days. |
| Morphological characterization and EPS detection |
| Plates containing Congo red or Calcofluor white were used to examine EPS production qualitatively [22,28] and morphological characterization comparisons. Briefly, 10 μL of a cell suspension with an optical density at 600 nm (OD600) of 0.5 was spotted onto RDM plates supplemented with either Congo red (25 μg mL-1) or a fluorescent brightener 28 (calcofluor white; Sigma-Aldrich) with an emission wavelength of 430 nm at a final concentration of 200 μg mL-1. This fluorescent pigment is specific to polysaccharides that contain β-1→4 or β-1→3 linkages [29]. These plates were incubated at 30°C for 10 days before documentation. The mucoidy of the colonies and EPS detection were determined visually. |
| Calcofluor white plates were examined under long-wave UV (430 nm) for fluorescence, which is indicative of dye binding and EPS production. All strains were compared with the MUTZC3 mutant strains of R. tropici [22], the positive control strain, that produce higher amounts of calcofluor-fluorescent exopolysaccharide under the same growth conditions. |
| Emulsifying activity |
| Emulsification activity and stability studies were performed using the culture medium and cell-free broth obtained from the centrifugation of cultures at 15,000 x g for 40 min. After centrifugation, the cell-free culture broth was cooled at –20°C and the emulsification activity was measured. The capacity of culture media and a substantially cellfree medium from a bacterial culture to stabilize emulsions containing several hydrophobic compounds was tested as described by Freitas et al. [30] with modifications. Briefly, the emulsification index of a cell-free supernatant and culture medium were mixed with each hydrophobic compound (2:3, v/v ratio) and stirred using a vortex. The mixture was mixed vigorously for 2 min and allowed to stand for 24, 48, 72 and 96 h at room temperature. The emulsification index was measured using the following equation and noted as E24, E48, E72 and E96, respectively: E24 = (he/hT) × 100; where he (mm) is the height of the emulsion layer, and hT (mm) is the overall height of the mixture. Emulsifying activity was expressed as the percentage of the total height occupied by the emulsion. All tests were performed in triplicate. The tested compounds included hydrocarbon (diesel oil) and soybean and sunflower oils purchased at the local supermarket. |
| The emulsions were observed under a light microscope to determine the size and uniformity of the drops in the oil phase. |
| Stability studies |
| Thermal Stability: To determine the effect of temperature and heat treatment on the emulsifying activity, the cell free broth was mixed with soybean oil (2:3, v/v ratio), then mixed vigorously for 2 min and allowed to stand for 24 h at room temperature and 37°C. In additional, the cell free broth was heated in autoclave to 120°C for 20 min and allowed to cool to room temperature. The emulsification capacity was measured and compared to the corresponding values before heat treatment. |
| Saline Stability: To investigate the salt tolerance on the emulsification capacity of the culture broth free of cells was also determined. This study used NaCl solutions with concentrations of 1, 10, 20 and 30% salt. The culture broth free of cell was divided into samples and each NaCl solution was mixed well with sample culture broth free of cells before addition of soybean oil at room temperature (30°C). After mixing, each concentration was left standing for 24 h before E24 was measured. |
| pH Stability: To study pH stability, a procedure was carried out to obtain emulsions at a broad pH range. The pH of a culture broth free of cell was adjusted to different pH values (2, 3, 4, 5, 6, 7, 8, 9 and 10) by the addition of 1 M HCl or 1 M NaOH, and gentle homogenization. After 1 h, emulsions prepared at different pH values, according to this procedure, were placed at 30°C. The height of emulsion layer was measured after 24 h to determine the emulsification index. |
| Results and Discussion |
| Detection of EPS and morphological characterization comparisons between wild-type and mutant strains of B. elkani SEMIA 587 |
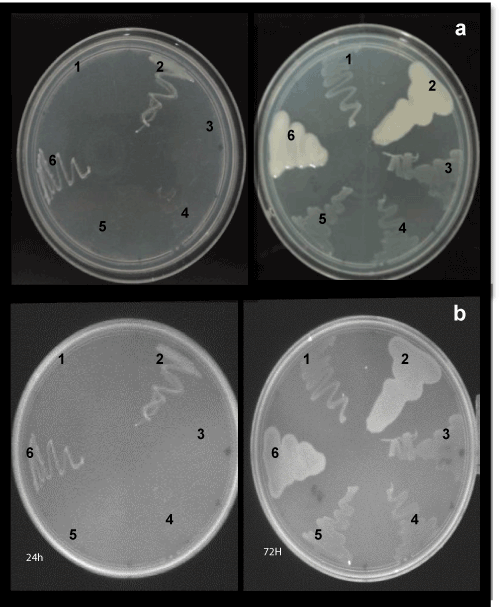
| The culture was first examined for purity microscopically. The results indicated that the culture was pure, and slower growing Bradyrhizobium strains. On solid growth medium RDM, the Bradyrhizobium strains produced smaller colonies, usually only 2 mm in diameter, and mucoid surface appearance compared to the smooth, larger the positive control (MUTZC3 mutant strain of R. tropici) colonies, after 72 hours, abundant after 10 days of incubation (Figures 1 and 2). Only one mutant (587::TnphoA50) of strain SEMIA 587 showed little difference in colony morphology (Figure 1e). Charoen et al. [31] noted an increasing tendency of Bradyrhizobium isolates to produce mucus as a reflection of the adaptation to the acid soils of the Brazilian tropical Savannah known as Cerrado. |
| Previous experience with Bradyrhizobium elkanii SEMIA587 in our laboratory have shown that this strain was the only one that never produced significant amounts of EPS in media, e.g., RDM and PSYL medium. Furthermore, when plated onto solid medium RDM containing Congo red, the strains of Bradyrhizobium produced translucent or cream-colored colonies (results not shown). Therefore, the presence of a translucent or creamy material involving a mucoid colony is indicative of the production of copious quantities of capsular exopolysaccharides in RDM medium. |
| The colonies were then grown on RDM containing the fluorescence brightener 28 (calcofluor) and observed by UV illumination at 430 nm (Figure 2). The aspect of the colonies was observed after 24 and 72 h of culture and after 10 days, and the EPS formation was measured. No differences in the morphological phenotypes were observed between the wild-type and the mutant strains of B. elkani. In all the cases, no significant EPS production was observed. By comparison, those strains showed less affinity to calcofluor white than that performed by MUTZC3 mutant strain of R. tropici cells (positive control). In turn, these results have indicated a lower level of exopolysaccharide production when grown on RDM containing calcofluor. |
| Emulsifying potential |
| In the present study, we examined the emulsifying activity of both culture media and a substantially cell-free medium from bacterial cell cultures in which B. elkanii SEMIA 587 or mutant strains had been grown using soybean oil, sunflower oil or diesel oil as a hydrocarbon. The ability of the culture medium and cell-free broth to emulsify and stabilize oil-in-water emulsions is shown in Table 1. For comparison, the same test procedure was performed with medium without inoculation. The culture medium and cell-free broth produced emulsions in which the oil phase drops were small and uniform in size, below the threshold of 100 μm established for fine emulsions (results not shown). |
| The good emulsification property is critical for biosurfactants to be promising in different environmental and industrial applications [3]. The criteria for determining the emulsion-stabilizing capacity of an emulsifier consists of evaluating its ability to maintain at least 50% of the original emulsion volume 24 h after its formation [32]. Considering this, the wild-type strain of B. elkanii SEMIA 587 possessed emulsionstabilizing capacity only for soybean oil, as shown by its emulsification index (E24) higher than 50%, with values of 65.22%. In contrast, all mutant (587::TnphoA24, 587::TnphoA31, 587::TnphoA33 and 587::TnphoA50) strains displayed lower emulsification index values for soybean oil, sunflower oil and diesel oil, except the 587::TnphoA50 strain with soybean oil (47.83%) (Table 1). Both the cell-free supernatant of wild-type and mutant strains showed low levels for diesel. Diesel oil was not emulsified effectively. |
| Based on Table 1, the E24 of cell-free medium from a bacterial cell culture in which mutant strains 587::TnphoA24 and 587::TnphoA31 had been grown was higher in soybean oil (88.64% and 72.34%, respectively) followed by the 587::TnphoA24 and 587::TnphoA31 strains, which had the highest emulsification indices observed for diesel oil (71.74% and 74.42%, respectively). The E24 of a bacterial cell culture from wild-type strain SEMIA 587 was highest for sunflower oil (95%), followed by the two mutant strains 587::TnphoA33 and 587::TnphoA50 (87.50%, both strains). This finding indicates a probable production of bioemulsificants that adhere to the cell wall of this bacterium and are extracellular. In general, bacteria are able to produce low molecular weight molecules such as glycolipids, lipopeptides [33] associated to efficient reduction of surface and interfacial tensions, as well as high molecular weight polymers that are efficient emulsifiers [34]. The same E24 value (79.17%) was observed in the case of soybean oil with culture medium from both the wild-type and the mutant strain 587::TnphoA50. Moreover, the emulsions of cell-free medium from a bacterial cell culture in which mutant strains had been grown were stable for several weeks, while the wild-type strain formed unstable emulsions that rapidly decomposed to water and oil after mixing within 72 h (data not shown). The emulsions of culture medium from all strains that had been grown were not stable after 24 h (Table 1). |
| If the specificity of the emulsifier is correct for the hydrocarbon, a murky "cream" will form that will remain stable over a period of minutes or, preferably, hours. The wild-type B. elkanii SEMIA 587 and mutant strains, however, produce different bioemulsifiers with different oils and hydrocarbon specificities for emulsification. |
| Effect of temperature, pH and NaCl on emulsifying activity of cell-free broth |
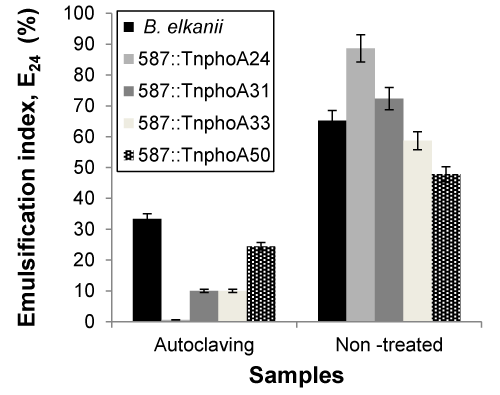
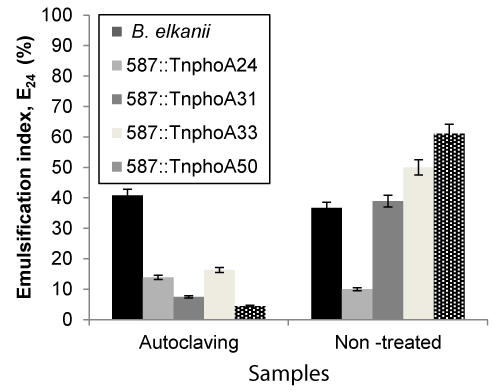
| The effects of these three key parameters that affect emulsifying activity were assessed on cell-free broth obtained by centrifuging the cultures at 15,000 x g for 40 min. All measurements were made at 24 h. In microbial-enhanced oil recovery processes, biosurfactants are used as coadjutants beside stable agents that are also required due the high temperature, pressure and salinity of the oil reservoirs [35]. As shown in Figures 3 and 4, samples were sterilised at high temperature 121°C, 20 min) and the capacity for emulsification were measured both prior and after sterilisation. The emulsification capacity were very sensitive to the temperature changes. The results showed that the samples were significantly different after sterilisation, representing that the high thermal stability was not observed on biosurfactant from B. elkanii strains. Asfora et al. [36] reported that the loss of emulsifying activity at 120°C can be explained by the denaturation on Microbactan protein fraction during heating [36], as seen with other microbial biosurfactants [37]. |
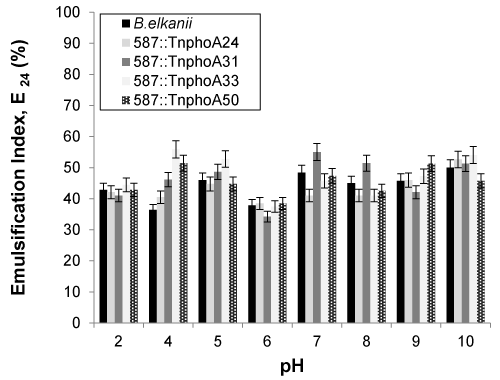
| The pH of the cell-free broth was varied from 2 to 10 to test the effect of pH on soybean oil emulsification capacity of the cell-free broth (Figure 5). An appreciable effect on activity was observed along the pH range, as it was observed that the emulsification activity for wild-type B. elkanii SEMIA 587 decreased at pH 4 and 6, whereas it was observed to increase at pH 10 for all oil emulsifications. For alkali initial pH values (8-10), a precipitate formed that interfered with emulsification activity and emulsifier stability determination as observed by other authors when they obtained a polymeric biosurfactant [38,39]. |
| An important property of bioemulsifiers is their effectiveness over a wide range of temperatures, pH values and salinities [3]. Previous studies proved that high-molecular-weight bioemulsifiers were always thermostable [2], as these bioemulsifiers exhibited the highest activity in the range of pH 9 to pH 12 and the activity was significantly inhibited under acidic conditions. In contrast, the bioemulsifier produced by Bacillus cereus was reported to be active only at a neutral pH [39]. Peralta-Yahya et al. [40] reported that the sharpest decrease in emulsification index occurred when the cell-free supernatant was adjusted to a pH value below 4.5, at which the minimum E24 value of 29.9% was achieved. |
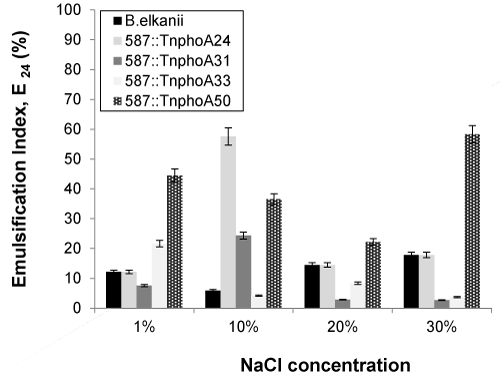
| The effect of added NaCl concentrations on soybean oil emulsification capacity of the cell-free broth is summarized in Figure 6. In the case of mutant (587::TnphoA24 and 587::TnphoA31) strains, an increase in emulsification activity was observed with the addition of up to 10% (w/v) sodium chloride to 57.58 and 24.32%, respectively. In contrast, wild-type SEMIA 587 did not display emulsion at a concentration of 10% (w/v) sodium chloride. In the case of 587::TnphoA33, a reduction of approximately 83% of the emulsification activity was observed with the addition of up to 30% (w/v) sodium chloride, showing a relative tolerance over these salt concentrations. Once the salinity decreases the viscosity, it is possible that the increase in NaCl concentration influenced the quality of the emulsion, thus reducing the emulsification capacity. Some literature reported that the emulsifying activity was significantly inhibited at a NaCl concentration greater than 50%. Liang et al. [41] reported that the structure of the biosurfactant molecule interacting with physicochemical factors, as salinity for example, was responsible by micelle formation ans shaping. By the way, the solubility of the biosurfactant could be affected decreasing its activity. Reductions in emulsification activity were also reported for other surfactants of microorganisms such as Candida lipolytica grown in n-hexadecane [42]. |
| Conclusion |
| In conclusion, this study clearly demonstrated that the culture medium and substantially cell-free medium from bacterial cell cultures of wild-type B. elkanii SEMIA 587 and mutant strains exhibited potential emulsifying properties for biotechnology applications that have promise for bioremediation. The bioemulsifiers of these mutant strains have the ability to emulsify a wide range of hydrocarbon and oils and to stabilize the emulsions once they have been formed. These strains likely represent many species, and among these, we can identify some new species with biotechnological and ecological agricultural importance. The significance of our research consists of their unique abilities in forming and/or stabilizing hydrocarbon-in-water emulsions, and these strains lend themselves to numerous and diverse applications. The B. elkanii SEMIA 587 and mutant strains have potential future applications such as thickening, emulsifying, dispersing or stabilizing agents, in addition to their use in sustainable economic processes. The findings of this study contribute significantly toward understanding applications of the use of the Bradyrhizobium spp. in environmental biotechnology and bioremediation. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15794
- [From(publication date):
November-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11065
- PDF downloads : 4729
