Emptying Mechanism of Perianal Glands of Ctenodactylus gundi
Received: 22-Nov-2017 / Accepted Date: 06-Mar-2018 / Published Date: 13-Mar-2018
Abstract
Previous studies on many mammalian species have confirmed the release of glandular secretion during scent marking behavior, however; the mechanism leading to extrusion of glandular exudates is still facing discrepancies by many authors in the field of ethology. Some investigators postulated that the secretion leaves the gland by replacement of older secretion by newly formed ones; others have always believed that scent glands must be squeezed somehow in order for the gland to release its secretory contents. No one however, showed evidence of such an active mechanism in any of the previously studied mammalian species. This study is intended to explain the exact mechanism causing the seeping of the secretion from the perianal glands during the scent marking behavior of mammals that adopt the act of anal and or hind quarters dragging. Skeletal muscles in the capsule and trabeculae of the perianal glands of Ctenodactylus gundi clearly indicate the active release of secretion from such glands voluntarily.
Keywords: Gundi; Perianal; Scent; Ethology; Anal dragging
Introduction
Cutaneous scent glands have been reported from 17 orders of the class mammalia, even though most of these organs are widely distributed on body surface, occupying more than 44 locations on different mammalian species, some are located within different body orifices such as the anal sacs of many carnivore species [1,2]; Different mammalian species processes different cutaneous scent glands, ranging from very tiny submicroscopic structures, to large well developed glands, very obvious to the naked eye [3]. Ctenodactylus gundi belongs to the order rodentia, the individuals of which are characterized by a wide range of scent marking behavior. Beavers have 2 primary scent structures, anal glands and castor sacs [4]. Anal gland secretion appears to serve as a family or individual identifier [1]. Castor fluid is the main source of chemical signals used for marking territories [5-7].
Although, rodent species have been reported to use their hind quarters in a number of social behaviors such as sand bathing, territorial marking, intra-specific identification, and mating rituals [1,8]; Literature is very poor concerning the behavioral role of scent glands of some rodent species, [4,9] have reported the lack of information on the morphology of the anal glands of a group of rodents (Hystricognathi ) that exhibit poorly understood social behavior. The behavioral and social interaction of these animals suggests the use of its eversible anal gland in communication between individuals; as far as Ctenodactylus gundi is concerned, most of the studies were descriptive, focusing mainly on the role of these animals as hosts for parasitic diseases. This study is intended to shed some light on the anatomical and histological structure of Ctenodactylus gundi perianal glands, with special reference to their emptying mechanism, since this issue was disputed by many authors in the field of ethology and animal communication.
Ctenodactylus gundi belongs to the family Ctenodactylidae, which comprises a group of small bulky gregarious rodents distributed from Morocco to Ethiopia along the rocky deserts across the Northern part of Africa. This species was first discovered in Libya in 1774 and were named “gundi mice” [10]. This fur bearing small mammal is considered one of economically important animal due to its destructive nature on crops, since they can eat almost every type of available plant.
Gundis are social animals living in colonies of up to a hundred or more individuals; they never build permanent dens, commonly sheltering in crevices of rocky terrain. They communicate through a low-pitched alert calls, which are carried well by the rocky terrain [10]. Other means of signals helping to bond the colony together are also evident, such as chemical signaling which is very well developed among these alert mammals. This is accomplished through the release of pheromones emitted from their perianal scent gland during certain behavioral rituals [9].
Literature has described the presence of perianal glands in a wide range of mammals; these glands are anatomically classified as bilobated or trilobated structures in their gross anatomy. Black-tailed prairie dogs have trilobted perianal glands [4,11]. Other mammals with trilobated perianal glands include several species of ground squirrels [12,13]. It has been reported that the number and arrangement of the secretory lobes in the glands of the aforementioned animals is identical, even-though the placement of the glands varies with the species. Ground squirrels for example have two lateral lobes and one ventral lobe [11]. Guinea pigs on the other hand have trilobated perianal scent glands the central one is located dorsal to the anal canal [1,5,11,14].
Although olfactory communication is believed to play a significant role in the sexual and social behavior of all rodent species [5,7,15], little information exists on scent marking behavior of Ctenodactylus species. Adult males and females of this species are well known for their hind quarters dragging during mating rituals. However, literature is very poor concerning the structure and function (anatomy, histology and histochemistry) of Ctenodacylus gundi perianal glands. Most studies were limited to geographical distribution, classification, and social activities such shelter marking, group numbers and territorialities.
Materials and methods
Healthy adult sexually mature male and female Gundi (Ctenodactylus gundi ) were trapped from different ecological niches in Libya. These animals were sacrificed, dissected, photographed, and investigated carefully for existence of possible scent glands. Two types of scent glands were found, photographed, and surgically removed. The glands were preserved in 10% formalin, labeled, processed and sectioned according to routine histological techniques.
Reference histological series of 6 μ thick sections were cut from paraffin wax embedded perianal glands of both males and females respectively. Sections were cut cross-sectionally to show the architectural arrangement of the secretory lobes around the circumference of the anal sphincter. Sections were stained with Harrie’s haemtoxylin and Eosin [16], using standard procedures. All sections processed in such a manner were examined microscopically, and carefully evaluated for detailed histological description of glands in hand. Special attention was given to the occurrence of muscular tissue in or out of these glands.
Results
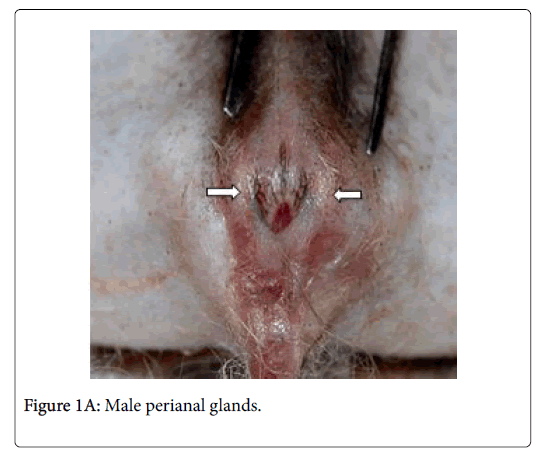
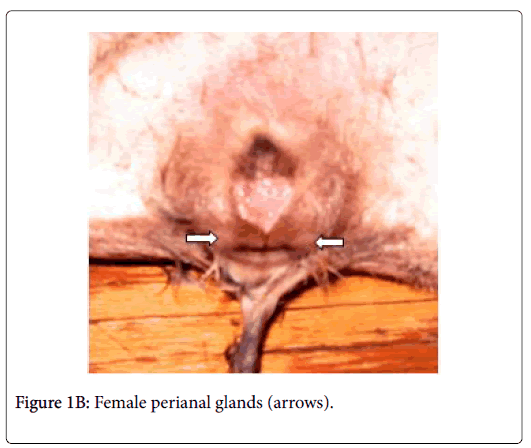
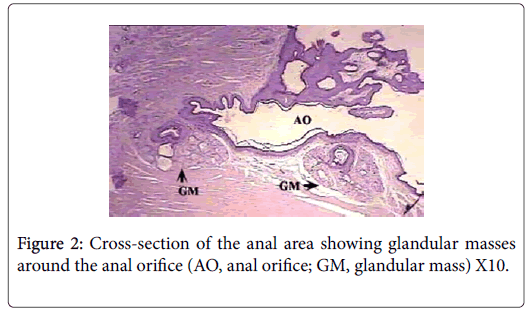
Anatomically, the perianal glands of Ctenodactylus gundi appear as a trilobated glandular mass distributed triangularly at the circumference of the anal orifice (Figure 1); Two large lobes are located at the inguinal region forming the base of the triangle, the third lobe is located at base of the tail. These glandular masses are embedded subcutaneously in the deep fascia of the dermis near the circumference of the anus (Figure 2). Each glandular mass opens on the skin surface through an independent opening (Figures 2 and 3).
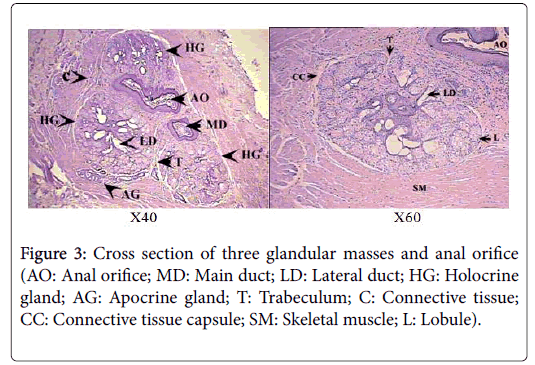
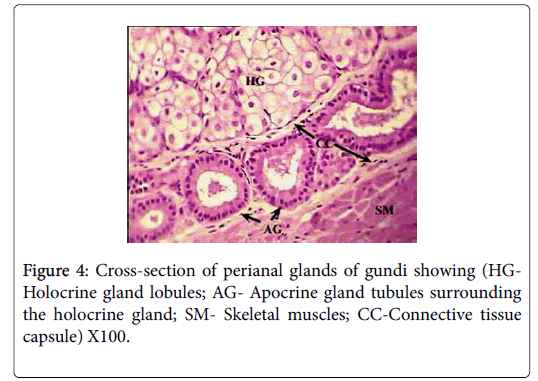
Histologically, each glandular mass represents a compound branched tubulo-alveoalr gland, composed of circumferential band of tubules surrounding a number of sebaceous lobes, giving the gland the appearance of a modified holocrine sebaceous enclosed by modified apocrine sweat gland (Figure 4). Each of the glandular masses is encapsulated by a thick band of dense irregular connective tissue, surrounded by another well-defined band of striated skeletal muscles.
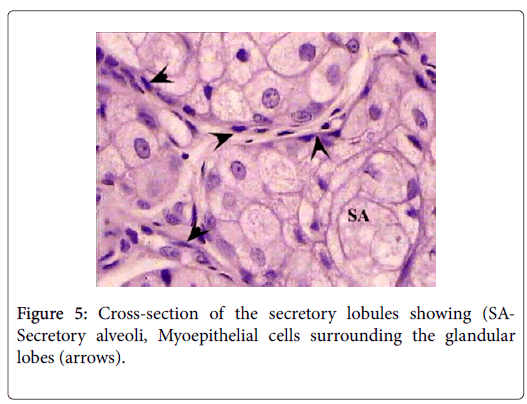
The sebaceous portion of the each perianal glandular mass of Ctenodactylus gundi is composed of three fairly large lobes surrounded by a thick capsule of connective tissue, Trabeculae arise from the connective tissue capsule dividing each sebaceous glandular portion into a number of lobes, each of which is further subdivide into a number of lobules, which intern consist of secretory alveoli (Figures 3 and 4). The parenchymal tissue of the lobules consists of epithelioid buds that will differentiate into secretory alveoli, and diffused myoepithelial cells arranged in layers of two cells thick, which encloses each secretory alveolus independently.
Holocrine breakdown of epithelial cells forming the secretory alveoli occurs in the center of the secretory alveoli, and proceeds toward the periphery emptying its contents in an alveolar duct. Alveolar ducts open directly into larger lobular ducts which intern conveys the sebaceous secretion into a main duct which leads to the surface of the skin surrounding the anal orifice (Figure 5).
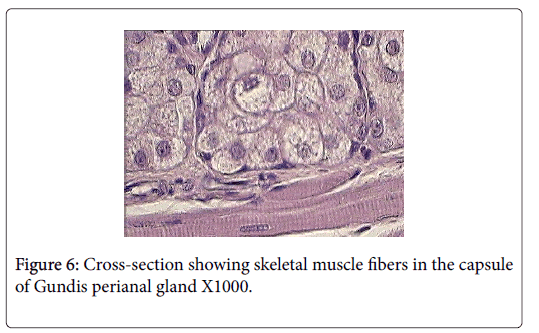
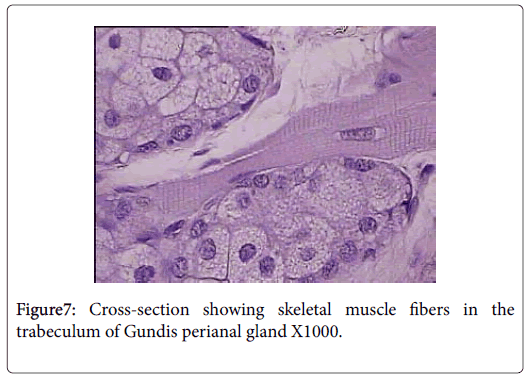
Skeletal muscle fibers are very evident in the capsule and trabeculae of Ctenodactylus perianal glands, this arrangement is also supported by the well pronounced appearance of myoepithelial cells surrounding the gland secretory components (Figures 6 and 7).
Discussion
Anatomically, perianal glands of Ctenodactylus gundi is similar to those found in Black-tailed prairie dogs, being trilobated circumferentially distributed around the anus. These glands are subcutaneously embedded in the deep fascia of the anal skin; each lobe represents a separate gland with its own opening which is in agreement with the findings of [11]. Histologically, each lobe gundis perianal glands represent a compound branched tubulo-alveolar gland consisting of an apocrine, modified tubular sweat gland, and an alveolar, holocrine, modified sebaceous gland. The apocrine part of the gland is similar to those found in the axillaries and groin regions of human. The holocrine part of gundis perianal glands is similar to those described for many Rattaus norvegicus , except for the fact that the glands in hand are surrounded by a thick capsule very rich in skeletal muscles. The non-continuous seeping of the secretion and the evidence of skeletal muscles in the capsule and trabeculae of Ctenodactylus perianl glands have lead us to strongly believe that these glands are different from regular sebaceous gland in their secretory mechanism. We strongly suggest the existence of some voluntary mechanism that controls the timing of secretion release. The quantity of skeletal muscle fibers in the capsule of these such glands strongly suggests the release of secretion due to muscle contraction; which occurs under the well of the animal, and only when such act is required, i.e. only during scent marking and not as volume replacement as suggested for other rodent scent glands by many authors in the field of ethology.
References
- Cloe AL, Woodley SK, Waters P, Zhou H, Baum MJ, et al. (2004) Contribution of anal scent gland and urinary odorants to mate recognition in the ferret. Physiol Behav 82: 871-875.
- de Haan CC (2003) Extrabuccal infralabial secretion outlets in Dromophis, Mimophis and Psammophis species (Serpentes, Colubridae, Psammophiini). A probable substitute for ‘self-rubbing, and cloacal scent gland function, and a cue for a taxonomic account. C R Biol 326: 275-286.
- McColl I (1967) The comparative anatomy and pathology of anal glands. Ann R Coll Surg Engl 40: 36-67.
- Frank R, Schulte BA (2004) Sexual dimorphism in the development of scent structures for the obligate monogamous Eurasian beaver (castor fiber). J Mammal 85: 1138-1144.
- Eisenberg JF, Kleiman DG (1972) Olfactory communication in mammals. Annu Rev Ecol Evol Syst 3: 1-32.
- Odend’hal S, Miller KV, Hoffmann DM (1992) Preputial glands in white tail deer Odocoileus virginianus. J. Mam 73: 299-302.
- Talamoni SA, Assis MAC, Freitas MMF, Godinho HP, Bazzoli N, et al. (2012) Seromucous anal gland in a New World hystricognath rodent Thrichomys apereoides (Lund, 1839). Acta Zoolocia 95: 133-136.
- Beaver DL (1963) Electron microscopy of the acinar cell of the that preputial gland’. Anat Rec 146: 47-59.
- Sleggs G (1926) The adult anatomy of the anal glands of Richardson’s ground squirrels, citellus richardsonii sabine. Anat Rec 32: 1-43.
- Londei T (2008) A gundi in the Fezzan, Southern Libya. J Mamm19: 73-75.
- Jones TR, Palkke RK (1981) The histology and histochemistry of the perianal scent gland of the reproductively quiescent Black-Tailed Prairie dog (Cynomys ludovicianus). J Mamm 62: 362-368.
- Kivett VK (1978) Integumentary glands of Columbian ground squirrels (Spermophilus Columbia nus). Can J Zool 56: 374-381.
- Kivett VK, Murie JO, Steiner AL (1976) A comparative study of scent gland location and related behavior in some North-western Nearactic ground squirrel species (Sciuridae): An evolutionary approach. Can J Zool 54: 1294-1306.
- Byrne KJ, Beauchamp GK, Preti G, Smithe AB, Wellington JL, et al. (1979) Perineal scent glands of wild and domestic guinea pigs. J Chem Eco 5: 737-751Â
- Drea CM, Dubay G, Scordato ES (2007) Chemical composition of scent marks in the ringtailed lemur (Lemur catta): Glandular differences, seasonal variation, and individual signatures. Chem Senses 32: 493- 504.
- Humanson GL (1979) Animal tissue techniques, Freeman and Company, USA pp: 661.
Citation: Mshiri OA, Berim NS (2018) Emptying Mechanism of Perianal Glands of Ctenodactylus gundi. J Biochem Cell Biol 1: 105.
Copyright: © 2018 Mshiri OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6659
- [From(publication date): 0-2018 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 5633
- PDF downloads: 1026