Review Article Open Access
Emerging Roles for Platelets in Inflammation and Disease
| Yancy Ferrer-Acosta1, Marieli González2, Mónica Fernández3 and Valance Washington A1,2* | ||
| 1 University of Puerto Rico, Rio Piedras Campus, San Juan, Puerto Rico, USA | ||
| 2 Universidad Central del Caribe, Bayamón, Puerto Rico, USA | ||
| 3 University of Puerto Rico, Mayagüez Campus, Mayagüez, Puerto Rico, USA | ||
| Corresponding Author : | Anthony Valance Washington Department of Biology University of Puerto Rico – Rio Piedras Campus, USA Tel: +1787764-0000 Fax: +1787264-2610 E-mail: valancew@gmail.com |
|
| Received May 19, 2014; Accepted June 18, 2014; Published June 24, 2014 | ||
| Citation: Ferrer-Acosta Y, González M, Fernández M, Washington AV (2014) Emerging Roles for Platelets in Inflammation and Disease. J Infect Dis Ther 2:149. doi: 10.4172/2332-0877.1000149 | ||
| Copyright: © 2014 Washington AV. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Platelets and their interaction with cells of the immune system contribute through a variety of molecular mechanisms to support hemostasis and inflammation. These simple yet essential cells exert their effects in lymphocytes, monocytes, and neutrophils, both recruiting and modulating their function after activation. Emerging evidence is starting to define the mechanisms that allow platelets to also play pivotal roles in host defense. For example, platelet cell-surface expression of toll-like receptors allows platelets to direct neutrophil activation toward extracellular trap formation and facilitate the elimination of blood pathogens. In addition to these well-known receptors, two of the most recently discovered platelet receptors, C-type lectin receptor 2 (CLEC-2), and TREM-like transcript-1 (TLT-1), have been shown to modulate hemostatic and inflammation-related roles in platelets. This review will discuss the evolution of our understanding of platelet functions from hemostasis to inflammation, and highlight novel mechanisms that platelets use to mediate hemostasis under inflammatory pressure.
| Introduction |
| Hemostasis, thrombosis, and inflammation are mediated by platelets. Platelets are the specialized cells circulating in the blood that maintain blood vessel integrity and contribute to thrombosis; the other side of hemostasis [1]. Anyone who has taken advanced biochemistry has learned the coagulation cascade; unfortunately the purpose and beauty of the cascade, which is to generate thrombin and form a clot, is lost in the intricacy of these enzymatic reactions as they are taken out of context for class. Platelets regulate thrombin generation and in turn, subsequent platelet activation, fibrin production, clotting, hemostasis, and thrombosis. The platelet’s role in inflammation on the other hand, is less direct, but equally important. |
| Even though platelets may seem simple, they contribute to inflammation through the recruitment and activation of leukocytes [2,3]. Within the platelet’s bag of tricks are inflammatory mediators such as Platelet factor 4 (PF4), RANTES (Regulated on Activation, Normal T Cell Expressed and Secreted), IL1β (Interlukin1β), and CD40L (CD40 Ligand), which promote innate immune activation and modulation of the adaptive immune system [4-8]. In neutrophils, platelets stimulate fast responses such as the release of reactive oxygen species, myeloperoxidase, and proteases [2]. In monocytes, platelets induce intracellular signaling that leads to prothrombotic and inflammatory gene expression [9]. In lymphocytes, platelets mediate class-switching on B-cells and increase cytotoxic T-cell activity [10]. Secondary to hemostasis, platelet interactions with leukocytes may contribute to vascular injury and tissue damage in several inflammatory diseases including: atherosclerosis, inflammatory lung, bowel, and skin diseases, ischemic and inflammatory hepatitis, cancer, arthritis, glomerulonephritis and sepsis [11]. As demonstrated below, platelet-leukocyte interactions are critical to the clearance of bacterial, viral, and parasitic infections. |
| Emerging evidence is beginning to define the mechanisms that allow platelets to play a key role in host defense. Platelet expression of toll-like receptors (TLR2, TLR4 and TLR9) allows them to directly detect and eliminate blood pathogens, both by secreting microbiocidal molecules and by directly phagocytosing them [12,13]. These new emerging roles of platelets require a better understanding on how these cells modulate their different responses, and call for new investigations into platelet-specific molecules that fine-tune these responses that they use to keep the delicate balance between hemostasis and thrombosis. Current studies have outlined a new paradigm that suggests platelets mediate an immune-derived hemostasis or an immunohemostasis, that elicits a different set of molecular programs than the classic tools used in basic hemostasis [14,15]. This review will summarize and expand on the involvement of platelets in acute and chronic inflammation and describe two promising candidate receptors, CLEC-2 and TREM-like transcript (TLT)-1, as targets to manipulate platelet function and as potential intervention during the inflammatory response. |
| Platelets: A Classic View |
| The coagulation cascade is one of the mechanisms that transfers vascular insult into action through the generation of a serine protease called thrombin. There are two well-described routes that can activate the clotting cascade: the extrinsic and the intrinsic pathway. Endothelial damage exposes tissue factor to Factor VII and initiates the extrinsic arm of the clotting cascade. The intrinsic pathway is initiated by phospholipids and Factor XII. Each of these pathways leads to membrane bound complexes that generate thrombin. The mechanisms of this serine protease cascade of activation are beyond the scope of this review, but for a more detailed explanation of these processes see references [16,17]. It is important to mention that these reactions in the fluid phase are kinetically unfavorable, but once the complexes that generate thrombin attach to the platelet’s surface, the rate of thrombin generation increases tremendously. After its generation, thrombin activates platelets and cleaves fibrinogen to fibrin. Together with red blood cells, these activated platelets form the basis of a clot. |
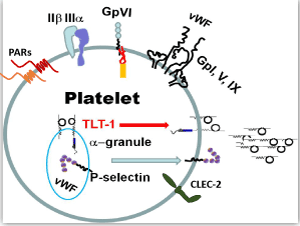
| The vast majority of platelet functions can be defined by a handful of molecules held within the platelet’s bag of tricks (Figure 1). Platelets are activated by many agonists. The important endpoint of platelet activation is the acquired competence of the integrin receptor glycoprotein (GP) IIβ IIIa to bind fibrin [18]. Activated GP IIβ IIIa mediates platelet adhesion through a GP IIβ IIIa-fibrin-GP IIβ IIIa interaction. Thrombin, ADP, and thromboxane A2, are platelet activators that exert their effects through G-protein coupled receptors. Thrombin is arguably the most prolific of these activators because it acts upon two protease activated receptors (PARs 1 and 4) that are coupled to three different activating G-proteins: Gq, Gi, G12&13 [19-21]. Collagen, found in the extracellular matrix, also activates platelets, and it exerts its effects though an Fc-coupled receptor called GP VI (GPVI). In contrast to the PAR receptors, GPVI/Fc receptor mediates platelet activation signaling cascades through an immune tyrosine activating motif (ITAM) that recruits the tyrosine kinase SYK [22,23]. |
| The platelet uses its bag of tricks to perform its multifunctional roles and relies on its receptors and granule contents. Among the most prominent molecules are the receptors that enable platelet activation (thrombin receptors-PAR 1 and 4 for thrombin; collagen receptor-GPVI), aggregation (the IIβ-IIIa fibrin-adhesion receptor) and adhesion (p-selectin; von Willebrand‘s Factor and its receptor GpI, V, IX) and recently described platelet receptors CLEC-2 and TLT-1. |
| Platelets activated by thrombin or bound to collagen, release their contents enclosed in their dense and a-granules, which expose more binding surfaces for coagulation factors such as prothrombin and factors V, VIII, IX&X promoting a positive feedback loop [24]. While the dense granules release small molecules such as ADP and serotonin, which aid in the coagulation process, the a-granules selectively release a much more complex mixture of mediators; mediators that we will refer to throughout the remainder of this review. |
| In platelets, GPIb-IX-V is an adhesion receptor complex that belongs to the leucine-rich repeat family. The major function of GPIb-IX-V complex is mediating the first step in platelet adhesion to the vessel wall. This complex binds to a collagen-tethered glycoprotein on the damaged sub-endothelial matrix, named von Willebrand Factor (vWF), to initiate thrombus formation at high-shear stress in flowing blood. High-shear results in increased GPIb-IX-V binding to vWF, which is rapidly secreted from platelet’s a-granules and endothelial cell’s Weibel-Palade bodies (storage granules) after tissue injury. Following binding of these proteins, platelets become activated. Platelet activation leads to shape change, spreading, and granule secretion, enabled by abrupt cytoskeletal rearrangements. This activation also promotes recruitment of additional platelets to the developing thrombus. The GPIb-IX-V complex can also bind to other platelet ligands such as thrombin, P-selectin, factor XI, factor XII, and high molecular weight kininogen [25,26]. |
| Platelets and Inflammation |
| Platelet a-granules harbor cytokines and membrane receptors that specifically mediate the inflammatory response. P-selectin (psel) is probably the most well studied a-granule resident protein. Used as a marker of platelet activation, psel binds to its ligand, Psel Glycoslated Ligand (PSGL-1), and initiates the platelet-endothelial and platelet-leukocyte interaction [27-31]. Integrin activation further stabilizes the platelet-leukocyte interaction. Thus, as leukocytes (monocytes and neutrophils) mediate the immune response, they have the added support of platelets as their “foot-soldiers”. |
| It is widely known that the presence of platelets increases immune function during in vitro immune studies. Immunology purists who study the innate immune response found that ethylenediaminetetraacetic acid (EDTA) is an important additive to their buffers [32]. EDTA chelates Ca++ and Mg++, inhibiting platelet activation and binding to leukocytes. Therefore, immune assays are often run without platelets and it is for these reasons that the platelet contribution to inflammation has gone unappreciated; that is, until recently. |
| The platelet-leukocyte interaction has shown to be more important than previously believed. In the spirit of “Retro”, classic studies are being revisited with a new twist: platelet depletion. Surprisingly, viral studies have shown that platelet depletion not only decreases the inflammatory response, but also the body’s ability to clear infection. Using two different models of viral infection in the liver, Iannacone et al. [33] demonstrated that platelet depletion lowered the inflammatory effects, but failed to clear the viral load. Re-introduction of platelets increased inflammation, but more importantly, resolved the viral infection. Apparently, platelets were important for the recruitment of cytotoxic T-cells to the site of infection. In a model using the mouse lymphocytic choriomeningitis virus (LCMV), it was found that platelet depletion changed both cytokine output and splenic organization, which led to systemic bleeding and death [34]. There are two series of studies that give insight into the mechanism behind the changes in T-cell function. The first begins with a study that establishes that platelets are the main source for CD40L in the blood [6]. An ensuing study investigated how platelets from wild type mice rescue the phenotype of CD40L null mice [10]. The authors convincingly show that CD40L on platelets enhances T-cell cytotoxicity and also mediates class-switching in B-cells. The second study shows that platelets have the ability to present antigen to T-cells in the context of class 1 [35]. These authors demonstrate that activated platelets exposed to antigen, presented the antigen to T-cells and promoted clearance of the parasite Plasmodium berghei, leading to 100% survival of the mice. On the other hand, B2 microglobin null platelets, under the same conditions, failed to raise a T-cell response, clear the infection, and all mice succumbed to the Plasmodium infiltration. From these studies it is clear that platelets are critical to the adaptive immune response and clearance of infectious disease. |
| Neutrophil endothelial traps or NETs, are a relatively new phenomenon by where activated neutrophils spew out their DNA to form intravascular traps during bacterial infection. NETs trap bacteria to coalesce them for more efficient removal [36]. They are found primarily in the pulmonary capillaries and liver sinusoids. NETs are visualized using fluorescent microscopy with stains for DNA (such as DAPI) and antibodies against histones. Clark et al. demonstrated that NETs are triggered by expression of TLR4 on platelets [37]. Platelet depletion greatly reduces NET presence and their ability to sequester bacteria [38]. Interestingly, NETs are remnant of evolutionary mechanisms found in invertebrates that used coagulation as a method to sequester foreign invaders [39]. |
| Emerging Roles |
| An emerging realization is that platelets use ulterior mechanisms than described by the handful of molecules to mediate hemostasis derived by inflammatory means. In an eloquent series of experiments it was shown that immune challenges in the absence of platelets causes bleeding, which in itself is not surprising [14]. The authors used the reverse arthus reaction as a model. This reaction is similar to the tuberculin test, where antigen is applied subcutaneously. If there are specific antibodies to recognize the antigen, leukocytes (mainly neutrophils) infiltrate the area causing edema and inflammation in reaction to the immune complexes at the site of injection. In a search to identify the hemostatic mechanism responsible for the bleeding, they performed the arthus reaction on mice deficient for many of the molecules used to define our understanding of hemostasis (B3, vWF, GPIV, psel, CaldagGEF and GP1b: Figure 1). Surprisingly, each of these mice maintained hemostasis, suggesting that the mechanisms used to clear infectious diseases initiate a differential hemostatic program. We finish our review on an emerging role of platelet function, and introduce two candidate receptors that may provide answers to this conundrum: CLEC-2 and TLT-1. |
| C-type lectin receptor 2 (CLEC-2) |
| It is only recently that platelets are beginning to be recognized as critical players of processes beyond their classical role in hemostasis. In this sense, a novel platelet receptor has been described with new unexpected roles. The C-type lectin receptor 2 (CLEC-2) is a newly identified type II membrane receptor with high mRNA levels found in megakaryocytes [40]. In addition to platelets, CLEC-2 is expressed in a variety of cells including monocytes, dendritic cells and granulocytes [41], opening the possibility of many yet to be identified roles. |
| There are several variations of CLEC-2. In mice, CLEC-2 splice variants lacking either exon(s) 2 or 2/4 have been identified [42]. These studies demonstrate that the transmembrane domain, coded by exon 2, may regulate its retention and localization in the cytoplasm. The full-length receptor is cleaved by a protease and releases a soluble form. The soluble form has been described by two groups in humans suggesting a potential conserved role for these fragments [42,43]. Although it has been shown that aprotinin and PMA inhibits CLEC-2 cleavage, the exact protease has not been identified nor has its significance of these ulterior forms of CLEC-2 been revealed in platelet function [42]. |
| The hunt for the ligand of the snake venom toxin rhodocytin (also called aggretin), led to the discovery of the CLEC-2 receptor [44]. Rhodocytin, a C-type lectin snake venom toxin similar to convulxin, induces robust platelet aggregation by inducing CLEC-2 tyrosine phosphorylation of its cytosolic tail and downstream activation of PLCγ2 [44,45]. |
| Recently, CLEC-2 has been found to bind Fucoidan, a sulfated polysaccharide derived from the brown seaweed Fucus vesiculosus [46]. It is awkwardly called a “non-anticoagulant” because it does not exhibit the anticoagulant properties of the sulfated polysaccharide heparin [47]. Fucoidan decreases bleeding time in mice and humans with hemophilia and thus holds promise as an intervention for this bleeding disorder [47]. The original mechanism of Fucoidan was thought to be due to inhibition of tissue factor pathway inhibitor (TFPI). However, in subsequent studies using CLEC-2 null mice it was shown that Fuciodan induces platelet aggregation in wild type mice but not in CLEC-2 null mice demonstrating that Fuciodan activates platelets through the receptor CLEC-2 [46]. While Fucoidan’s binding of CLEC-2 does not exclude any effects it may have on TFPI, these studies do outline a potential role for CLEC-2 in the treatment of hemophilia. |
| Early studies on CLEC-2 highlighted its similarities to the platelet collagen receptor GPVI, which has an established role in clot formation by mediating stable platelet adhesion and aggregation in response to collagen [48]. However, subsequent investigations determined that although GPIV and CLEC-2 regulate Syk phosphorylation via ITAM and hemITAM sequences respectively, they have unrelated physiological ligands and distinct roles [49-51]. The physiological ligand for CLEC-2, podoplanin (PDPN), is expressed in a variety of tissues, but not on vascular endothelial cells [52-54]. The absence of PDPN in the vessel wall strongly suggests that unlike GPVI, CLEC-2’s role might not be primary hemostasis. In fact, there is conflictive evidence about whether CLEC-2 plays a significant role in hemostasis and thrombosis. While antibodies against platelet CLEC-2 have no significant effect on platelet aggregation to other agonists, CLEC-2-depleted platelets showed reduced platelet aggregation to collagen and reduced thrombus formation in mice after a ferric chloride injury model [55]. However, reduced collagen-dependent platelet aggregation in vitro was not reproducible in a separate CLEC-2 deficient mouse model, putting in question CLEC-2’s function in vivo [56]. |
| Recent evidence supports a role for CLEC-2 in the separation of blood and lymphatic vasculature [57-59]. The importance of the interaction between CLEC-2 and PDPN was established when PDPN null mice bled from the high endothelial venules (HEVs) after immunization [45,60,61]. HEVs are specialized blood vessels that permit leukocyte infiltration into the lymph nodes for immune surveillance. PDPN as well as CLEC-2 deficient mice, showed spontaneous bleeding in both the mucosal lymph nodes and the draining peripheral lymph nodes after immunization. The loss of vessel integrity was explained by a reduced release of sphingosine-1-phosphate from CLEC-2 or PDPN null mice, leading to reduced induction of vascular endothelium cadherin (VE-cadherin) in surrounding fibroblastic reticular cells. VE-cadherin or vascular endothelium cadherin, is a calcium-dependent cell-cell adhesion glycoprotein that regulates the integrity and permeability of the cellular junctions. |
| Subsequent studies supported a role for the CLEC-2/PDPN axis in the regulation of a newly described form of hemostasis termed: lymphovenous hemostasis. Under conditions of impaired lymphovenous valve function, which prevents the blood’s blackflow into the lymphatic system, platelet CLEC-2/PDPN interaction can stabilize thrombi in the lymphatic endothelium to prevent retrograde blood flow [62]. Furthermore, in inflammation-induced hemorrhage, mice deficient in CLEC-2 bled when challenged with the arthus reaction, pointing to a role for CLEC-2 and ITAM-dependent signaling in immune-derived bleeding [63]. |
| Taken together, strong evidence points towards a role of platelet immune-like receptors in settings outside primary hemostasis. These new realizations may have potential implications in our understanding and treatment of diseases such as sepsis and dengue, where immune derived bleeding may have a negative impact on clinical outcome. |
| The Triggering Receptor Expressed on Myeloid cells-like transcript-1 (TLT-1) |
| TLT-1 was first characterized as a putative inhibitory receptor because it contains an Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) in its cytoplasmic domain and it was hypothesized to inhibit other TREM family members [64-67]. Its role in hemostasis is much less defined than CLEC-2. |
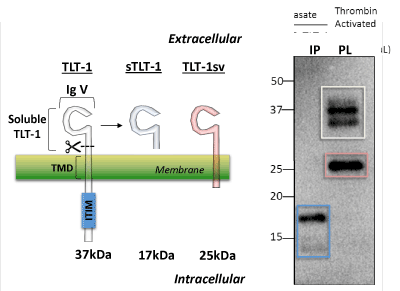
| Four TLT-1 isoforms have been isolated to date (Figure 2). In platelets, three isoforms have been reported: TLT-1 full length (TLT-1) and TLT-1 splice variant (TLT-1sv); the third is the soluble isoform that is released upon platelet activation [64,66,68]. This third soluble isoform corresponds to the extracellular sequence of the protein. The fourth isoform (TLT-1s) has only been identified in pre-osteoclasts and contains a very short extracellular domain fragment, the transmembrane and the intracellular domain [69]. Although the full length TLT-1 has been shown to bind SHP-1 and SHP-2 in model cell lines, to date, no inhibitory functions have been attributed to this motif in platelets [64,66]. Full length TLT-1 is a 34 kDa Ig domain-containing receptor expressed in megakaryocytes and platelets. Although splice variants have been identified in other cell types [69], the extracellular Ig domain-containing form has only been found in platelets (Figure 2). Our discussion will focus on the platelet isoforms. Interestingly, sTLT-1 is the platelet’s fourth most abundantly released molecule and has been detected in the serum but not in plasma of healthy mice and humans [68,70]. |
| Left panel, Representation of the two isoforms reported for TLT-1 on platelets and the cleaved soluble fragment generated upon platelet activation. These proteins consist of a common extracellular immunoglobulin V domain (IgV). The full length form of TLT-1 (TLT-1, white, 37 kDa) and isoform TLT-1sv (red, 25 kDa) both contain a transmembrane domain (TMD) but TLT-1 harbors an immunoreceptor tyrosine-based inhibition motif (ITIM). Soluble TLT-1 (sTLT-1, blue, 17 kDa) which is released from platelets upon activation. Right panel, In lane 1 (IP) immunoprecipitation followed by western blot of sTLT-1 from platelet releasate after activation with thrombin (0.01 U/mL). In lane 2, TLT-1 and TLT-1sv isoforms from platelet lysate. The two bands observed in TLT-1 (white square) suggest posttranslational modifications of TLT-1. |
| The first evidence that TLT-1 plays a role in the hemostatic mechanism of platelets was identified with the use of a single chain fragment (scFv) called C10 [71]. TLT-1 specific antibodies were isolated from a scFv library of which C10 showed the greatest binding affinity. In aggregation assays, C10 demonstrated a dose-depended inhibition of platelet activation with low-dose thrombin activated platelets. Higher doses of thrombin overcame C10 mediated inhibition. Consistent with these findings, platelets from treml1-/- mice also displayed reduced aggregation to ADP, collagen, and thrombin. TLT-1 null mice have increased tail-bleeding time when compared to controls and their platelets show decreased fibrinogen binding in vitro [72]. |
| The soluble fragment of TLT-1 seems to play a distinct role in platelet function as well. Although the mechanism of sTLT-1 function remains to be elucidated, we have shown that sTLT-1 increases both platelet adherence and actin polymerization that appears to lead to enhanced platelet aggregation and contact with endothelial cells [73]. As pointed out above, sTLT-1 is not found in the plasma of healthy individuals, therefore when sTLT-1 levels are elevated in plasma it suggests both platelet activation and TLT-1 involvement, which urges further investigation into a potential role for TLT-1 in that particular disease. The first such evidence was identified in patients with sepsis. |
| TLT-1 as possible modulator of Sepsis |
| Sepsis is a systemic inflammatory response caused by severe infection [74]. This disease can be accompanied by a low platelet count or thrombocytopenia, which can predict mortality in septic patients. Sepsis manifestation occurs when mechanisms meant to fight infection become unbalanced, leading to an acute state of inflammation. Severe sepsis may lead to septic shock, which results in decreased vascular pressure and multiple organ failure, leading to death. Disseminated Intravascular Coagulation (DIC), a condition often associated with sepsis, further increases mortality. In sepsis-associated DIC, platelets are systemically activated leading to increased fibrin deposition. D-dimers, which are fibrin degradation products, are used in the diagnosis of DIC. Dysregulation of clot formation and clot degradation results in consumption of coagulation factors, disruption of hemostasis, and can lead to simultaneous bleeding and clotting [75]. In these settings, inflammation and coagulation are intrinsically linked, and because of this, an understanding of how these factors are associated is needed to identify possible therapeutic targets. |
| Our early studies evaluated sTLT-1 levels in septic patients. A cross-sectional study of 13 patients diagnosed with sepsis and compared to healthy individuals revealed a stark difference in the levels of sTLT-1 [72]. While healthy patients had virtually undetectable levels, patients with sepsis had levels that averaged over 300 ng/mL in plasma. A longitudinal study showed similar results and allowed a comparison with D-dimer levels [72]. Interestingly, sTLT-1 levels correlated with the presence of DIC better than D-dimers, suggesting sTLT-1 as a new candidate biomarker to test for DIC. |
| These findings prompted an investigation into a sepsis mouse model. Using a Lipopolysaccharide (LPS) model of sepsis we found that the treml1-/- mice succumb faster and in greater numbers than wild type mice. However, studies using the Shwartzman reaction, which mimics the mechanisms of DIC in a localized lesion, demonstrate that the treml1-/- mice bleed from the inflammatory insult, where the wild type mice do not. These results suggested that TLT-1 may be one of the mechanisms that platelets use to manage immune-derived hemostasis. Follow up studies using the treml1-/- mouse demonstrated that the treml1-/- mice are more susceptible to bacterial infection and that the use of a peptide derived from TLT-1, named LP17, can modulate the outcome to microbial infection in vivo; similar results were also achieved in a swine model [76,77]. These studies confirm that TLT-1 plays a role during the progression of bacterial sepsis, but moreover, suggest that it may be a therapeutic target. |
| Ongoing studies in the laboratory suggest that TLT-1 may modulate more than just platelet function. Additional potential targets for TLT-1 function are leukocytes and endothelial cells. It would be interesting to see if TLT-1 inhibitory antibodies such as C10 could induce the same outcomes as the LP17 peptide in the microbial studies. Our findings in studies of infectious disease may have long-reaching effects in cancer, atherosclerosis, and other inflammatory conditions. |
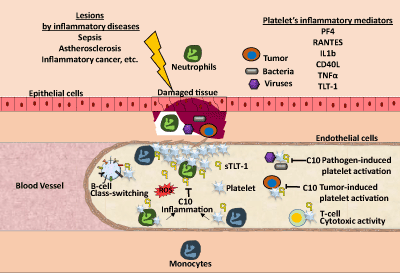
| The generation of C10 [71], which specifically binds and inhibits sTLT-1 effect on thrombin-induced platelet activation, opens a new possible target to TLT-1 mediated contribution in inflammatory hemostasis (Figure 3). Thus, in conditions such as sepsis, this antibody holds promise as a new therapeutic treatment to modulate aberrant platelet activation. In addition to its potential in sepsis, our expanded view of diseases in which TLT-1 could play a role, suggest that in acute or chronic inflammatory conditions, modulation of platelet activation by C10 will provide a possible approach to control inflammation and hemostasis. |
| Platelets take on different roles upon the disruption of hemostasis and the promotion of inflammation in the vascular system. Platelets mediate B-cell class switching and increase T-cell cytotoxic activity. Once tissue damage is detected after infection or inflammation, platelets become activated at the site of damage and release factors that assist in their pro-inflammatory functions. Platelets recruit neutrophils and monocytes to the site of damage. While neutrophils release reactive oxygen species (ROS), monocytes promote the expression of pro-inflammatory gene expression. When regulation of this inflammatory response cannot be properly controlled by the endothelial cells this results in increased tissue damage. In the case of pathogen-induced platelet activation, this can cause a septic shock when platelet activation is excessive and hemostasis can be lost. In this review, we propose that C10 antibody can help diminish this excessive platelet activation through the blockade of soluble and platelet-bound TLT-1, modulating the inflammatory response and helping to restoring hemostasis. |
| Acknowledgement |
| Support for this work these projects was obtained through grant (1R01HL090933) from the National Heart Lung and Blood Institute. |
References
- George JN (2000) Platelets. Lancet 355: 1531-1539.
- Zarbock A, Polanowska-Grabowska RK, Ley K (2007) Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 21: 99-111.
- Gawaz M, Langer H, May AE (2005) Platelets in inflammation and atherogenesis. J Clin Invest 115: 3378-3384.
- Semple JW, Italiano JE Jr, Freedman J (2011) Platelets and the immune continuum. Nat Rev Immunol 11: 264-274.
- Hartwig D, Härtel C, Hennig H, Müller-Steinhardt M, Schlenke P, et al. (2002) Evidence for de novo synthesis of cytokines and chemokines in platelet concentrates. Vox Sang 82: 182-190.
- Henn V, Slupsky JR, Gräfe M, Anagnostopoulos I, Förster R, et al. (1998) CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391: 591-594.
- Kameyoshi Y, Dörschner A, Mallet AI, Christophers E, Schröder JM (1992) Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med 176: 587-592.
- Eisman R, Surrey S, Ramachandran B, Schwartz E, Poncz M (1990) Structural and functional comparison of the genes for human platelet factor 4 and PF4alt. Blood 76: 336-344.
- van Gils JM, Zwaginga JJ, Hordijk PL (2009) Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J LeukocBiol 85: 195-204.
- Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, et al. (2003) Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 19: 9-19.
- Michelson A.D. (2013). Platelets, 3 edn. Elsevier).
- Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, et al. (2005) Platelets express functional Toll-like receptor-4. Blood 106: 2417-2423.
- Cognasse F, Hamzeh H, Chavarin P, Acquart S, Genin C, et al. (2005) Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol 83: 196-198.
- Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O'Donnell E, et al. (2008) Inflammation induces hemorrhage in thrombocytopenia. Blood 111: 4958-4964.
- Ho-Tin-Noé B1, Goerge T, Wagner DD (2009) Platelets: guardians of tumor vasculature. Cancer Res 69: 5623-5626.
- Butenas S, Mann KG (2002) Blood coagulation. Biochemistry (Mosc) 67: 3-12.
- Hoffman MM1, Monroe DM (2005) Rethinking the coagulation cascade. CurrHematol Rep 4: 391-396.
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, et al. (2003) Talin binding to integrin beta tails: a final common step in integrin activation. Science 302: 103-106.
- Brass LF, Manning DR, Cichowski K, Abrams CS (1997) Signaling through G proteins in platelets: to the integrins and beyond. ThrombHaemost 78: 581-589.
- Brass LF (2003) Thrombin and platelet activation. Chest 124: 18S-25S.
- Offermanns S (2003) G-proteins as transducers in transmembrane signalling. ProgBiophysMolBiol 83: 101-130.
- Watson SP, Auger JM, McCarty OJ, Pearce AC (2005) GPVI and integrin alphaIIb beta3 signaling in platelets. J ThrombHaemost 3: 1752-1762.
- Nieswandt B, Watson SP (2003) Platelet-collagen interaction: is GPVI the central receptor? Blood 102: 449-461.
- Heemskerk JW, Bevers EM, Lindhout T (2002) Platelet activation and blood coagulation. ThrombHaemost 88: 186-193.
- Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC (2003) Glycoprotein Ib-IX-V. Int J Biochem Cell Biol 35: 1170-1174.
- Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK (2001) The vascular biology of the glycoprotein Ib-IX-V complex. ThrombHaemost 86: 178-188.
- McEver RP, Martin MN (1984) A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J BiolChem 259: 9799-9804.
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF (1985) A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol 101: 880-886.
- Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, et al. (1986) A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest 78: 130-137.
- Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, et al. (1994) The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J BiolChem 269: 23318-23327.
- Furie B, Furie BC (1995) The molecular basis of platelet and endothelial cell interaction with neutrophils and monocytes: role of P-selectin and the P-selectin ligand, PSGL-1. ThrombHaemost 74: 224-227.
- Noble PB, Cutts JH, Carroll KK (1968) Ficoll flotation for the separation of blood leukocyte types. Blood 31: 66-73.
- Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, et al. (2005) Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med 11: 1167-1169.
- Loria GD, Romagnoli PA, Moseley NB, Rucavado A, Altman JD (2013) Platelets support a protective immune response to LCMV by preventing splenic necrosis. Blood 121: 940-950.
- Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, et al. (2012) Platelets present antigen in the context of MHC class I. J Immunol 189: 916-923.
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532-1535.
- Clark SR1, Ma AC, Tavener SA, McDonald B, Goodarzi Z, et al. (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13: 463-469.
- McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P (2012) Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12: 324-333.
- Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, et al. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16: 887-896.
- Senis YA, Tomlinson MG, García A, Dumon S, Heath VL, et al. (2007) A comprehensive proteomics and genomics analysis reveals novel transmembrane proteins in human platelets and mouse megakaryocytes including G6b-B, a novel immunoreceptor tyrosine-based inhibitory motif protein. Mol Cell Proteomics 6: 548-564.
- Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, et al. (2001) A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol 31: 3493-3503.
- Xie J, Wu T, Guo L, Ruan Y, Zhou L, et al. (2008) Molecular characterization of two novel isoforms and a soluble form of mouse CLEC-2. BiochemBiophys Res Commun 371: 180-184.
- Fei M, Zhou L, Xie J, Ruan Y, Xu J, et al. (2012) The production of soluble C-type lectin-like receptor 2 is a regulated process. Glycoconj J 29: 315-321.
- Colonna M, Samaridis J, Angman L (2000) Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol 30: 697-704.
- Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, et al. (2010) Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J BiolChem 285: 24494-24507.
- Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, et al. (2013) Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2). J BiolChem 288: 7717-7726.
- Liu T, Scallan CD, Broze GJ Jr, Patarroyo-White S, Pierce GF, et al. (2006) Improved coagulation in bleeding disorders by Non-Anticoagulant Sulfated Polysaccharides (NASP). ThrombHaemost 95: 68-76.
- Watson SP, Herbert JM, Pollitt AY (2010) GPVI and CLEC-2 in hemostasis and vascular integrity. J ThrombHaemost 8: 1456-1467.
- Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, et al. (2013) Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. ArteriosclerThrombVascBiol 33: 926-934.
- Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, et al. (2008) Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J ThrombHaemost 6: 2152-2159.
- Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, et al. (2009) The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J ThrombHaemost 7: 1192-1199.
- Chaipan C, Steffen I, Tsegaye TS, Bertram S, Glowacka I, et al. (2010) Incorporation of podoplanin into HIV released from HEK-293T cells, but not PBMC, is required for efficient binding to the attachment factor CLEC-2. Retrovirology 7: 47.
- Christou CM, Pearce AC, Watson AA, Mistry AR, Pollitt AY, et al. (2008) Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J 411: 133-140.
- Hou TZ, Bystrom J, Sherlock JP, Qureshi O, Parnell SM, et al. (2010) A distinct subset of podoplanin (gp38) expressing F4/80+ macrophages mediate phagocytosis and are induced following zymosan peritonitis. FEBS Lett 584: 3955-3961.
- May F, Hagedorn I, Pleines I, Bender M, Vögtle T, et al. (2009) CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood 114: 3464-3472.
- Hughes CE, Navarro-Núñez L, Finney BA, Mourão-Sá D, Pollitt AY, et al. (2010) CLEC-2 is not required for platelet aggregation at arteriolar shear. J ThrombHaemost 8: 2328-2332.
- Bénézech C, Nayar S, Finney BA2, Withers DR1, Lowe K2, et al. (2014) CLEC-2 is required for development and maintenance of lymph nodes. Blood 123: 3200-3207.
- Finney BA1, Schweighoffer E, Navarro-Núñez L, Bénézech C, Barone F, et al. (2012) CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood 119: 1747-1756.
- Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, et al. (2010) Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116: 661-670.
- Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, et al. (2013) Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502: 105-109.
- Osada M, Inoue O, Ding G, Shirai T, Ichise H, et al. (2012) Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J BiolChem 287: 22241-22252.
- Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, et al. (2014) Platelets mediate lymphovenoushemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest 124: 273-284.
- Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, et al. (2013) Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest 123: 908-916.
- Washington AV, Quigley L, McVicar DW (2002) Initial characterization of TREM-like transcript (TLT)-1: a putative inhibitory receptor within the TREM cluster. Blood 100: 3822-3824.
- Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J (2003) The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol 33: 567-577.
- Barrow AD, Astoul E, Floto A, Brooke G, Relou IA, et al. (2004) Cutting edge: TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatoryimmunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J Immunol 172: 5838-5842.
- Ford JW, McVicar DW (2009) TREM and TREM-like receptors in inflammation and disease. CurrOpinImmunol 21: 38-46.
- Gattis JL, Washington AV, Chisholm MM, Quigley L, Szyk A, et al. (2006) The structure of the extracellular domain of triggering receptor expressed on myeloid cells like transcript-1 and evidence for a naturally occurring soluble fragment. J BiolChem 281: 13396-13403.
- Yoon SH, Lee YD, Ha J, Lee Y, Kim HH (2012) TLT-1s, alternative transcripts of triggering receptor expressed on myeloid cell-like transcript-1 (TLT-1), Inhibits the triggering receptor expressed on myeloid cell-2 (TREM-2)-mediated signaling pathway during osteoclastogenesis. J BiolChem 287: 29620-29626.
- Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, et al. (2011) Deciphering the human platelet sheddome. Blood 117: e15-26.
- Giomarelli B, Washington VA, Chisholm MM, Quigley L, McMahon JB, et al. (2007) Inhibition of thrombin-induced platelet aggregation using human single-chain Fv antibodies specific for TREM-like transcript-1. ThrombHaemost 97: 955-963.
- Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, et al. (2009) TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest 119: 1489-1501.
- Morales J, Villa K, Gattis J, Castro W, Colon K, et al. (2010) Soluble TLT-1 modulates platelet-endothelial cell interactions and actin polymerization. Blood Coagul Fibrinolysis 21: 229-236.
- Levi M1, de Jonge E, van der Poll T (2003) Sepsis and disseminated intravascular coagulation. J Thromb Thrombolysis 16: 43-47.
- Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8: 776-787.
- Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, et al. (2012) Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol 188: 5585-5592.
- Derive M, Boufenzer A, Bouazza Y, Groubatch F, Alauzet C, et al. (2013) Effects of a TREM-like transcript 1-derived peptide during hypodynamic septic shock in pigs. Shock 39: 176-182.
Figures at a glance
 |
 |
 |
||
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 15857
- [From(publication date):
August-2014 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11282
- PDF downloads : 4575
