Research Article Open Access
Elevated Interleukin-17 Levels in Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Chunhua Chen1#, Yebo Shao1#, Xiuling Wu3, Cheng Huang4 and Weiqi Lu2*
1Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN , USA
2Department of General Surgery, Zhongshan Hospital, Shanghai, China
3Department of Endocrinology, Fuyang Cancer Center, Fuyang, China
4Drug Discovery Lab, School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, China
5#Contributed equally to this study
- *Corresponding Author:
- Weiqi Lu
Department of General Surgery
Zhongshan Hospital, Shanghai, China
Tel: +86-21-64041990
E-mail: lu.weiqi@zs-hospital.sh.cn
Received date April 29, 2016; Accepted date June 15, 2016; Published date June 22, 2016
Citation: Chen C, Shao Y, Wu X, Huang C, Lu W (2016) Elevated Interleukin-17 Levels in Patients with Newly Diagnosed Type 2 Diabetes Mellitus. Biochem Physiol 5:206. doi: 10.4172/2168-9652.1000206
Copyright: © 2016 Chen C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Interleukin (IL)-17 is a newly described T cell-derived inflammatory cytokine. Recent studies have shown that inflammatory cytokines play important roles in the pathogenesis of type 2 diabetes mellitus (DM). However, whether IL-17 plays a prominent role in type 2 DM remains unclear. Here we measured serum concentrations of IL-17, and mRNA expression of IL-17 and its transcription factor RORγt in peripheral blood mononuclear cells (PBMC) from 30 patients with newly diagnosed type 2 DM and 20 healthy subjects. Results have shown that serum levels of IL-17 were significantly higher in patients than in healthy subjects (10.44 ± 6.47 vs. 2.99 ± 1.68 pg/mL, P<0.01); and mRNA levels of IL-17 and its transcript factor RORγt were also upregulated in PBMC from patients (P<0.001). Further, IL-17 mRNA expression was correlated with TNF-α mRNA expression in PBMC from patients (r=0.6988, P<0.001). These results indicated that IL-17 might contribute to the inflammation process in type 2 diabetes as an inflammation factor.
Keywords
Interleukin-17; Type 2 diabetes mellitus; Inflammation cytokines
Introduction
Inflammation is defined as the local physiological response to tissue injury and regulated by pro-inflammatory cytokines. Recently, chronic inflammation has received considerable attention as an important pathophysiological mechanism in type 2 diabetes mellitus (DM). Inflammatory cytokines have been postulated to be important pathogenic factors in the development of type 2 DM [1,2].
Interleukin (IL)-17 (IL-17A) is a newly identified inflammatory cytokine produced by activated and memory T lymphocytes [3]. It has pleiotropic activities including the induction of diverse inflammatory cytokines (e.g. IL-6 and TNF-α) and chemokines (e.g. CCL2/MCP- 1, CXCL1/KC, and CXCL2/MIP-2) from a large variety of cells [4,5]. IL-17 acts as highly potent inflammatory cytokine that initiates tissue inflammation and induces the infiltration of other inflammatory cells into the target organs [6,7].
Increasing evidence suggests that IL-17 plays a crucial role in various inflammatory responses and autoimmune diseases [8-10]. Recent studies have shown that increased IL-17 levels were detected in STZ-induced diabetic animal models and non-obese diabetic (NOD) mice from insulitis to diabetes [11,12]. However, to date, there are little published data evaluating the role of IL-17 in type 2 DM.
Based on these observations, in this study, we investigated mRNA levels of IL-17 and its transcription factor RORγt in peripheral blood mononuclear cells (PBMC) and serum concentrations of IL-17 from patients with newly diagnosed type 2 DM. Meanwhile, we analyzed the correlation between IL-17 mRNA expression and mRNA expression of inflammation cytokines involved in type 2 DM in PBMC from diabetic patients. Our results suggest that IL-17 might be involved in the pathogenesis of type 2 DM as an inflammation cytokine.
Materials and Methods
Subjects
Thirty patients with newly confirmed type 2 diabetes mellitus were collected from out-patient department or epidemiology investigation. The control subjects, who were healthy examinees from the hospital, comprised 20 healthy non-diabetic subjects of similar age. This study conformed to the approved institutional guidelines. Informed consent was obtained from all participants. These patients have been not received the treatment of antidiabetic drugs before diagnosis with diabetes. Microvascular and macrovascular diseases were assessed by clinical history and examination including fundoscopy through dilated pupils (retinopathy), proteinuria and creatinine (nephropathy), electrocardiogram and heart color ultrasound and peripheral vascular ultrasound (heart and peripheral vascular disease).
The diagnosis of type 2 diabetes was based on diagnostic criteria for diabetes of the World Health Organization (WHO) 1999. These are positive cases from any two of the following tests on different days: symptoms of diabetes mellitus plus casual plasma glucose concentration ≥ 11.1 mmol/L or fasting plasma glucose (FPG) ≥ 7.0 mmol/L or 2 h post-prandial plasma glucose (2h PPG) ≥ 11.1 mmol/L after a 75 g glucose load. All demographic and clinical data were extracted from patients’ files. Body mass index (BMI) was calculated as the ratio of the weight in kilograms to the square of the height in meters (kg/m2).
Clinical and biochemical measurements
Venous blood samples were collected from each subject in the morning after a 12 h fasting. The collection tubes were promptly centrifuged, and serum was separated and stored at -80ºC for the measurement of cytokines. Fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL) cholesterol, and glycosylated haemoglobin (HbAlc) were measured by using routine clinical laboratory procedures. Insulin was determined by radioimmunoassay Kit (DPC, Los Angeles, CA).The insulin sensitivity was determined by Homeostasis Model Assessment Model (HOMA) index with formula: HOMA-IR=fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5 [13]. Low HOMA-IR values indicate high insulin sensitivity, whereas high HOMA values indicate low insulin sensitivity (insulin resistance).
PBMC Isolation from blood samples
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized peripheral blood obtained from normal subjects and patients by density gradient centrifugation using Ficoll-Hypaque (density: 1.077; Pharmacia, Dübendorf, Switzerland). Mononuclear cells at the interface were carefully transferred into a Pasteur Pipette, and washed twice in PBS (137 mmol/L NaCl, 2.7 mmol/L KCL, 10 mmol/L Na2HPO4, 2 mmol/L KHPO4). Cells were suspended at a density of 2×106 cells/ml and used for RNA isolation.
ELISA for serum IL-17
Serum IL-17 levels were assayed by ELISA Kit (Human IL- 17 immunoassay, eBioscience, San Diego, USA) according to the manufacturer’s instructions. The optical density was measured at 450 nm with an automatic ELISA reader. The minimum detection limit was 4 pg/ml for IL-17. To minimize the effect of inter-assay variation, samples from diabetes patients and controls were equally represented on each ELISA plate. All samples were analyzed in duplicates, and the mean of the duplicates was used for the statistical analysis. The intraassay and inter-assay coefficients of variation for IL-17 were 6.4 and 14.3%, respectively.
Quantitative real time PCR
Total RNA was isolated from cell pellets using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genomic DNA was removed from total RNA using the RNase-free DNase set (Qiagen, Hilden, Germany). The first strand cDNA was synthesized by using the cDNA synthesis kit (Promega, Madison, WI). All reactions were performed on ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). The gene expression levels were analyzed by real time PCR using SYBR Green master mix (Applied Biosystems, Foster City, USA). The PCR conditions comprised an initial holding at 50°C for 2 min, and 95°C for 10 min followed by a two-step PCR program consisting of 95°C for 15 s, and 60°C for 60 s for 40 cycles. For each sample, mRNA expression level was normalized to the level of GAPDH gene. The sequences of primers were showed in the Table 1.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) or median (interquintile range). Differences between groups were tested with the Student’s t-test or the Mann–Whitney U test for skewed data. Spearman’s correlation coefficient by rank test was calculated to assess the correlations between variables. The values of P < 0.05 were considered significant.
| Gene | Acc Num | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | ||
|---|---|---|---|---|---|
| GAPDH | NM_002046 | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | ||
| IL-17 | NM_002190 | AATCTCCACCGCAATGAGGA | ACGTTCCCATCAGCGTTGA | ||
| RORγt | NM_001001523 | ACCGATGTGGACTTCGTTTTG | CGGTGTGCTGCGGAAACT | ||
| IL-6 | NM_000600 | GTGGCTGCAGGACATGACAA | TGAGGTGCCCATGCTACATTT | ||
| TNF-α | NM_000594 | GAGATCAATCGGCCCGACTA | CGTTTGGGAAGGTTGGATGT | ||
| IL-1β | NM_000576 | CTTTGAAGCTGATGGCCCTAAA | AGTGGTGGTCGGAGATTCGT | ||
Table 1: The sequence of primers for real time PCR.
Results
Basic clinical characteristics of patients
The clinical and demographic data of patients with type 2 DM in this study were shown in Table 2. Age and sex distributions were similar between patients and control subjects. Among the 30 recruited patients, 16 were men and 14 were women. The average age of the patients was 53.24 ± 7.54 years. Healthy controls were 20 subjects with mean age 49.48 ± 6.54 years, including male 12 and female 8 subjects. The diabetic patients were characterized by markedly higher levels of fasting blood glucose (FBG), glycosylated haemoglobin (HbAlc), C-peptide and C-reactive protein (CRP) compared to control subjects.
| Clinical feathers | DM | CON |
|---|---|---|
| N (M/F) | 30(16/14) | 20(12/8) |
| Age (years) | 53.24 ± 7.54 | 49.48 ± 6.54 |
| BMI (kg/m2) | 25.36 ± 4.23 | 24.17 ± 2.9 |
| SBP (mm Hg) | 136.57 ± 17.11 | 128.73 ± 9.2 |
| DBP (mm Hg) | 80.29 ± 10.28 | 78.23 ± 5.84 |
| FBG (mmol/l) | 7.10 ± 1.23* | 4.83 ± 0.86 |
| 2h FBG (mmol/l) | 11.53 ± 1.77 | - |
| HbA1c (%) | 8.91 ± 1.93* | 4.92 ± 1.21 |
| TC (mmol/l) | 4.49 ± 0.73 | 4.27 ± 1.26 |
| Triglyceride (mmol/l) | 2.12 ± 1.48 | 1.63 ± 0.78 |
| HDL (mmol/l) | 1.22 ± 0.22 | 1.24 ± 0.61 |
| LDL (mmol/l) | 2.63 ± 0.66 | 2.18 ± 0.94 |
| CRP (mg/l) | 2.92 ± 0.46* | 1.26 ± 0.38 |
| C-peptide (ng/ml) | 2.81 ± 0.97* | 1.21 ± 0.32 |
| 2h C-peptide (ng/ml) | 4.80 ± 1.26 | - |
| ALT (IU/l) | 24 ± 9.71 | 21.82 ± 7.36 |
| Cr (μmmol/l) | 62.05 ± 18.54 | 64.32 ± 12.61 |
| BUN (mmol/l) | 5.59 ± 1.45 | 4.89 ± 1.05 |
| UA (μmmol/l) | 261.33 ± 80.62 | 248.28 ± 74.23 |
| Ualb (mg/l) | 23.7 (16.85-28.7) | 14.85 (8.24-21.81) |
| Ualb/Ucr (g/mol) | 1.88 (1.26-2.16) | 1.13 (0.62-1.57) |
DM: Type 2 Diabetic Patients; CON: Healthy Controls; F: Female Subjects; M: Male Subjects; BMI: Body Mass Index; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; FBG: Fasting Blood Glucose; 2h FBG: Blood Glucose After Meal Two Hours HbA1c: Glycosylated Haemoglobin; TC: Total Cholesterol; HDL: High-Density Lipoproteins; LDL: Low-Density Lipoproteins; CRP: C-Reactive Protein; 2h C-peptide: 2h Postprandial C-Peptide; ALT: Alanine Aminotransferase; Cr: Blood Creatinine; BUN: Blood Urea Nitrogen; UA: Blood Uric Acid; Ualb: Urinary Microquantitative Albumin; Ualb/Ucr: Urinary Albumin/Creatinine Ratio. Data are expressed as mean ± SD or median (interquintile range); Differences between CON and DM: *P<0.05.
Table 2: Clinical features of type 2 diabetic patients.
To access liver and renal tubular function, we examined some clinical parameters including alanine aminotransferase (ALT), blood creatinine (Cr), blood urea nitrogen (BUN), blood uric acid (UA), urinary microquantitative albumin (Ualb) and urinary albumin/ creatinine ratio (Ualb/Ucr). These clinical parameters reflecting liver and renal function did not exceed the upper limits of normal range in patients with type 2 diabetes (data not shown).
In all of patients, glutamic acid decarboxylase antibody (GAD) and islet cell antibody (ICA) and insulin antibody (IAA) were negative. No patients have apparent diabetic complications including microvascular and macrovascular complications.
Serum levels of IL-17 in patients with type 2 diabetes
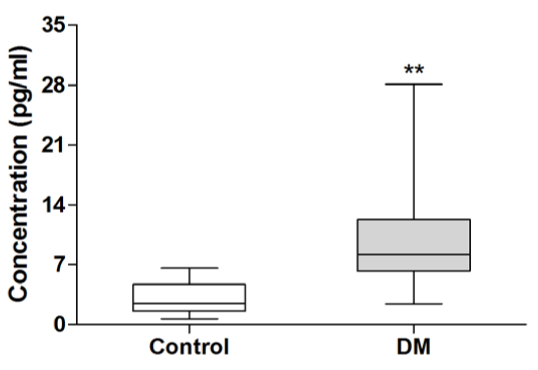
To investigate the role of IL-17 in type 2 diabetes, we examined serum concentrations of IL-17 in patients with newly diagnosed type 2 diabetes. As shown in Figure 1, serum IL-17 levels in patients with type 2 diabetes were significantly elevated compared to healthy subjects (10.44 ± 6.47 vs 2.99 ± 1.68 pg/mL, P<0.01).
mRNA expression of IL-17and RORγt in PBMC from diabetic patients
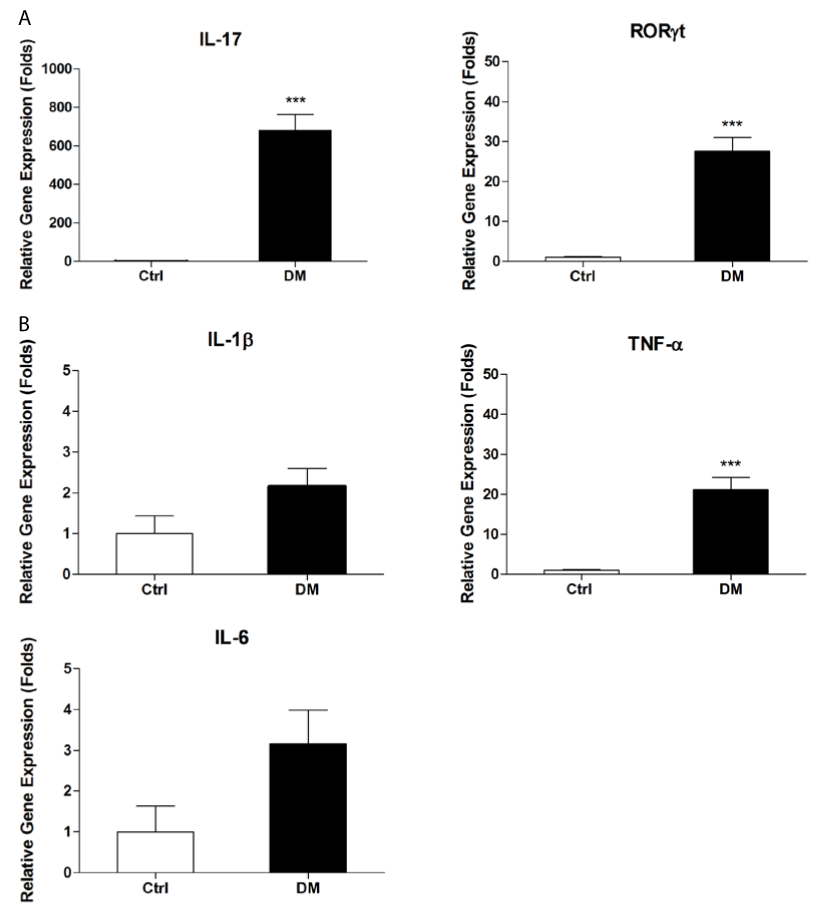
PBMC is a major source of IL-17. To test whether serum IL-17 is secreted by PBMC, we further investigated mRNA levels of IL-17 and its upstream regulator RORγt in PBMC from patients with type 2 diabetes. As shown in Figure 2A, mRNA levels of IL-17 were dramatically higher in diabetic patients than in control subjects (P<0.001); and the expression of RORγt gene in diabetic patients were also markedly increased compared with control subjects (P<0.001).
Relationship between IL-17 and IL-6 or TNF-α or IL-1β mRNA expression levels in PBMC from diabetic patients
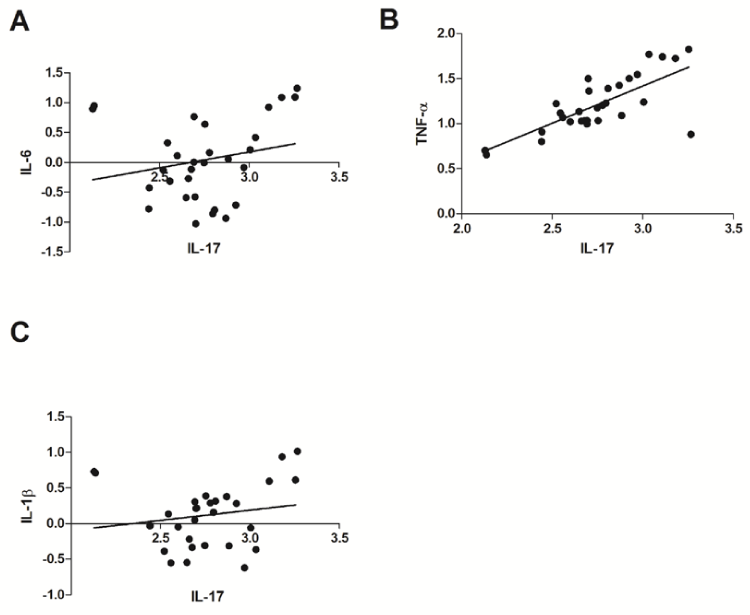
To clarify the relationship between IL-17 and other inflammation cytokines on mRNA levels, we first examined mRNA expression of inflammation cytokines such as IL-6, TNF-α and IL-1β in PBMC from diabetic patients using real time PCR. Our results showed mRNA levels of TNF-α was significantly upregulated in patients with type 2 DM (Figure 2B). Although mRNA levels of IL-6 and IL-1β were increased, but no statistical difference was observed between control and type 2 DM group. We further analyzed the correlation between mRNA expression of IL-17 and inflammation cytokines including IL-6, TNF-α and IL-1β. No significant relationship between the expression of IL-17 gene and that of IL-6 or IL-1β gene was observed in PBMC from diabetic patients (Figure 3A and Figure 3C). However, there was significant correlation between IL-17 gene expression and TNF-α gene expression in PBMC from diabetic patients (r=0.6988, P<0. 001) (Figure 3B).
Discussion
This study was designed to investigate IL-17 levels in patients with newly diagnosed type 2 diabetes. There are several notable findings in the present study. First, we found that serum levels of IL-17 in newly diagnosed patients with type 2 DM were significantly increased compared to healthy subjects. Second, we demonstrated that mRNA expression of IL-17 and its transcription regulator RORγt were markedly upregulated in PBMC from diabetic patients. Final, our data showed that IL-17 gene expression correlated with TNF-α gene expression in PBMC from diabetic patients. These results suggested that IL-17 might participate in the inflammatory process of type 2 diabetes and have a crucial role in the pathogenesis of type 2 DM.
Chronic low-grade inflammation is closely involved in the pathogenesis of type 2 diabetes. Elevated concentrations of various inflammatory markers such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 and IL-1β have been reported in patients with type 2 diabetes [14-16]. There is evidence that inflammatory mediators not only represent markers of metabolic aberrations [17,18] but may also contribute to insulin resistance and β-cell dysfunction [19]. TNF-α as well as IL-6 are considered the major adipocyte cytokines [20]. Both can alter insulin sensitivity by triggering different key steps in the insulin signaling pathway [21-23]. IL-1β negatively affects β-cell function and plays a role in the pathogenesis of progressive β-cell failure in type 2 diabetes [24]. Our results demonstrated that mRNA expression of TNF-α, IL-1β and IL-6 were increased at different levels in PBMC from patients with newly diagnosed type 2 diabetes. Moreover, we found that IL-17 gene expression correlated with TNF-α gene expression in PBMC from diabetic patients. These results suggested IL-17 might participate in the pathogenesis of type 2 DM together with other inflammation cytokines.
Here we demonstrated that the expression of IL-17 is increased in patients with newly diagnosed type 2 DM. The exact role of IL-17 in the pathogenesis type 2 DM has not been explored. IL-17 is capable of inducing the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, chemokines and adhesion molecules, which mediate tissue infiltration and tissue destruction. In addition to stimulating the secretion of other pro-inflammatory cytokines, IL-17 is involved in the induction of potentially harmful mediators of inflammation, such as free radical nitric oxide [25]. Recent studies have described an ongoing process of β cell destruction by apoptosis in animal models of type 2 diabetes and in human type 2 DM [26,27]. In human islets, IL-1β has been shown to impair insulin release and to promote the apoptosis of β cell [28]. Several studies have pointed to the evidence that some cytokines such as TNF-α are involved in the apoptosis of β cell in synergy with IL-1β [29]. Further, Miljkovic D reported IL-17 could augment the expression of inducible nitric oxide synthase (iNOS) and cause NO-dependent toxicity in MIN6 cells and mouse pancreatic islets [11]. Therefore, it is possible that IL-17 might participate in the local inflammation and lead to the destruction of β cell in the pancreas in synergy with these inflammation cytokines.
In the study, our data indicated that IL-17 may be involved in the inflammation process of type 2 diabetes. But whether IL-17 is involved in the progression of type 2 diabetes is not conclusively clarified. Several issues are still unsolved. Recent study reported that the plasma level of IL-17 is increased in obese women [30]. Obesity is considered the main environmental cause for the development of insulin resistance and type 2 DM. Obesity is defined as BMI>25 kg/m2 in Asian. In our experiment, the average level of BMI in all patients is about 25.42 kg/ m2; and BMI levels were similar between diabetic patients and health subjects. As a consequence of narrow selection criteria, the subdivision of the patients’ groups was hardly enough to make clear estimations. Further study should be performed in a large number of diabetic patients including diabetic patients with low-level BMI (BMI<25) and diabetic patients with high-level BMI (BMI ≥ 25)in order to investigate the relationship between IL-17 and clinical parameters. Moreover, further studies is required to investigate the circulating levels of IL-17 in groups with impaired glucose tolerance (IGT) and diabetic patients with complications. Serum IL-17 level might be useful to predict an early detection of risk for type 2 diabetes, as well as being potential marker of established diabetic complications. On the other hand, we need to further explore the detailed mechanism of IL-17 involved in the pathogenesis of type 2 diabetes.
Figure 2: Gene expression in peripheral blood mononuclear cells from patients with type 2 DM. PBMC were separated from patients with type 2 diabetes and healthy controls and subjected to real time PCR analysis for mRNA expression of IL-17 and its transcript factor RORγt, IL-6, TNF-α and IL-1β. Data are presented as relative expression of genes in reference to GAPDH. DM: type 2 diabetic patients; Control: Healthy controls. Data are representative of three experiments. Asterisks represent statistical differences between groups; ***P<0.001.
In conclusion, we demonstrated that serum levels and mRNA levels of IL-17 are increased in patients with newly diagnosed type 2 DM. Our results suggested IL-17 might promote the inflammatory state of patients, and participate in the pathogenesis of type 2 DM. Further studies are necessary to clarify the crucial role of IL-17 in the pathogenesis of type 2 DM and whether IL-17 is a prognostic factor for the development of type 2 DM.
Figure 3: Relationships between mRNA expression of IL-17 and inflammation cytokines in peripheral blood mononuclear cells from patients with type 2 DM. A: Relationship between expression levels of IL-17 and IL-6 in PBMC from diabetic patients (r=0.2529, P=0.1775); B: Relationship between expression levels of IL-17 and TNF-α in PBMC from diabetic patients (r=0.6988, P<0.001); C: Relationship between expression levels of IL-17 and IL-1β in PBMC from diabetic patients (r=0.2516, P=0.1798). r=correlation coefficient; The P values were calculated by Spearman’s correlation coefficient by rank test.
References
- Crook M (2004) Type 2 diabetes mellitus: A disease of the innate immune system? An update. Diabet Med 21: 203-207.
- Haffner SM (2003) Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol 92: 18J-26J.
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, et al. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123-1132.
- AggarwalS, Gurney AL (2002) IL-17: prototype member of an emerging cytokine family. J LeukocBiol 71: 1-8.
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, et al. (1996) T cell interleukin-17 induces stromal cells to produce pro-inflammatory and hematopoietic cytokines. J Exp Med 183: 2593-2603.
- Kolls JK, Lindén A (2004) Interleukin-17 family members and inflammation. Immunity 21: 467-476.
- Korn T, Oukka M, Kuchroo V, Bettelli E (2007) Th17 cells: effector T cells with inflammatory properties. SeminImmunol 19: 362-371.
- Kurts C (2008) Th17 cells: a third subset of CD4+ T effector cells involved in organ-specific autoimmunity. Nephrol Dial Transplant 23: 816-819.
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, et al. (2008) Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 172:146-155.
- Paradowska A, MaÅ›liÅ„iski W, Grzybowska-Kowalczyk A, Å√?¬Āacki J (2007) The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch ImmunolTherExp (Warsz) 55: 329-334.
- Miljkovic D, Cvetkovic I, Momcilovic M, Maksimovic-Ivanic D, Stosic-Grujicic S, et al. (2005) Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse beta cells. Cell Mol Life Sci 62: 2658-2668.
- Vukkadapu SS, Belli JM, Ishii K, Jegga AG, Hutton JJ, et al. (2005) Dynamic interaction between T cell-mediated beta-cell damage and beta-cell repair in the run up to autoimmune diabetes of the NOD mouse. Physiol Genomics 21:201-211.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412-419.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327-334.
- Pickup JC, Chusney GD, Thomas SM, Burt D (2000) Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 67: 291-300.
- Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, et al. (2008) Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31 Suppl 2: S161-164.
- Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27: 813-823.
- Kolb H, Mandrup-Poulsen T (2005) An immune origin of type 2 diabetes? Diabetologia 48: 1038-1050.
- Donath MY, Størling J, Maedler K, Mandrup-Poulsen T (2003) Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med (Berl) 81: 455-470.
- Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R (2003) Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448-1454.
- Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol 25: 4-7.
- Ruan H, Lodish HF (2003) Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev 14: 447-455.
- Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, et al. (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J BiolChem 278: 13740-13746.
- Donath MY, Størling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T (2008) Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev 29: 334-350.
- Miljkovic D, Trajkovic V (2004) Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev 15: 21-32.
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, et al. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102-110.
- Donath MY, Gross DJ, Cerasi E, Kaiser N (1999) Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomysobesus during development of diabetes. Diabetes 48: 738-744.
- Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG (1998) Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1beta and Fas ligation. Diabetes 47:727-732.
- Rabinovitch A1, Sumoski W, Rajotte RV, Warnock GL (1990) Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J ClinEndocrinolMetab 71: 152-156.
- Sumarac-Dumanovic M1, Stevanovic D, Ljubic A, Jorga J, Simic M, et al. (2009) Increased activity of interleukin-23/interleukin-17 pro- inflammatory axis in obese women. Int J Obes (Lond) 33: 151-156.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 13680
- [From(publication date):
June-2016 - Dec 04, 2024] - Breakdown by view type
- HTML page views : 12657
- PDF downloads : 1023