Research Article Open Access
Electro-Oxidation and Detection of Phenol on Metals Modified Carbon Paste Electrodes
Maallah R1, Hafid A2, Barakat A3 and Chtaini A1*1Equipe of Molecular Electrochemistry and Inorganic Materials, Faculty of Science and Technology of Beni Mellal, Sultan Moulay Slimane University, Morocco
2Organic Chemistry and Analytical Laboratory, Faculty of Sciences and Techniques Beni Mellal, University Sultan Moulay Slimane, Morocco
3ETE laboratory and Environment, Faculty of Science and Technology Beni Mellal, University Sultan Moulay Slimane, Morocco
- *Corresponding Author:
- A Chtaini
Equipe of Molecular Electrochemistry and Inorganic Materials
Faculty of Science and Technology of Beni Mellal
Sultan Moulay Slimane University, Morocco
Tel: +21252348511
Fax: +212523485201
E-mail: a.chtaini@usms.ma
Received date: March 29, 2016; Accepted date: April 21, 2016 Published date: April 29, 2016
Citation: Maallah R, Hafid A, Barakat A, Chtaini A (2016) Electro-Oxidation and Detection of Phenol on Metals Modified Carbon Paste Electrodes. Toxicol open access 2:111. doi:10.4172/2476-2067.1000111
Copyright: © 2016 Maallah R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Toxicology: Open Access
Abstract
A variety of electrodes based on the modification of the carbon paste electrode by various metals such as, Ti, Natural phosphate (NP), kaolin, were prepared. Cyclic voltammetry and square wave voltammetry tests were performed to evaluate the electrochemical performance. The results confirmed that the modification of cabon paste electrode by metals was successful. The presence of metal in the electrode matrix enhanced the electrode stability and increases its performance for the phenol oxidation. The electrochemical study of the complex mediated graphite electrode revealed its fast electron transfer property.
Keywords
Cyclic voltammetry; Square wave voltammetry; Modified electrode; Phenol
Introduction
Phenol is considered as one of the most serious environmental contaminants rejected by industrial plants such as pharmaceuticals, formaldehyde resins, pesticides, textiles, petroleum refineries, chemical industry and agricultural activities. Phenol is one of 129 chemical compounds considered important pollutants listed by the Agency for Environmental Protection (EPA) [1,2]. Various methods for the treatment of phenolic wastewater have been used, such as wet air oxidation [3], adsorption [4], chemical oxidation [5], photocatalyst [6], biological treatment [7], and ozone oxidation [8]. But there are few sufficiently efficient processes for the removal of phenol.
The electrochemical oxidation methods has become a promising method for the toxic, bio refractory and highly concentrated organic wastewater treatment because of its simplicity, easy control, strong oxidation performance and environmental compatibility [9]. For anodic oxidation reactions, that the organic matter electro oxidation process, hydroxyl radicals (.OH), produced by water discharge through the retardation of oxygen at high electrode over potential on metal/ particle electrode surface, are required for organic matter elimination [10,11]. Recently, electrochemically activated carbon electrodes such as glassy carbon and screen printed carbon electrode (SPCE) have been broadly used for the detection of various analytes [12-15]. The activated carbon electrodes have showed more surface area and high electrochemical conductivity than that of bare electrodes due to the presence of more surfaces detects on the electrode surface. However, the electrochemically activated screen printed carbon electrode (EASPCE) has never been used for the detection of phenol in the industrial pollutants.
The goal of this work was to examine possible beneficial effect of metals modified carbon paste electrode in the electro oxidation of phenol.
Experimental
Voltammetric experiments were performed using a voltalab potentiostat (modelPGSTAT 100, Eco Chemie B.V., Utrecht, The Netherlands) driven by the general purpose electrochemical systems data processing software (voltalab master 4 software) run under windows 2007. The three electrode system consisted of a chemically modified carbon paste electrode as the working electrode a saturated calomel electrode (SCE) serving as reference electrode, and platinum as an auxiliary electrode.
Reagents and chemicals
All chemicals were of the highest quality. Graphite powder (spectroscopic grade RWB, Ringsdorff-Werke GmbH, Bonn-Bad Godesberg, Germany) was obtained from Aldrich and was used without further purification, Titanium powder was obtained from Merck chemicals. Deionised water was used to prepare all solution.
Electrode preparation
Modified electrodes were prepared by mixing a carbon powder and the desired weight of Natural Phosphate (NP), titanium and/or clay. A 4% of modifiers was used thorough this work.
The body of the working electrode for voltammetric experiments was a PTFE cylinder that was tightly packed with carbon paste. The geometric area of this electrode was 0.1256 cm2. Electrical contact was made at the back by means of a bare carbon. A Natural Phosphate (NP) used in this work was obtained in the Khouribga region (Morocco). Prior to use, this material was treated by techniques involving attrition, sifting, calcinations (900°C), washing, and recalcination. Kaolin was obtained in the north of Cameroun region.
Procedure
The initial working procedure consisted of measuring the electrochemical response at Metal-CPE at a fixed concentration of phenol. Standard solution of phenol was added into the electrochemical cell containing 100 mL of supporting electrolyte. The mixture solution was kept for 20 s at open circuit and deoxygenated by bubbling pure nitrogen gas prior to each electrochemical measurement. The cyclic voltammetry was recorded in the range from -1.8V to 1.8V. Optimum conditions were established by measuring the peak currents in dependence on all parameters. The square wave voltammetry was recorded in the range from -1.8V to 1.8V, for which the scan rate is 1 mV.s-1, step potential 50 mV, amplitude 2 mV and duration 0.1 s. All experiments were carried out under ambient temperature. Supporting electrolytes have been known to enhance the reaction response of modified electrodes towards the electro oxidation of phenol by increasing either the current response and/or lowering the reaction potential. We studied the electrochemical response characteristics of the prepared electrodes in 0.1 mol L−1 Na2HPO4, 0.1 mol L−1 Na2SO4, and the best results were obtained with Na2SO4.
Results and Discussion
Electrode Ti-CPE
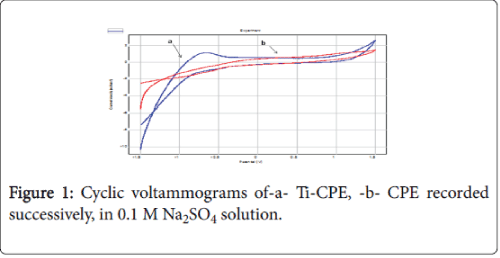
As shown in Figure 1 on the Titanium modified carbon paste electrode (Ti-CPE), the cyclic voltammograms (CV’s) recorded respectively carbon paste electrode (CPE) and Ti-CPE in 0.1 M Na2SO4 solution, demonstrated two different behaviours. In the absence of Titanium, the work electrode shows no peaks (curve b). In the presence of Titanium, the CV shows an anodic peak, at about -0.3V, which probably corresponds to the surface oxidation.
Phenol oxidation
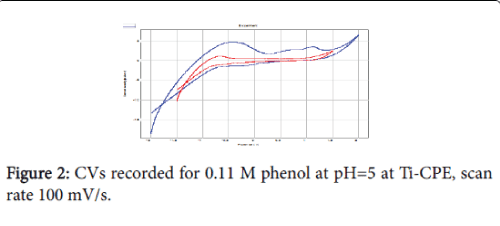
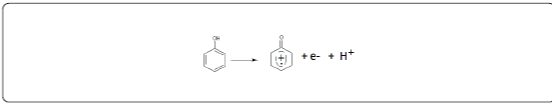
Cyclic voltammetry (CV) at a scan rate of 100 mV/s was the electrochemical technique applied to study the oxidation behaviour of phenol (Figure 2). We can see that in the presence of phenol, the current densities are remarkably very important. The CV shows the appearance of three peaks, two in the anodic scan, at about 0.6 V and 1.7 V and a reduction peak at -0.1 V. Taking into account the positions of the anodic peaks and the cathodic peak, we can conclude that it is not the same redox system. The oxidation of phenol is thus irreversible. The proposed mechanism is the following:
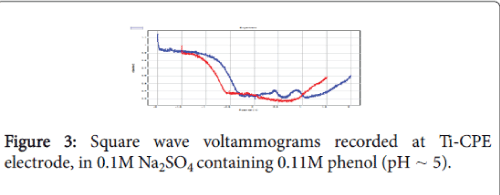
The ability of the Ti-CPE to oxidizing phenol was also studied by square wave voltammetry (SWV). Figure 3 shows the SWV obtained in 0.1 mol L-1 Na2SO4 in presence and absence of phenol. When the electrolytic solution was enriched by phenol, square wave voltammetry shows two well defined peaks. These peaks were due to the oxidation of phenol.
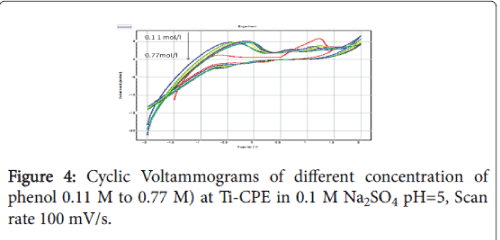
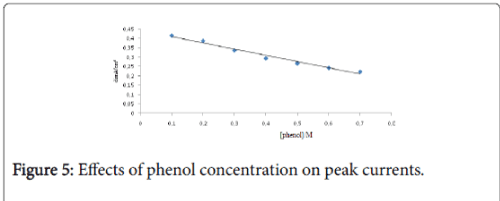
The increase in the concentration of phenol decreased the current density of the anodic peaks, which can be explained by the saturation of the electrode surface (Figure 4). The Figure 5 shows the linear relationship between the scan rate anodic peak currents of phenol at Ti-CPE, deducted from SWV’s.
Electrode kaolin-CPE
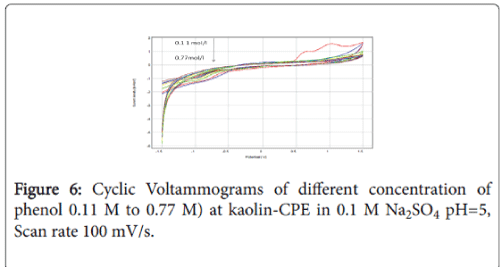
Typical metal matrix modified carbon paste electrode have been shown to be able to participate in the electro oxidation of phenolic solution, such as, NP and kaolin. Figure 6 shows the CV’s recorded respectively at NP-CPE electrode, in electrolytic solution containing phenol (curve b) and in without phenol (curve a). A strong peak can be observed at a potential range from around 0.5 to 1.5 V, as it can be seen the double oxidation of phenol is characterized by two well defined peaks. Current densities are 10 times higher than the one observed on Ti-CPE electrode. The same behavior was observed when the concentration of phenol increase, the oxidation current densities decrease, which confirms the saturation of the electrode surface.
Electrode NP-CPE
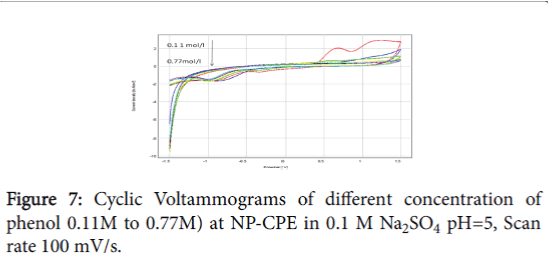
When the clay is replaced by the NP, the oxidation current densities are slightly improved. The structure is characterized by the presence of connection witch facilitate the adsorption of the molecule of phenol, but at high concentrations of the phenol, we find a power drop due to the passivation of the electrode surface (Figure 7).
Conclusion
This work the electro oxidation of phenol on various prepared electrodes was carried out. On all studied electrodes, the oxidation of phenol is characterized by the appearance of two well defined anodic peaks. With clay and NP, as modifiers, oxidation current densities of the phenol are 10 times greater than in the case of Ti-CPE electrode. The porous nature of metallic matrix, NP and kaolin, facilitate the adsorption of phenol at electrode surface and catalyzes the oxidation. We noted that the current density of the anodic peaks falls remarkably when the concentration of phenol becomes significant, probably due to saturation of the electrode surface, which confirms that the adsorption of phenol constitutes the decisive step of the reaction of oxidation. Nevertheless there is a strong relationship between the peak current density and phenol concentration.
References
- Keith LH, Telliand W (1979) ES &T special priority pollutants: I-a perspective view. Environ Sci Technol 13: 416-423.
- Zhang F, Li M, Li W, Feng C, Jin Y, et al. (2011) Degradation of phenol by a combined independent photocatalytic and electrochemical process. Chemical Engineering Journal 175: 349-355.
- Lefèvre S, Boutin O, Ferrasse JH, Malleret L, Faucherand R, et al. (2011) Thermodynamic and kinetic study of phenol degradation by a non-catalytic wet air oxidation process. Chemosphere 84: 1208-1215.
- Su J, Lin H-f, Wang QP, Xie ZM, Chen ZL (2011) Adsorption of phenol from aqueous by organomontmorillonite. Desalination 269: 163-169.
- Manojlovic D, Ostojic DR, Obradovic BM, Kuraica MM, Krsmanovic VD, et al. (2007) Removal of phenol and chlorophenols from water by new ozone generator. Desalination 213: 116-122.
- Xu X, Yi Z, Chen D, Duan X, Zhou Z, et al. (2012) Evaluation of photocatalytic production of active oxygen and decomposition of phenol in ZnO suspensions. Rare metals 28: 347-348.
- Ahmad SA, Shamaan NA, Arif NM, Koon GB, Shukor MY, et al. (2012) Enhanced phenol degradation by immobilized Acinetobacter sp. strain AQ5NOL 1. World J Microbiol Biotechnol 28: 347-352.
- Joshi MG, Shambaugh RL (1982) The kinetics of ozone phenol reaction in aqueous solution. Water Res 16: 933-938.
- Zhu X, Tong M, Shi S, Zhao H, Ni J (2008) Essential explanation of the strong mineralization performance of boron-doped diamond electrodes. Environ Sci Technol 42: 4914-4920.
- Liu H, Liu Y, Zhang C, Shen R (2008) Electrocatalytic oxidation of nitrophenols in aqueous solution using modified PbO2 electrodes. J Appl Electrochem 38: 101-108.
- Rice RJ, McCreery RL (1989)Quantitative Relationship between Electron Transfer Rate and Surface Microstructure of Laser-Modified Graphite Electrodes AnalChem 61: 1637-1641.
- Sánchez S, Fàbregas E, Pumera M (2009) Electrochemical activation of carbon nanotube/polymer composites. Phys Chem ChemPhys 11: 182-186.
- Prasad KS, Muthuraman G, Zen JM (2008)The role of oxygen functionalities and edge plane sites on screen-printed carbon electrodes for simultaneous determination of dopamine, uric acid and ascorbic acid. Electrochem Commun 10: 559-563.
- Karuppiah C, Palanisamy S, Chen SM, Veeramani V, Periakaruppan P (2014) A novel enzymatic glucose biosensor and sensitive non-enzymatic hydrogen peroxide sensor based on graphene and cobalt oxide nanoparticles composite modified glassy carbon electrode. Sensors and Actuators B: Chemical 196: 450-456.
- Palanisamy S, Karuppiah C, Chen SM, Periakaruppan P (2014)Highly sensitive and selective amperometric nitrite sensor based on electrochemically activated graphite modified screen printed carbon electrode. J Electroanal Chem 727: 34-38.
Relevant Topics
- Aflatoxins
- Cardiac Toxicity
- Chemical Toxicology
- Developmental Toxicology
- Drug Toxicity
- Heavy Metal Toxicity
- Heavy Metal Toxins
- Industrial Hygiene Toxicology
- Insecticides Toxicology
- Metal Toxicology
- Nano Toxicology
- Pesticidal Toxicology
- Renal Toxicity
- Reproductive Toxicology
- Skin Toxicology
- Tetanus Toxin
- Toxicogenomics
- Toxicology Reports
- Toxicology Testing
Recommended Journals
Article Tools
Article Usage
- Total views: 12646
- [From(publication date):
May-2016 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 11672
- PDF downloads : 974