Review Article Open Access
Electrolytic Metal Manganese Industry in Chongqing City of China
Wensheng L1,2, Zuohua L1,2, Changyuan T1,2*, Xuming W3*and Miller JD31College of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China
2Chongqing Key Laboratory of Chemical Process for Clean Energy and Resource Utilization, Chongqing, China
3Department of Metallurgical Engineering, College of Mines and Earth Sciences, University of Utah, Salt Lake City, USA
- *Corresponding Authors:
- Changyuan Tao
College of Chemistry and Chemical Engineering
Chongqing University, Chongqing, 400044, China
Tel: + 8613983053187
E-mail: aocy@cqu.edu.cn - Xuming W
Department of Metallurgical Engineering
College of Mines and Earth Sciences
University of Utah, Salt Lake City, UT 84112, USA
Tel: 18015851797
E-mail: x.wang@utah.edu
Received Date: November 04, 2015; Accepted Date: November 16, 2015; Published Date: November 30, 2015
Citation:Wensheng L, Zuohua L, Changyuan T, Xuming W, Miller JD (2015) Electrolytic Metal Manganese Industry in Chongqing City of China. J Powder Metall Min 4:139. doi:10.4172/2168-9806.1000139
Copyright: © 2015 Wensheng L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Powder Metallurgy & Mining
Abstract
Electrolytic metal manganese (EMM) is the main industry for Chengkou and Xiushan countries in Chongqing, which accounts for local GDP about 85% and 75% respectively. In 2013, hydrometallurgical produced about 200 thousand tons EMM from about 2.0 million tons ore. But the Energy consumption is over 6000 kwh per ton of metal with the DC electrolytic efficiency of less 75%, and simultaneously producing 10 ~ 12 tons of solid residue. With the development of the EMM, the manganese content of the ore continuously decreased to 13%, and impurities in the ore become complex. The present article introduces the current status of the local industry in Chongqing, scientific advances and problems are also discussed. The sustainable development is necessary for the local EMM industry; new products related to manganese industry are also proposed.
Keywords
Electrolytic metal manganese; Hydrometallurgy; Chongqing
Introduction
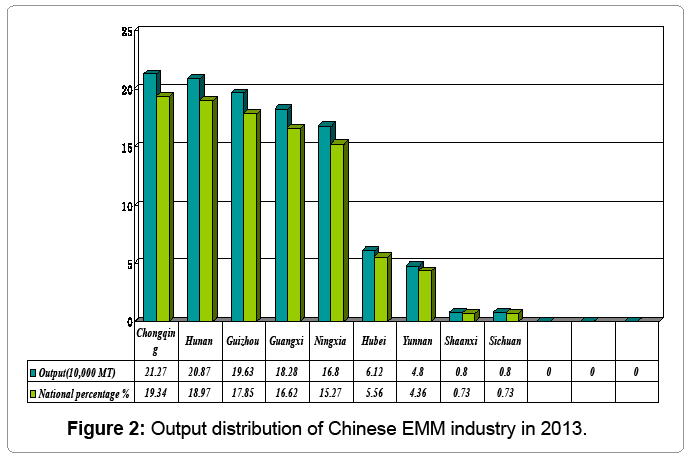
Manganese is an important element for iron-steel industry, and it is widely said “no manganese, no steel”. There are hydrometallurgical and pyrometallurgical routes for the processing manganese ore. Hydrometallurgical processes can meet this demand with low content manganese ore. The actual output in 2013 was 1.1 million tons of EMM, down by 60,000 tons or 5.2% over 2012 (Figure 1). Although the decrease for a succession of two years was unprecedented in the Chinese EMM industry, it is still the biggest producer in the world, accounting for over 98.5% of the total output in the world [1]. Except for China, only South Africa has ore EMM plant with annual capacity of 30 kt. Chongqing City, located in the southwest of China, ranks second with its rhodochrosite ore, accounting about 83.74 million tons with content 16.55-27.96% manganese ore (Figure 2). There are mainly two countries processing manganese ore in Chongqing City to produce EMM as shown in Table 1, and manganese industry accounts for 85% and 75% of local GDP for Chengkou County and Xiushan County, respectively. But the sustainable development of the EMM industry is necessary to meet the social and environmental requirements associated with advances in science and technology.
| Order | Region | Capacity(10,000 MT/year) | Output (10,000 tons) |
|---|---|---|---|
| 1 | Xiushan, Chongqing | 25.9 | 19.27 |
| 2 | Zhongning, Ningxia | 20 | 16.8 |
| 3 | Songtao, Guizhou | 14.2 | 10.25 |
| Total | 60.1 | 46.32 |
Table 1: Main counties of Chinese EMM manufacturers.
Hydrometallurgical Route for EMM
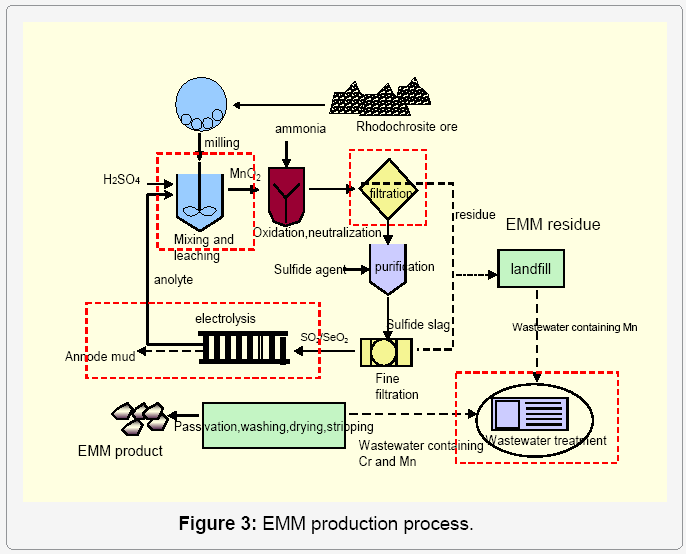
The hydrometallurgical process for EMM is shown in Figure 3, with main materials including rhodochrosite (MnCO3), H2SO4, (NH4)2SO4, ammonia, and some additives. The main unit operations are milling, mixing and leaching, purification and filtration, electrolysis, wastewater treatment, etc. Production of 1 t of EMM, requires about 8 ~ 10 t milled ore powder, 1.70 ~ 1.95 t sulfuric acid (95 wt%), 80 kg ammonia, 1.2 ~ 2.0 kg SeO2, and 5800 ~ 7000 kWh DC electricity, and discharges 1.0 ~ 1.3 t CO2 into the air during leaching and 10 ~ 12 t of solid residue containing NH3-N and MnSO4, etc. The wastewater from the in-situ and the landfill leachate is about 130 t product and is prone to harm to the environment. Especially during in wet season, the landfill leachate containing NH3-N and heavy metals is a disaster for the water resources. The chemical reactions for EMM production are as follows:
Leaching
For the leaching process, the anolyte recycling from the electrolysis and the fresh high concentrated H2SO4 are pumped into the reactor to form slurry.
MnCO3 + H2SO4 = MnSO4 + H2O + CO2↑ (1)
CaCO3 + H2SO4 = CaSO4 + H2O + CO2↑ (2)
MgCO3 + H2SO4 = MgSO4 + H2O + CO2↑ (3)
The other impurities in the ground ore (~ 200 mesh), include Al, Fe, Cu, Co, Ni, Zn, all of which will be reacted to solution. The gangue SiO2 will go into the EMM residue without reaction.
Purification
After the leaching of the ore, ammonia is pumped into the slurry to neutralize the over dosage of H2SO4. Al3+ is easy to remove by precipitation with ammonia. Because it is hard to remove Fe2+ by Fe(OH)2, traditionally it is oxidized to Fe3+ with pyrolusite (MnO2) or anode mud from the electrolytic cell, then it is removed by precipitate ion of Fe(OH)3.
MnO2 + 2Fe2+ + 4H+ = 2Fe3+ + Mn2+ + H2O (4)
Fe3+ + 3NH3 + 3H2O = Fe(OH)3↓ + 3NH4+ (5)
Al3+ + 3NH3 + 3H2O = Al(OH)3↓ + 3NH4+ (6)
The heavy metal ions, Co2+,Ni2+, Cu2+,Pb2+,Zn2+, etc., will do much harm to the electrolytic process, and ore removed as sulfide compounds using SDD, (NH4)2S. In industry process, the concentration of the Co2+and Ni2+ should be controlled lower than 1.0 mg/L, or the deposited metal manganese will be redissolved into the aqueous MnSO4 solution.
M2+(Co2+,Ni2+, Cu2+,Pb2+,Zn2+,etc.) + SDD = MSDD↓ (7)
M2+(Co2+,Ni2+, Cu2+,Pb2+,Zn2+,etc.) + (NH4)2S = MS↓+NH4 + (8)
Electrolysis process
The aqueous MnSO4 solution is controlled at 33~35 g/L Mn2+, 110 ~ 130 g/L (NH4)2SO4, and (NH4)2SO3 or SeO2 is added during the electrolysis cell to elevate the over-potential of H2, which can inhibit byproduct reactions. The concentration of the electrolyte Mn2+ should be controlled at about 12 ~ 14 g/L in the electrolysis cell. The manganese will be electrodeposited on cathode, and anode mud (MnO2) will be produces on the anode. In fact, on the cathode, the [Mn(NH3)4]2+ will lose electrons and metal manganese will be formed and crystallize on the surface layer of MnSe at the cathode surface.
Cathode reaction
Mn2+ + 4NH4+ = [Mn(NH3)4]2++4H+ (9)
[Mn(NH3)4]2+-2e = Mn+4NH3↑ (10)
2H+-2e = H2↑ (11)
Mn+SeO2+2H2 = MnSe+2H2O (12)
Anode reaction
4OH- + 4e = O2↑+H2O (13)
[Mn(NH3)4]2+ + 2e + O2 = MnO2 + 4NH3↑ (14)
Produce 1.0 t EMM, the output of anode mud will be about 50 ~ 150 kg, which can be used for Mn-Si alloy production. If the anode mud can be converted to α-MnO2, β-MnO2, orγ-MnO2 from the amorphorous state, MnO2 products valuable to prepare electrode material for battery production.
Advances for the EMM Industry in Chongqing City
Leaching with new mixing reactor
The leaching reactor is gas-liquid-solid mixing system, which plays vital a role for MnCO3 utilization. The average mixing tanks are about 180 m3, and some are over 400 m3 for batch operation. But poor mixing performance may lead to “column circulation”, and the ore particles (~ 200 mesh) will sink to the bottom of the tank for the traditional operation. In the present industrial operation, the air is used as both oxidative reagents for Fe2+ and stripper for CO2 removal from the reaction between H2SO4 and carbonates (MnCO3, CaO3, MgCO3, etc.) in the ore. The traditional process to remove Fe2+ with MnO2 is replaced by air, operating at pH 5 ~ 6 for about 2 h, which can reduce solid waste discharge and lower the operation cost.
Fe2+ + O2 + H2O = Fe(OH)3↓ (15)
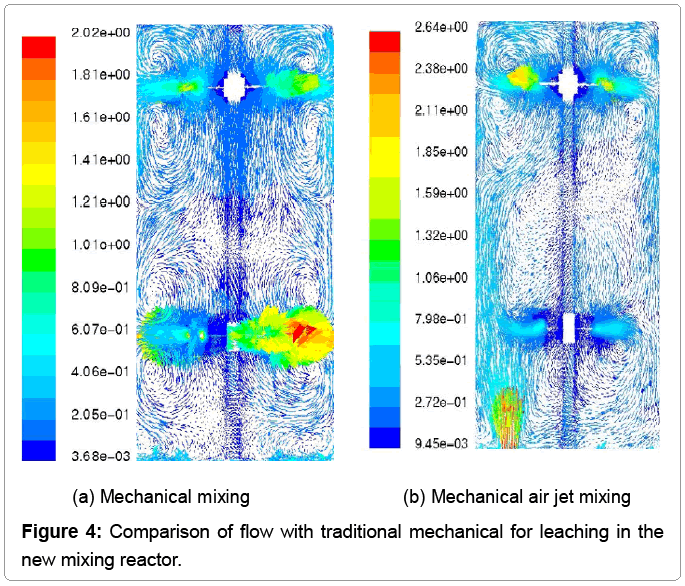
The air is eccentrically pumped into the slurry, which breaks the symmetric flow field, and enhances the chaotic mixing. Zuohua Liu [2,3] studied the flow field by computational fluid dynamics (CFD) with software Fluent 6.0. The eccentric mechanical air jet mixing can reduce leaching time from 10 ~ 12 h to 4 ~ 6 h per batch operation. Develop continuous leaching process and equipment to reduce the mixing tank size is needed and to improve the leaching efficiency (Figure 4).
Purification of MnSO4 solution
For the electrolyte solution MnSO4 -(NH4)2SO4, concentrations of Fe and Co are known as controlling parameters, but the complex salts are prone to crystallize in the formation of (NH4)2Mn(SO4)2, (NH4)2Mg(SO4)2, NH4AlSO4, etc. (NH4)2SO4 has the characteristic of secondary nucleation and it can form very hard crystal. Because there is no purification method to remove Mg2+ from the system and it will accumulate in the recycling anolyte. Especially in winter, both pipeline and diaphragm will often be blocked by the complex salts, which occur when the concentration of Mg2+ is over 30 g/L. The complex salts are manually removed and much solid waste will go into the landfill site, resulting in loss of manganese and ammonium salts. The solvent extraction or precipitation with NH4F can’t satisfy the industrial process. However we found that to mix high phosphorous rhodochrosite contributes to the removal of Mg2+ by NH4MgPO4. Nevertheless technology to reduce the high concentration Mg2+ is needed with little loss of manganese and ammonia.
Electro-deposition and nonlinear kinetics
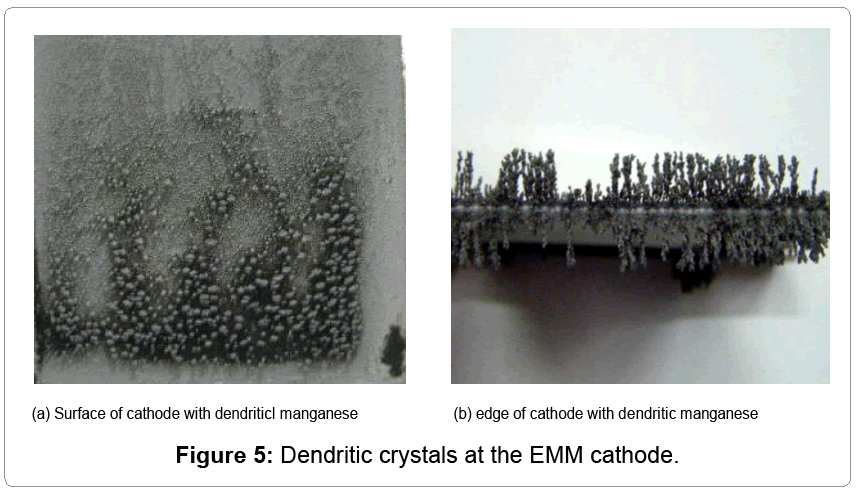
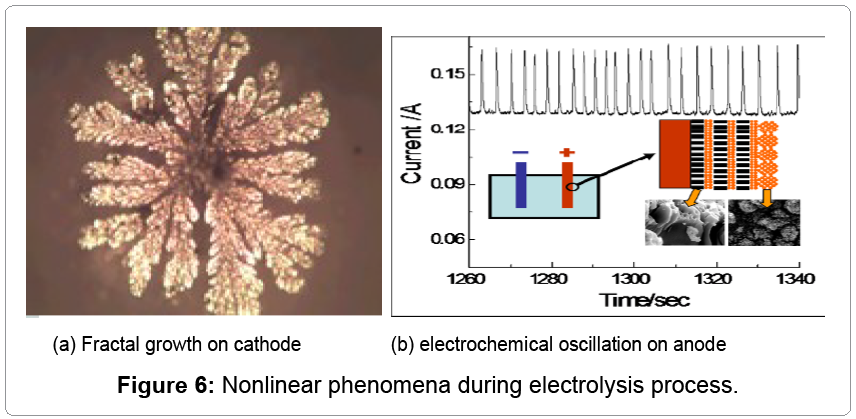
The hydrometallurgical electrolytic process for the production of manganese is typically far away from equilibrium state, and there are many reactions occurring simultaneously, which leads to nonlinear phenomena. Obviously, there are tree-like crystals on the cathode of 316L stainless steel plate, shown in Figure 5, which may result in short circuit and lower the electrolytic efficiency.
Tao [4-6] found that there were chemical oscillations on the anode and fractal growth on the cathode, shown in Figure 6, which may result in non-electrolytic energy consumption. The electrolytic process contains multi-field coupling and nonlinear chemical reactions.
The mechanism for nonlinear kinetics can be explained based on the following chemical reactions:
Mn2+ + 2H2O – 2e- = MnO2 + 4H+ (16)
H2O – 4e- = O2+4H+ (17)
Mn2++4H2O–5e- = MnO4 - + 8H+ (18)
2MnO4- + 3Mn2+ + 2H2O = 5MnO2 + 4H+ (19)
Mn2+ – e- = Mn3+ (20)
Mn3+ – e- = Mn4+ (21)
Mn4+ + 2H2O = MnO2 + 4H+ (22)
Mn3+ + 2H2O = MnOOH + 3H+ (23)
MnOOH – e- = MnO2 + H+ (24)
Hence, to realize energy saving during electrolysis process, it is vital to control the nonlinear chemical reactions. Tao [4-6] found that pulse DC electrolytic with porous anode contributed to improve the output by 5 ~ 10%, and saving 100 ~ 300 kWh per ton EMM. But the optimal model and large scale pulse DC electrolytic equipment need to be developed for energy saving and emission reduction.
Solid waste residue treatment and comprehensive utilization
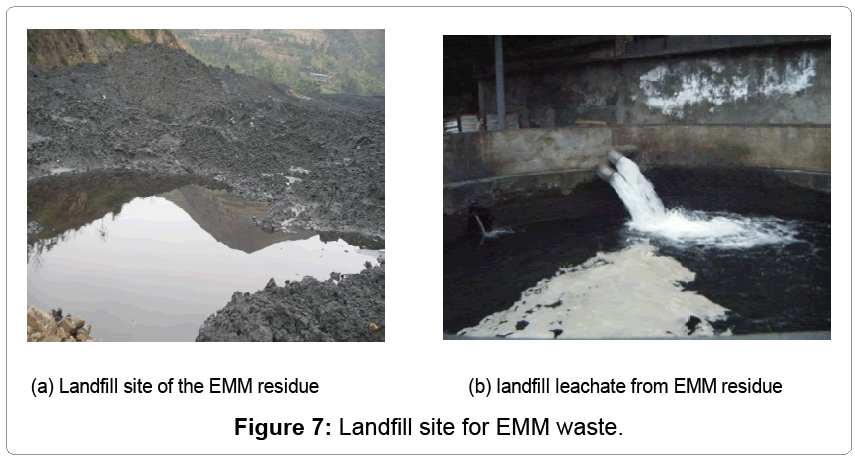
EMM residue from the leaching and purification units, which leads to serious environmental pollution, is the headache problem for the local governments, shown in Figure 7. As above mentioned, previously EMM residue contains Mn, Fe, Ca, Mg, Al, Si, NH3-N, etc., and water content is in the range of 23 ~ 28%. Many scientists found there were MnSO4, (NH4)2SO4, CaSO4, Fe(OH)3, with heavy metals and sulfide, etc. They developed some technologies to utilize the solid EMM waste has been developed, such as pavement base, preparation of brick, and so forth. But up till now, there is no feasible way to meet both economic and technical requirements. Tao [4-6] studied washing the EMM waste with water to recover part of the MnSO4 and (NH4)2SO4 , but it would break the water balance of the processing system. The soluble manganese in the EMM residue is in the form of complex salt, which cannot be washed out by limited water, but can enter local water resources by rainfall. In fact, EMM residue contains many complex salts, which must be controlled or decomposed during the leaching, to solve the loss of manganese and ammonium salts.
Wastewater and landfill leachate
Wastewater from passivation of manganese contains K2Cr2O7, and the dilute solution containing Cr(VI) is collected and chemically treated with FeSO4-Ca(OH)2. The other wastewater steam from electrolysis cell washing is precipitated with Ca(OH)2 or Na2CO3, and the manganese can be recovered to produce EMM. The landfill leachate containing 2-12 g/L Mn2+ can be pumped to prepare slurry. Dissolved manganese can be precipitated by chemical reagents, but it is a big problem for the wastewater containing low concentration NH3-N. If it is recovered by adding NaOH and air stripping, for NH3 collection, it is not cost effective. There is some membrane separation strategy for the wastewater purification, but it is hard to solve the problem, for the complex salt will block the pores of the membrane in a short time.
14H+ + Cr2O72- + 6Fe2+ = 2Cr3+ + 6Fe3+ + 7H2O (25)
Cr3+ + 3OH- = Cr(OH)3↓ (26)
Fe3+ + 3OH- = Fe(OH)3↓ (27)
Mn2+ + OH-= Mn(OH)2↓ (28)
Mn(OH)2 + CO3 2-= MnCO3↓ + 2OH- (29)
Scientific and Technical Problems
CO2 capture and utilization
In the leaching process, carbonates (MnCO3, MgCO3, CaCO3, etc.) react with H2SO4 and much CO2 is discharged into the air. For the Chinese EMM’s output 1.1 million tons, the discharged CO2 is about 1.1 ~ 1.3 million tons. The environmental protection agencies will consider the process strategies to control the CO2 emission. The capture and fixation of CO2 is still under study for the EMM residue.
Comprehensive utilization of low grade and high impurity ore
With the development of EMM industry, high content Mn ore have been consumed and the commercial ore grade has lowered from 20% in 2006 to 13% at present. Before 2006, ore with Mn content below 18% was discarded. Now, some plants begin to use ore with a Mn content of 9%, which produces too much EMM residue and consumes too much sulfuric acid. But in the mining district, there are plenty of Mn ores in the content range of 5 ~ 10%, which needs to be treated by mineral processing to produce a high grade concentrate suitable for hydrometallurgical process beneficiation technology.
On the other hand, there are complex refractory manganese ores, which contain high content P or S, shown in Tables 2 and 3, and they also need to be concentrated beneficiation process. In the hydrometallurgy process for manganese ore with high sulfur, H2S will go out from the leaching slurry. In January 9th, 2008, 5 workers died, 13 workers poisoned by the H2 S gas during the operation in a plant of Chongqing City. Zuohua Liu [2,3] studied the desulfurization of manganese ore with concentrated NaOH solution, the desulfurization ratio was over 90%, it was found that polysulfide (S2 O6 2-, S4 O6 2-, etc.), in the leaching solution would lead to low electrolytic efficiency, as well as H2S release in the cathode.
| Element | O | Mn | Si | Mg | Ca | Al | Fe | K | Na | S |
| wt´╝? | 39.3 | 29.3 | 9.7 | 3.2 | 12.3 | 0.87 | 1 | 0.31 | — | 0.95 |
| Element | P | Ti | V | Sr | Ba | Zn | Co | Cr | Rb | Ni |
| wt´╝? | 2.7 | 0.07 | — | 0.04 | 0.11 | 0.02 | — | — | — | 0.02 |
Table 2: Composition of rhodochrosite with high phosphorous content by XRF.
| Element | O | Mn | Si | Mg | Ca | Al | Fe | K | Na | S |
| wt´╝? | 39.27 | 22.40 | 12.86 | 4.65 | 12.89 | 1.66 | 0.65 | 0.34 | 0.18 | 4.04 |
| Element | P | Ti | V | Sr | Ba | Zn | Co | Cr | Rb | Ni |
| wt´╝? | 0.28 | 0.048 | — | 0.024 | — | 0.015 | — | 0.014 | — | 0.018 |
Table 3: Composition of rhodochrositewith high sulfur content by XRF.
Mn-related high-tech products
Although Chongqing City is an important EMM manufacturing base, there is a lack of high-tech products. The Mn-related magnetic recording material is a key for laptop and microelectronic industry. Some other Mn-related products are in great demand, such as LiMnO4, MnFeO4, MnZnFeO4 , BaMnFeO4, etc. In recent years, MnOx related catalyst for deNOx has been developed, which has a great potential market for air pollution control.
Summary
Although EMM is an important industry in Chongqing City, it should solve the problems of energy saving and emission reduction, industry upgrade, resource guarantee, etc., which contributes to industry’s sustainable development. Comprehensive resource utilization is needed with the development of beneficiation technologies. Because only metal manganese is extracted from the ore, some new methods can be used to recover associated elements.
For the environment problems, capture and utilization of CO2, deNH3-N can be solved using traditional procedures. For EMM residue, reduction, harmless, reclamation may be the way out, and the goal can be obtained by multidisciplinary cooperation.
Acknowledgement
This research was supported by the Chongqing Science and Technology Committee (cstc2012jcsf-jfzhX0014).
References
- Duan N, Fan W, Changbo Z, Chunlei Z, Hongbing, Y (2010) Analysis of pollution materials generated from electrolytic manganese industries in China Resources. Conservation and Recycling 54: 506-511.
- Liu Z, Li Y, Zhou XX, Du J, Tao C (2014) Research Progress of Electro-Oxidation Intensification Leaching for Refractory Ore. Advanced Materials Research 236-238: 775-780.
- Liu Z, Zheng X, Liu D, Wang Y, Tao C (2014) Enhancement of liquid–liquid mixing in a mixer-settler by a double rigid-flexible combination impeller. Chemical Engineering and Processing: process intensification 86: 69-77.
- Tao C, Jun H, Xing F, Jun D, Zuohua L (2012) Anodic potential oscillation in electrolytic manganese process. Journal of the Chinese Rare Earth Society 30: 266-271.
- Tao C, Min L, Liu Z, Jun D, Yan C, et al. (2012) Study on Manganese extraction from complex residue. Journal of the Chinese Rare Earth Society 30: 565-570.
- Tao C, Si S, Zuohua L, Jun D, Xing F (2012) Study on crystal inhibitor on electrolytic manganese qualified solution. Journal of the Chinese Rare Earth Society 30: 225-230.
Relevant Topics
- Additive Manufacturing
- Coal Mining
- Colloid Chemistry
- Composite Materials Fabrication
- Compressive Strength
- Extractive Metallurgy
- Fracture Toughness
- Geological Materials
- Hydrometallurgy
- Industrial Engineering
- Materials Chemistry
- Materials Processing and Manufacturing
- Metal Casting Technology
- Metallic Materials
- Metallurgical Engineering
- Metallurgy
- Mineral Processing
- Nanomaterial
- Resource Extraction
- Rock Mechanics
- Surface Mining
Recommended Journals
Article Tools
Article Usage
- Total views: 15528
- [From(publication date):
December-2015 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 14430
- PDF downloads : 1098