Research Article Open Access
Electrolyte Effects on Polyacrylic Acid-Polyvinylpyrrolidone Aqueous Glycol Mixtures for Use as De-Icing Fluids
Yuchen Wang1, Richard A Pethrick2, Nicholas E Hudson2 and Carl J Schaschke3*
1Department of Chemical and Process Engineering, University of Strathclyde, Glasgow, Scotland, UK
2Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, Scotland, UK
3School of Science, Engineering and Technology, Abertay University, Dundee, Scotland, UK
- *Corresponding Author:
- Carl J Schaschke
School of Science
Engineering and Technology
Abertay University, Dundee
Scotland DD1 1HG, UK
Tel: 0044(0)1382308488
E-mail: c.schaschke@abertay.ac.uk
Received date: January 27, 2016; Accepted date: February 01, 2016; Published date: February 06, 2016
Citation: Wang Y, Pethrick RA, Hudson NE, Schaschke CJ (2016) Electrolyte Effects on Polyacrylic Acid-Polyvinylpyrrolidone Aqueous Glycol Mixtures for Use as De-Icing Fluids. Ind Chem 2:115. doi:10.4172/2469-9764.1000115
Copyright: © 2016 Wang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Industrial Chemistry
Abstract
Rheological and wind tunnels measurements are presented for mixtures of polymer polyacrylic acid [PAA] and polyvinylpyrrolidone [PVP] polymers dispersed in water-1,2 propylene glycol mixture to examine their use as potential aircraft de-icing fluids. PAA solutions which form the basis of de-icing fluids are known to result in undesirable gelation which may lead to undesirable and catastrophic consequences in such applications. In this study, we examine the blending of PVP with PAA blends as alternative de-icing fluid formulations that can reduce the likelihood of forming such irreversible gel deposits. Through adjustment of the electrolyte concentration, the ratio of PAA to PVP as well as the molecular weight of PVP, it is possible to achieve a required viscosity profile to that exhibited by a model de-icing fluid across a range of appropriate temperatures. Wind tunnel tests indicate that the mixtures are capable of meeting the necessary requirements for boundary layer depletion as well as having sufficient capability of retaining a stable layer required during aircraft taxiing.
Keywords
De-icing fluids; Poly(acrylic acid); Poly(vinyl pyrrolidone); Water-glycol mixtures; Electrolytes; Wind tunnel
Introduction
Aircraft de-icing fluids used where there is a risk of ice build-up, are largely based on water-glycol mixtures containing polyacrylic acid [PAA] as a thickening agent [1-7]. After a long flight at high altitude or if re-fuelled with very cold fuel at temperatures in the presence of high humidity, the wings can gain deposits of ice. Ice on flight control surfaces reduces their aerodynamic efficiency, reducing lift and manoeuvrability during or after take-off. Industrial best practice in relation to de-icing and anti-icing is summarized in the Association of European Airlines and US directives [1-3]. The fluid is applied at approximately 60°C with the aim of dispersing ice deposited on the aircraft and also to provide protection whilst the aircraft is at its stand and during taxiing to the hold area prior to take-off. The applied fluid is required to form a thin stable layer while the aircraft is static yet rapidly removed during the initial stages of take-off.
Various fluids have been developed to address different levels of severity of weather. An aqueous mixture of 40% propylene glycol, for example, may have a melting point of -5°C, which is lowered to -25°C by raising the glycol content to 50% glycol, and down further to -60°C with a 60% mixture. Such base fluids, however, have insufficient viscosity to be retained on the aerodynamic profiles of the aircraft and therefore require thickening with PAA. Certain types of aircraft when operating for prolonged periods of inclement weather are prone to the build-up of gel deposits particularly around the ailerons. The loss of flight control in a number of incidents has been attributed to the deposition of insoluble PAA residues in the region below the wings [8,9].
In a previous paper, the concept of blending PAA with polyvinylpyrrolidone [PVP] as a thickener has been proposed [10]. PVP is water soluble, although unlike PAA, it is less sensitive to the presence of electrolyte [11]. The formation of the insoluble gel is believed to be associated with either dilution of the de-icing fluid with electrolytes present in hard water or its interaction with runway de-icing fluids.
In the case of PAA-type fluids, the variation in the concentration of a monovalent electrolyte has a significant effect on the temperature dependence of the viscosity and wind tunnel behaviour [12]. In this paper, the effects of the variation of the level of added electrolyte on the rheological and wind tunnel behaviour are reported for blends of PAA with PVP in water-glycol mixtures with added electrolyte in which the viscosity of PAA is sensitive to the presence of electrolyte. This is important since monovalent cations are able to interact with the carboxylic acid groups of the polymer backbone and influence the electrostatic interactions between these groups which, in turn, change the hydrodynamic volume of the polymer molecule in solution and its flexibility. PAA is noted as being capable of increasing its effective hydrodynamic volume by forming a ladder-type of complexes thereby significantly influencing the elongational viscosity [13]. It is therefore proposed that replacement of a proportion of the PAA by PVP may reduce the sensitivity to the electrolyte without compromising the rheological properties, which is key to the effectiveness of the polymers as thickeners. In this paper, the effects of change of electrolyte and molecular weight of the PVP on both the rheological and wind tunnel characteristics are therefore explored with the aim of this investigation being to determine whether it is possible to reduce the sensitivity to electrolyte effects by reduction of the PAA thickener in the fluids.
Materials and Methods
Blends of PAA with PVP polymer were formulated with a 50:50 water-to-glycol mixture to give a total polymer concentration of 0.30 g/dL. Ratios of PAA-to-PVP studied were prepared as 40:60, 50:50 and 60:40 on a weight:weight basis. The PAA polymer (Carbomer A) was supplied by Lubrizol (Brussels, Belgium) as flocculated solid particles with a diameter of 0.2 μm and a nominal molar mass of 4 × 106 g/ mol. A detailed characterisation of this material has been previously reported [10]. To achieve dissolution, solid polymer was dispersed using a Silverson L4R high shear mixer placed in a 1.0-litre beaker containing 600 g of de-ionized water and operated at 4800 rpm for 5 minutes. On completion the rotation speed was reduced to 3600 rpm and stirring continued for a further 55 minutes. Three PVP polymers were used in this study with molecular weights of 300 (purchased from Aldrich, Gillingham, UK), 700 (purchased from BDH, Poole UK) and 1300 (purchased from ISP Technologies (Calvert City, US), and each dispersed as powders in de-ionized water at a concentration of 1.22 g/dL until fully dissolved using a magnetic stirrer. A water/1,2- propylene glycol mixture was prepared as a 50:50 wt/wt ratio. The PAA gel intermediate was first added to the water-glycol mixture and fully mixed followed by the PVP solution and again fully mixed. The solutions were then neutralized with 5.0M potassium hydroxide solution to attain a pH of 7.0. The viscosity profile of the solution was adjusted using 5.0 M sodium chloride solution to give a profile similar to that observed for the standard PAA thickened de-icing fluid (Table 1). The solutions were allowed to stand and achieve thermodynamic equilibrium over a period of 5 days.
Viscosity measurements were carried out using a shear stresscontrolled Carri-Med CSL2500 rheometer (TA Instruments, Crawley, UK) using a 4-cm parallel plate fitted with a solvent trap. The temperature was controlled between 15°C and -15°C by the Peltier effect with an anti-freezing bath maintained at 0°C. Temperature ramp tests were carried out at a rate of 1.0°C per two minutes from and initial temperature of 15°C down to -15°C, and then back to 15°C, repeated at shear stresses of 5 Pa and 10 Pa. Steady shear stress tests were also carried out at 0°C, -5°C, -10°C and -15°C at 0.1 to 100 Pa, with a tenfold increase in stress per 10 minutes, and subsequent decrease.
Temperature-controlled wind tunnel tests were carried out to determine the rate at which a layer of the de-icing fluid can be removed from a flat surface simulating the air flow and removal at the point of aircraft take-off. The wind tunnel consisted of a duct through which temperature-controlled air was blown. The effectiveness of the removal of the fluid is evaluated using pressure sensors along the duct to determine the boundary layer displacement thickness (BLDT), which is a measure of the remaining film thickness on the surface during its continuous removal by the increasing flow of air.
Samples of test fluid were initially applied as a coating on the floor of the duct to a uniform thickness of 1.5 mm to simulate the coating which would be deposited by a spray application. The temperature of the test facility was thermostatically controlled and tests were carried out at 0°C, -5°C, -10°C and -15°C. At the start of each run, the air velocity was increased from stationary to 65 ms-1 at a linear rate over a period of 25 seconds. This is the typical time and velocity profile between the end of the taxiing process and point of take-off for a small passenger aircraft. The average thickness of the BLDT was then determined from the measurement of pressure differences along the wind tunnel between 27 and 33 seconds after the start of the increase of pressure and it reaching its maximum velocity. Each test was carried out in triplicate.
Results
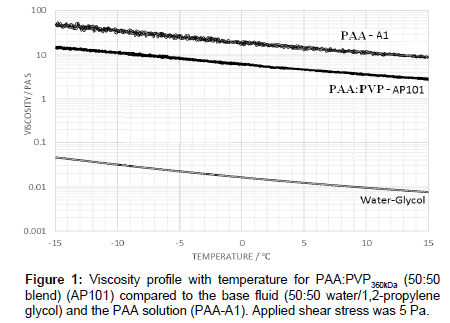
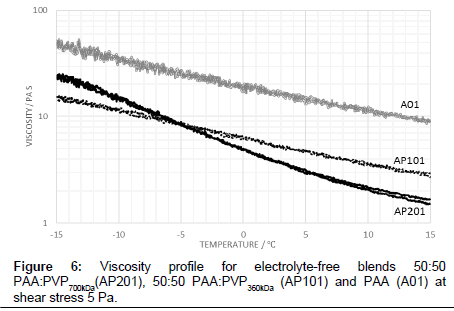
As a baseline fluid, the viscosity profile with temperature of a 50:50 PAA:PVP360kDa blend in the absence of the electrolyte for an applied shear stress of 5 Pa is shown in Figure 1. The profile is noted as being similar to that of the pure PAA although the viscosity for the blend is substantially reduced and significantly above that of the water-glycol mixture alone. The viscosity of the PAA:PVP360kDa mixture (AP101) ranges from 2.68 Pas at 15°C to 15.24 Pa s at -15°C, compared to 9.37 Pa s at 15°C and 48.64 Pa s at -15°C for the reference PAA solution.
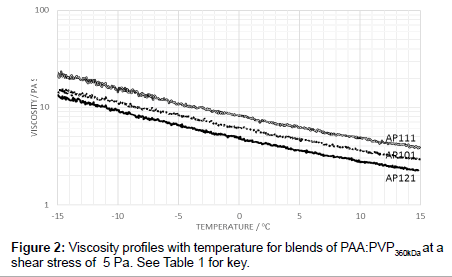
To examine the effects of PVP addition, blends were prepared with PAA in the ratios of 40:60, 50:50 and 60:40 for the PVP360kDa in the absence of the electrolyte. As expected, the 60:40 blend is more viscous than that of the 50:50 blend and likewise, the 40:60 blend is less viscous (Figure 2). With no electrolyte added, the 60:40 blend has a higher viscosity than the 50:50 blend but, to be expected, it is less viscous than the pure PAA. Changing the weight ratio therefore increased the molar ratio of PAA to PVP from 0.36:1 for the 50:50 mixture to 0.54:1 for the 60:40 mixture.
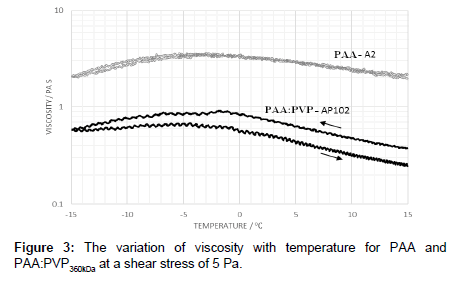
The effect of electrolyte was examined in the PAA:PVP360kDa under low shear stress and compared to pure PAA. The viscosity was noted to be less than the pure PAA solution. However, by varying the ramped temperature up and then back down to -15°C, a hysteresis profile was found in which the viscosity was higher on the return profile (Figure 3).
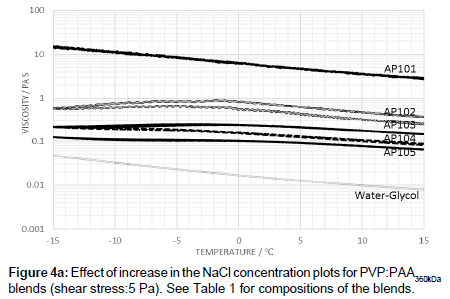
The addition of electrolyte to PVP:PAA360kDa blends showed that the viscosity is progressively reduced with increasing salt concentration. A peak temperature in the viscosity-temperature profile is also observed along with a small level of hysteresis (Figure 4a).
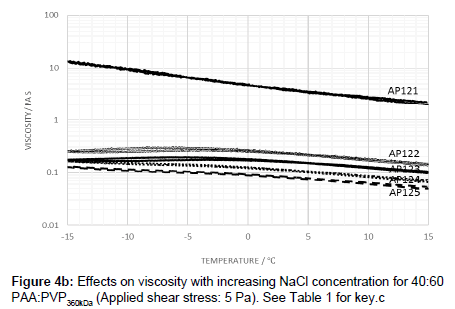
The viscosity profile was also examined for a blend of 40:60 PAA:PVP360kDa with electrolyte. While the same trend was noted in which the addition of salt lowers the viscosity, a peak temperature and hysteresis are also observed.
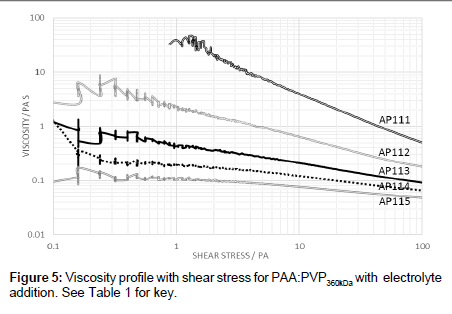
Measurements of the viscosity of the 40:60 PAA:PVP360kDa blend were also undertaken as a function of shear stress at a temperature of 0°C (Figure 5). It was found that the addition of electrolyte not only reduces the viscosity but appears to shift the point at which shearthinning occurs. The shear-thinning characteristics are important to allow for the effective removal of a thin film of the fluid deposited on the wings during the initial stages of take-off. The dynamics of polymer chains are influenced by both the molecular weight and changes in the size of thepolymer chain can have profound effects on the viscosity.
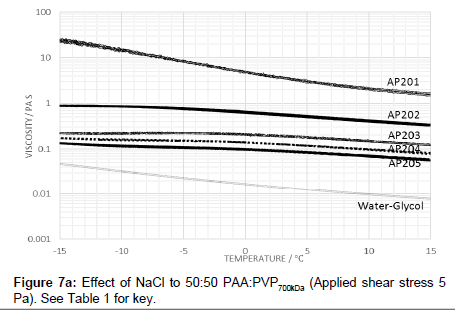
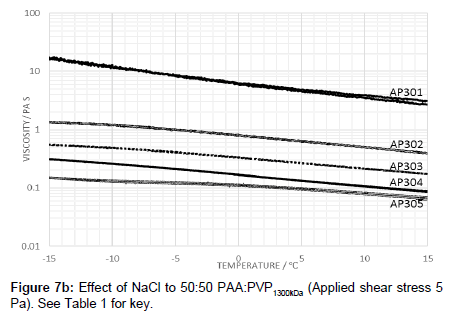
The effect of raising the molecular weight of the PVP in the blend was also examined in which the viscosity profile is shown in Figure 6. The addition of electrolyte with the high molecular weight PVP lowers the viscosity and creates a peak in the viscosity-temperature profiles (Figure 7a). The profiles are similar to those for lower molecular weight of 360 kDa (i.e., AP112) although the peak value for the viscosity at -15°C is lower.
Raising the molecular weight of PVP in the blend with PAA reduced the viscosity and creates a peak in the viscosity-temperature profiles (Figure 7a). The profiles are similar to those for lower molecular weight of 360 kDa (i.e., AP112) although the peak value for the viscosity at -15°C is lower.
Repeating the same examination with a higher molecular weight of PVP of 1300 under the showed that the viscosity is further reduced with the salt addition further reducing the viscosity of the fluid.
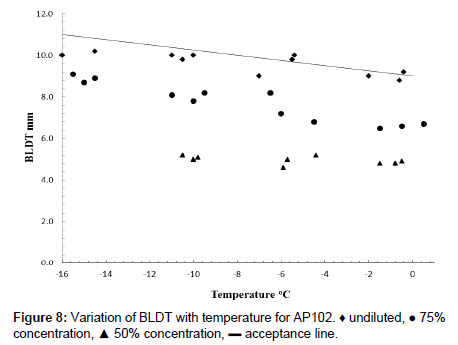
Boundary layer displacement thickness tests were carried out for PAA:PVP360kDa blends with electrolyte in which the undiluted fluid failed the acceptance test on a several samples. Dilution of the fluid with water, however, reduced the viscosity to within the acceptance levels (Figure 8). For a de-icing fluid to be effective, ice formation is required to be suppressed for a time period equivalent to that of the “hold-over time”. The fluids were tested by coating the un-chilled fluid onto an inclined aluminium plate. Both the plate and surrounding air were maintained at a temperature of -5.0°C. Cooled water was then sprayed onto the coated surface for which the wet spray endurance time [WSET] is defined as the time for ice formation to reach the failure zone. This is the area 25 mm below the upper edge and 5 mm in from either side of the test plate, or the formation of slush on 10% of the plate surface, with a water spray intensity onto the testing area corresponding to approximately 5.0 g dm-2 h-1. In these tests, it was found that the fluids could be effectively removed.
Discussion
The rheological characteristics of the de-icing fluids are achieved by polymer-polymer interaction creating a virtual polymer with a large hydrodynamic volume. We have previously shown that mixtures of PAA with PVP exhibit similar characteristics to those of a commercial de-icing fluid [10]. In this paper, we examined the effects of change of molecular weight of the PVP, the ratio of PAA to PVP and the electrolyte concentration on the rheological characteristics in 50:50 water-glycol mixtures. The mixing ratios for PAA to PVP were 40:60, 50:50, and 60:40 using PVP molecular weights of 360 kDa, 700 kDa and 1300 kDa (Table 1). The electrolyte, sodium chloride, was also progressively added to the blends at levels equating to allow maximum viscosity enhancement for the PAA.
| Blend name | PVP MW kDa | PAA:PVP ratio | NaCl g/100 mL |
|---|---|---|---|
| AP101 | 360 | 50:50 | 0.000 |
| AP102 | 360 | 50:50 | 0.015 |
| AP103 | 360 | 50:50 | 0.030 |
| AP104 | 360 | 50:50 | 0.045 |
| AP105 | 360 | 50:50 | 0.060 |
| AP111 | 360 | 60:40 | 0.000 |
| AP112 | 360 | 60:40 | 0.018 |
| AP113 | 360 | 60:40 | 0.036 |
| AP114 | 360 | 60:40 | 0.054 |
| AP115 | 360 | 60:40 | 0.072 |
| AP 201 | 700 | 50:50 | 0.000 |
| AP202 | 700 | 50:50 | 0.015 |
| AP203 | 700 | 50:50 | 0.030 |
| AP204 | 700 | 50:50 | 0.045 |
| AP205 | 700 | 50:50 | 0.061 |
| AP211 | 700 | 60:40 | 0.000 |
| AP212 | 700 | 60:40 | 0.018 |
| AP213 | 700 | 60:40 | 0.036 |
| AP214 | 700 | 60:40 | 0.054 |
| AP215 | 700 | 60:40 | 0.072 |
| AP301 | 1300 | 50:50 | 0.000 |
| AP302 | 1300 | 50:50 | 0.015 |
| AP303 | 1300 | 50:50 | 0.030 |
| AP304 | 1300 | 50:50 | 0.045 |
| AP305 | 1300 | 50:50 | 0.061 |
Table 1: Composition of PAA/PVP mixtures studied and electrolyte concentration.
The viscosity profile shown in Figure 1 for the 50:50 PAA:PVP360kDa blend in the absence of the electrolyte shows that as the temperature is lowered the interaction between the PAA molecules increases with a corresponding increase in viscosity. The molecular weight of the PVP polymer is significantly lower than that of the PAA polymer leading to a reduction in the hydrodynamic volume of the entities in solution and a corresponding reduction in the viscosity for the PAA:PVP blend. A similar behaviour is observed when the shear stress is increased to 10 Pa in which the observed decrease is associated with a reduction in the degree of complexation of the PAA, previously described through rheological modelling studies [12].
As a result of the issues of insoluble gel deposit formation, it is desirable to achieve a viscosity profile with as low a level as possible of PAA in the blend. The viscosity characteristics are the consequence of the balance of PAA complexation with itself and hydrogen bonding with the PVP polymer. Previous studies [14] suggest that PAA and PVP will form a complex when the monomer molar ratio is around 1:1.
The addition of sodium chloride solution to the PAA mixtures electrostatically screens the carboxylic acid groups thereby reducing both the intra- and inter-polymer chain interactions [10] and causes a reduction in viscosity. As the polymer is heated it is able to undergo conformational change and alter the degree to extent of association depends on the applied shear stress. Cooling the solution does not immediately allow relaxation to occur in which the polymer exhibits a large hydrodynamic volume and corresponding increased apparent viscosity. Cooling does alter the conformational preference and the extent of association leading to a more contracted pre-gelation conformation with a lower viscosity. At higher shear stresses, a hysteresis is observed in which conformational changes are promoted as indicated by the change in location of the peak maximum on the heating and cooling cycle (Figure 3).
The nature of the interactions between the two types of polymer with temperature is complex. This is due the carboxylic acid groups on the PAA chain being screened by interaction with the sodium cation. Lowering the temperature reduces the screening by salting out of the electrolyte. Unscreened carboxylic acid groups have the potential for hydrogen bonding with the PVP and increasing the hydrodynamic volume of the associated complex formed.
The low level of hysteresis for PAA shown in Figure 3 suggests that the polymer is able to fairly rapidly recover its equilibrium state, whereas this process is somewhat slower in the blends. Whilst the temperature dependence appears to be primarily determined by the PAA, hydrogen bonding interactions between PAA and PVP enhance the hysteresis effects. In the pure PAA fluids, hydrogen bonding occurs between nonscreened groups whereas in blends, however, hydrogen bonding occurs between non-screened carboxylic acid groups and the pyrrolidone ring. These are likely to be sensitive to both stress levels and temperature. The reduction in viscosity at low temperatures can be associated with a combination of the de-shielding of the carbonyl groups associated with salting out effects and changes on conformation leading to a pregelation state prior to gel formation [15-17]. A further increase in the electrolyte level, leads to a decrease in the viscosity in comparison to that of the pure PAA. As the level of electrolyte is increased the peak in the viscosity profile also shifts to lower temperatures (Figure 4a). Such observations indicate that, while the process of dilution effectively reduced the polymer concentration resulting in a lower probability of polymer entanglement, the lowering of the electrolyte level changes the extent of polymer-polymer interaction, and hence shift in ‘peak’ temperature. Previous studies [18-20] suggest that the polymer may swell by absorbing water when diluted with de-ionized water.
Increasing the level of electrolyte increases the screening of the carboxylic acid groups and increases the stability of the polymer in solution and, as a consequence, suppresses the point at which the polymer enters a pre-gelation stage. Increasing the electrolyte level reduces the extent to which intra- and inter-PAA complexation can take place, and leads to a lowering of the viscosity level with the PAA behaving as an isolated polymer molecule rather than an extended complex with itself. At the higher applied shear stress of 10 Pa, the disentanglement of the PAA occurs to a greater extent while the viscosity is lowered.
The addition of the electrolyte to the 40:60 blend resulted in a peak in the viscosity-temperature profile in which the temperature at which the peak occurs being dependent on the amount of electrolyte added. As with the 50:50 PAA:PVP360kDa blend, the lowest level of electrolyte addition produced curves which exhibited, to a small extent, hysteresis behaviour (Figure 4b). Increasing the electrolyte level leads to a lowering of the viscosity and where the peak virtually disappears. As with the observations at the higher ratios, the PAA plays a dominant role in dictating the temperature dependence, and once a critical level of electrolyte is present, interaction between the PAA and PVP appears to be minimal. An excess of electrolyte minimizes interaction between the PAA polymers with the rheology being dictated purely by the characteristics of the individual polymer molecules.
The shear-thinning effects of the electrolyte addition to the 40:60 PAA:PVP360kDa blends (Figure 5) are important to allow for the effective removal of the thin film deposited on the wings during the initial stages of take-off. The dynamics of polymer chains are influenced by both the molecular weight and changes in the size of the polymer chain which can have profound effects on the viscosity.
Since the polymer-polymer interactions influence the rheological characteristics de-icing by creating a virtual polymer with large hydrodynamic volume, further measurements were made by increasing the molecular weight of the PVP with the intention of increasing the probability of hydrogen bonding interactions with the PAA. PVP with a molecular weight of 700 kDa was therefore used with the 50:50 blend, thus doubling the molecular weight (Figure 6). Increasing the molecular weight increases the viscosity at the lower temperatures suggests but lowers the viscosity at elevated temperatures suggests that the hydrogen bonding formed between the carbonyl group of the pyrrolidone ring and the carboxylic group of PAA influences the observed viscosity profile. At higher temperatures, fewer polymer entanglements are formed by the PAA chains resulting in a lower viscosity being observed. At lower temperatures, however, the bonding between PAA and PVP is effectively preventing the collapse of the PAA forming a gel complex with the hydrogen bonding between the PAA and PVP chains leading to a more extended structure and hence a higher viscosity.
The reduced viscosity profile for the higher molecular weight of PVP in the blend with PAA with increased electrolyte (Figure 7a) shows that the sodium chloride content is less effective at screening the carboxylic acid groups and enables, with the higher molecular weight PVP, the creation of species with a larger hydrodynamic volume. The absence of a hysteresis is also consistent with the increased role of the PVP polymer and the lower sensitivity to the effects of stretching of the chain on the pre-gelation structure. The profiles also indicate that the viscosity at a particular temperature decreases with increase in the electrolyte concentration. The addition of electrolyte suppresses the magnitude of the increase in the viscosity on decreasing the temperature across the range 15°C to -15°C, indicating that screening effects contribute to the stabilisation of viscosity. With the larger molecular weight PVP blends, the gelation effect is suppressed and may account for the diminished peak across the series.
It is noted that compared to the molecular weight of PAA, PVP is a significantly smaller polymer even though larger networks are created leading to the observation of higher viscosities both for the solutions with and without the electrolyte. Using PVP with a higher molecular weight of 1300 kDa, the viscosity profiles without added electrolyte were found to be similar to those of PAA:PVP360kDa (Figure 7b). A shifting of the peak to lower temperatures implies that the PVP is, through hydrogen bonding, able to lower the temperature at which the salting out effect occurs to a temperature of below -15°C. The hysteresis effect of the PAA:PVP360kDa blends was reduced significantly. Adjustment of the electrolyte concentration from 0.015 g NaCl/100 mL to 0.045 g NaCl/100 mL caused the expected decrease in the viscosity.
An effective de-icing fluid is required to be form a layer on a surface that is able to suppress the further formation of ice yet be effectively removed in the critical period prior to rotation. To be fit for purpose the de-icing fluid is required to have a boundary layer displacement thickness (BLDT), [δ*], obtained from wind tunnel tests of less than 11 mm at a temperature of -16°C and less than 9 mm at 0°C. The data for the model system prepared using low shear in Figure 8 largely fall below the acceptance line and reduce further with dilution with water. Whilst the 60:40 PAA:PVP360kDa exhibited some values of [δ*] above the acceptance line, dilution of the fluid was able implies that the fluids exhibit the desired removal characteristics. On dilution to 75% and 50% of the original concentrations by the addition of water, the values of [δ*] clearly drop below the acceptability limit.
Conclusions
PAA is the dominant contributor to the viscosity behaviour in the PAA:PVP blends studied. Reducing the PAA content, however, permits lower levels of electrolyte to be employed. The characteristics of a peak in the viscosity-temperature profile is observed in a number of blends. These features indicate that the PAA:PVP blends have potential for use as de-icing fluids. The proposition that the incorporation of the PVP would suppress the gelation is demonstrated through the viscosity measurements and confirmed through a lack of observation of gel particles after extended time studies. This study demonstrates the potential of the PAA:PVP blends as de-icing fluids, but also indicates that further work is required before a viable formulation is developed. This study has, however, identified the potential of PAA:PVP blends with electrolyte for this application.
Acknowledgements
The financial support and use of the wind tunnel testing facilities of Kilfrost Limited is gratefully acknowledged.
References
- Blau K (2011) Recommendations for De-icing/Anti-icing Aeroplanes on the Ground. 26thedn. AEA Publications.
- Simonot M, Mischka G, Holland N, Fieandt J, Pontinen J, et al. (2004) Recommedations for De-icing/Anti-icing of aircraft on the ground. 19thedn. AEA Publications.
- D'Avirro J, Chaput MD (2011) Optimizing the Use of Aircraft Deicing and Anti-Icing Fluids.
- Beisswenger A, Laforte J, Tremblay M, Perron J (2007) Investigation of a New Reference Fluid for Use in Aerodynamic Acceptance Evaluation of Aircraft Ground De-icing and Anti-icing Fluid. US Department of Transportation, Federal Aviation Administration.
- Jenkins R, Bassett D, Lightfoot R, Boluk M (1997) Aircraft anti-icing fluids thickened by associative polymers. EP 0860490 A1.
- Seiler M, Bernhardt S (2011) De-icing agent and/or anti-icing agent. US 7875203 B2
- Dow J (2005) Understanding the stall-recovery procedure for turboprop airplanes in icing conditions, Flight Safety Digest. Flight Safety Foundation. pp: 1-17.
- Hille J (2007) De-icing and Anti-Icing Fluid Residues. Aero. Boeing Commercial Airplanes Group.pp: 14-21.
- Schwark T, Severin K, Grellner W (2008) "I am flying to the stars"- suicide by aircraft in Germany. Forensic SciInt 179: e75-78.
- Wang Y, Pethrick R, Hudson N, Schaschke C (2014) Poly(acrylic acid)-Poly(vinyl pyrrolidone)-Thickened Water/Glycol De-icing Fluids. Cold Regions Science and Technology 101: 24-30.
- Jin S, Liu M, Chen S, Chen Y (2005) Complexation between poly(acrylic acid) and poly(vinylpyrrolidone): Influence of the molecular weight of poly(acrylic acid) and small molecule salt on the complexation. Eur Polym J 41: 2406-2415.
- Wang Y, Pethrick R, Hudson N, Schaschke C (2013) Rheology of poly(acrylic acid) in water/glycol/salt mixtures. Ind Eng Chem Res 52: 594-602.
- Hudson N, Ferguson J, Warren B (1988) Polymer Complexation Effects in Extensional Flows. J Non-Newton Fluid Mech 30: 251-266.
- Lau C, Mi Y (2002) A study of blending and complexation of poly(acrylic acid)/poly(vinyl pyrrolidone). Polymer 43: 823-829.
- Arguelles-Monal W, Perez-Gramatges A, Peniche-Covas C, Desbrieres J, Rinaudo M (1998) Conductimetric and viscometric study of the macromolecular complex between poly(acrylic acid) and poly(vinyl pyrrolidone).Eur Polym J 34: 809-814.
- Henke A, Kadlubowski S, Wolszczak M, Ulanski P, Boyko V, et al. (2011) The Structure and Aggregation of Hydrogen-Bonded Interpolymer Complexes of Poly(acrylic acid) with Poly(N-vinylpyrrolidone) in Dilute Aqueous Solution. Macromol Chem Phys 212: 2529-2540.
- Kaczmarek H, Szalla A, Kaminska A (2001) Study of poly(acrylic acid)-poly(vinylpyrrolidone) complexes and their photostability. Polymer 42: 6057-6069.
- Bromberg L, Temchenko M, Moeser GD, Hatton TA (2004) Thermodynamics of temperature-sensitive polyether-modified poly(acrylic acid) microgels. Langmuir 20: 5683-5692.
- Dobrynin A (2004) Phase Diagram of Solutions of Associative Polymers. Macromolecules 37: 3881-3893.
- Miquelard-Garnier G, Demoures S, Creton C, Hourdet D (2006) Synthesis and rheological behavior of new hydrophobically modified hydrogels with tunable properties. Macromolecules 39: 8128-8139.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13078
- [From(publication date):
March-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11591
- PDF downloads : 1487