Research Article Open Access
Electrochemically Activated Tap Water Induced Effects of Genomic Instability in Various Living Objects In Vitro and In Vivo
Faina Ingel1*, Olga Zatsepina2, Anatoly Stekhin1, Galina Yakovleva1, Olga Savostikova1, Anna Alekseeva1 and Tatiana Iksnova11Federal State Budget Organization, A.N.Sysin Research Institute for Human Ecology and Environmental Hygiene, Ministry of Health, Russaian Federation, Moscow
2Closed Joint-Stock Company “Clean water”, Samara, Russian Federation, Moscow
- *Corresponding Author:
- Faina Ingel
Senior fellow researcher
Institute of Human Ecology and Environmental Hygiene
AN Sysina Ministry of Health
Russian Federation, Moscow
Tel: 7-499-246-4813
E-mail: fainaingel@mail.ru
Received date: November 20, 2013; Accepted date: December 12, 2013; Published date: December 19, 2013
Citation: Ingel F, Zatsepina O, Stekhin A, Yakovleva G, Savostikova O, et al. (2013) Electrochemically Activated Tap Water Induced Effects of Genomic Instability in Various Living Objects In Vitro and In Vivo. Occup Med Health Aff 1:143. doi: 10.4172/2329-6879.1000144
Copyright: © 2013 Ingel F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Abstract
Results of the study of genetic safety of drinking waters with altered physical properties, obtained after electrochemical activation are present. The effects of genomic instability were detected on standard biological models: human blood cells, cultivated with Cytochalasin B, Drosophila male gametes and mice bone marrow in dynamics of a subchronic exposure
Keywords
Catholyte; Anolyte; Redox potential; Electrochemical activation; Electroconductivity; Luminal-depended chemiluminescence
Introduction
Problems of drinking water quality at the present stage of development of a civilization are a key because they are directly connected with state of health of now living people and with health of future generations. However it is known that quality of tap water is defined not only by its chemical composition, content of microorganisms etc, but also by physical properties [1]. Therefore now various new technologies for improving physical and chemical properties of drinking water were elaborated. More often for change of physical and chemical parameters devices for electrochemical activation of water are used. More often for changing of physical and chemical parameters are used devices for electrochemical activation of water. As a rule, for this goal are applied an electrolyzer (with or without diaphragm) where water decays under the influence of an electric current. Under such influence in the cathode area the electron-donor water (catholyte), which is characterized by alkali pH values and negative values of redox potential (RP), is being accumulated. And in the area of anode the electron-acceptor water (anolyte), with acidic pH and increased positive values for RP, is accumulated. It’s well known that under electrolysis conditions of water in the area of electrodes are accumulate plenty of ions, present in electrolysis medium and manifesting a frank toxicity (for instance, metal ions, ions containing chlorine, sulfur, etc,). To reduce their concentration different devices for contact-free water activation were constructed. In them for electro-chemical activation is used pure drinking water. To reduce their concentration different devices for noncontact water activation were constructed. In them for electro-chemical activation is used pure drinking water. This water for activation is placed into closed thin-walled polyethylene bins located in water collected in the area of electrodes. Therewith, the water, contained in these bins, is changing its RP, pH, an extent of structuredness and other physical and chemical properties similarly to the water, presenting around the electrodes though the principal mechanisms of the process of charge transfer under such membrane electrolysis are not clear all through [2].
Over the past few decades a little number of researches has been done upon the study of biological activity of electrochemically contactfree activated waters (ECFAW). Basically, they are the investigations on plants and hydrobionts of various trophic levels [3]. In spite of the fact that there is a lack of information about any regular investigation of safety or at least some toxicological characteristics of drinking waters with altered physical and chemical properties, obtained from contact or contact-free electrochemical activation, in Internet and in literature there is a large number of publications describing positive results of ECFAW application for treatment a wide range of deceases, although information about checking the safety of this treatment, we could not find [4-8]. At the same time it was demonstrated, that physical and chemical properties of water exert an influence on the processes of formation, stabilization and functioning of various biological structures, including cellular membranes, proteins and DNA [9]. For us these facts assumes the possibility of an induction of genotoxic effects. That’s why we did an attempt of regular study of genetic safety of ECFAW on biological models of different levels, accepted by OECD and FDA as standard tests for evaluation of chemical compounds safety: gametes of drosophila males, mice bone marrow cells in vivo and culture of human blood lymphocytes.
Materials and Methods
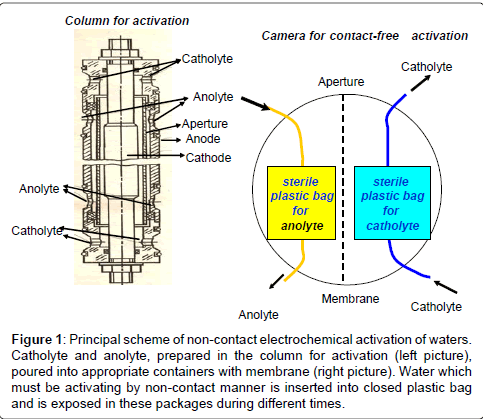
In experiments we used two types of drinking water: Moscow tap water and osmotic artesian water obtained in ZAO “Pure Water” (Samara city). Moscow tap water was passed through the filter system for coarse and fine pre-treatment; 15 min boiled, allowed to stand at room temperature for 24 hours and then was poured into plastic hermetically sealed sterile bags of 500 ml (Nasco Whirl-Pak, USA) which were able to be tightly closed. These packs were installed into one by one bins with catholyte or anolyte waters, received by contact electrochemical activation (activator device “Izumrud”, NPP “IZUMRUD”, Russia) (Figure 1). In order to receive various ECFAW, the packs with water, prepared as described above, were exposed for 5, 20 or 40 minutes to obtain different catholytes or 35 min - to obtain anolyte.
Figure 1: Principal scheme of non-contact electrochemical activation of waters. Catholyte and anolyte, prepared in the column for activation (left picture), poured into appropriate containers with membrane (right picture). Water which must be activating by non-contact manner is inserted into closed plastic bag and is exposed in these packages during different times.
The artesian water purified in a reversed osmosis unit, was boiled for sterilization, put into sterile packs and non-contact activated under the same modes as the Moscow tap water.
Physical and chemical parameters of these activated waters (pH, RP, electro conductivity, luminal-depended chemiluminescence and levels of structuredness) were detected by standard methods.
1. To assess the frequency of dominant lethal mutations in germ cells we used standard and one of the most studied object of classical genetics - fruit fly Drosophila melanogaster (D- 32 strain from the collection of N.K.Koltsov’ Institute of Developmental Biology of Russian Academy of Sciences, Russia; the strain is maintained in FSBO “A.N.Sysin Research Institute for Human Ecology and Environmental Hygiene” Ministry of Health of Russian Federation, Moscow, Russia).
In the experiments the young males of Drosophila (50 individuals in each group) during 48 hours were fed 5% sucrose dissolved in catholytes and anolytes, received by non-contact electrochemical activation of Moscow tap water (solutions were prepared with and replaced every 24 hours).
In order to obtain test results the young males of Drosophila (50 individuals in each group) during 48 hours were drinking catholytes or anolytes received by contact-free electrochemical activation of Moscow tap water, prepared as it was described above (we prepared and replaced water after every 24 hours). Then, during 6 hours the males from exposed and control groups were mated with virgin females of the same line (1 male and 2 females), after what the females were placed for oviposition into special cabins with removable cuvettes with feed as a bottom. The cuvettes were replaced every 12 hours during 60 hours, the number of laid eggs was tallied and the cuvettes with eggs were left in humidified chambers for 36 hours at room temperature. Then we tallied a number of non- developed eggs in each of the cuvettes. Eggs, that didn’t change their original look, were determined as early embryonic death (EED), and eggs with yellow coloration as the late embryonic death (LED) [10]. It is commonly supposed that EEDs occur as a result of a complex toxic and genotoxic influence of the factor being studied on the gametes of Drosophila mates, and LEDs occur as a result of exclusively mutational events (the genotoxic effect). The experiment was carried out in 4 replications. The significance of differences was evaluated through comparison between the exposed and the control series according to X2 criterion. All the calculations were carried out on the preliminary encoded cuvettes. It is important to note that the test on induction of dominant lethal mutations in the Drosophila mate gametes is not only an analog of the test on laboratory rodents, but gives similar results [10]. That’s why the results, obtained in it, offer an opportunity for extrapolation (at least) onto animals.
2. For evaluation of the level of induction of chromosome aberrations in mice bone marrow cells (F1 from mating of CBA x C57Bl6/j, 6 animals in each group) the mice were allowed to drink ad libitum contact-free catholytes and anolyte prepared on the basis of Moscow tap water. Mice of control groups drunk Moscow tap water, which was previously filtered, boiled and settled.
The experiment was carried out for 30 days; new portions of water were prepared and poured out into water once a day. A volume of water drunk out in each of the waterers was recorded every day. Euthanasia of animals through cervical dislodgement was done a day before the beginning of the experiment, a day after its beginning and further on 8, 15 and 30 days.
Slides for metaphase analysis were prepared by standard method and got encoded. On each preparation we analyzed 100 metaphase plates with good dispersion of chromosomes and modal number 40 ± 2. We took into account single and double fragments and also chromosome exchanges. Frequencies of single-stranded breaks (achromatic gaps) were tallied individually and were not included into the number of chromosome aberration. Besides, on each of preparations, when analyzing 1000 nuclei, we defined mitotic index (MI) and the number of megalokaryocytic nuclei. Statistical assessment of the results was done by comparison between the experimental control series according to the X2 criteria.
3. For cultivation of human blood cells we used venous blood of healthy young non-smoking donor. In the samples of electrochemically contact-free activated artesian water we dissolved standard sterile dry medium RPMI-1640 with glutamine (reconstructed medium, [11]. For preparation of control medium we used purified and boiled artesian water, not subjected to activation. This fragment of the study was being done in micronucleus test with Cytochalasin B (6 μkg/ml), which was added into cultures at 44 hours after the beginning of the experiment [12].
All cultures were fixed at 72-th hour. Cytogenetic slides were got encoded and analyzed under microscope (10x100, oil immersion) with application of the international protocol of the micronuclei test, which includes the assessment of frequency of binuclear cells with micronuclei and nucleoplasmic bridges [12]. Additionally we defined frequency of genetic damages in all dividing cells, proliferative activity, spectrum of dividing cells, asymmetry of cellular division among cells of the 2 mitotic cycles (cells with 3 and 4 nucleus), frequency of apoptosis and mitotic index in total 33 indexes [13,14]. To evaluation of the changing of the effects under additional genotoxic load in parallel cultures, prepared using each water, at 24 hours after the beginning of cultivation we added 3 or 6 μkg/ml N-methyl- N’-nitro-N-nitrosoguanidine (MNNG, Amersham). For statistic analysis of the obtained data we used Spearman correlations and Mann-Witney criterion.
Results
1. The experiments with Drosophila flies showed that males’ fertility varied between groups, but according to dynamics of eggs depositing only one group differed from the control one, i.e. the males who drank catholyte obtained under minimum exposition duration. But this difference against the control one dynamics of oviposition in no way reflected on frequency of mutations.
The main results of this test are given in Table 1. As it’s seen from this table, in all the groups, except of that, where the males were exposed to the catholyte of middle efficiency (20 min), the number of deposited eggs considerably exceeded the control level. And only in this case (the exposure of flies to the catholyte of middle efficiency) we observed significant enhance of both LEL frequency against the control level, and LEL share in the spectrum of mutation events.
| Water title | Redox potential. RP (mV) | Number of eggs deposited, pcs | Fertility. (% of control) | Frequency of dominant lethal mutations (%) | The share in the total frequency of mutations | ||
| EEL | LEL | EEL | LEL | ||||
| Control | 322.0 | 776 | 100 | 8.38 ± 0.10 | 0.52 ± 0.10 | 0.94 | 0.06 |
| Anolyte | 275.0 | 1029 | 132.60** | 8.94 ± 0.10 | 0.58 ± 0.10 | 0.94 | 0.06 |
| Catholyte 5 min | 58.8 | 1425 | 183.63** | 6.67 ± 0.16 | 0.63 ± 0.16 | 0.91 | 0.09 |
| Catholyte 20 min | -16.6 | 663 | 85.44* | 3.62 ± 0.20 | 0.90* ± 0.20 | 0.80 | 0.2* |
| Catholyte 40 min | - 62.9 | 1179 | 151.93** | 5.00 ± 0.20 | 0.25 ± 0. 20 | 0.95 | 0.05 |
**) significance in comparison with control, p≤ 0,01
Table 1: Influence of electrochemically contact-free activated waters on Drosophila melanogaster males fertility and frequencies of lethal mutations in their gametes.
In the course of discussion of these data it is worthy, first of all, to be noted that the statistical analysis revealed a high-level correlation between the RP values of waters, those the fly males were exposed to, and the frequencies of occurrence of EEL in their gametes (R=0.90; p=0.006); therewith, the highest frequencies of EEL were detected in the control group. It is important that an increase of fly fertility in exposition by 5 min catholyte and 40 min catholyte didn’t induce an increase in frequencies of the late lethal mutations. At the same time, a frank genotoxic activity was marked in gametes of the male who drank catholyte of medium efficiency (20 min). This implies that from all the studied variants of water activation only the catholyte of medium activity can present a real menace to the genetic structures of fly male gametes.
The own experience, gained while working with drosophulas, and the practice gained by other scientists showed that mutations in gametes and somatic cells will occur in parallel [10]. And as far as the test for induction of dominant lethal mutations in drosophila gametes is an analogue of a relevant test on mammals, we supposed that under application of catholyte of middle efficiency genetic damages may occur in somatic cells of not only flies, but animals, too. That is why the next series of experiments was carried out with the purpose to check this supposition.
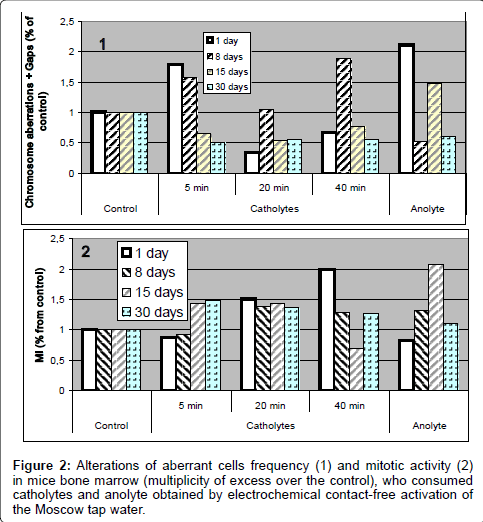
2. The results of assessment of chromosome aberrations frequency in mice bone marrow cells showed that all the studied types of water caused genotoxic effects and also influenced on mitotic activity (Figure 1).
So, already over 24 hours after beginning the test on bone marrow of animals, consumed various types of catholytes, we marked an increase of frequency of achromatic blanks, expressed to different extent; after 8 day of giving them contact-free activated waters we noticed an increase of frequency in aberrant cells; therewith, a maximum effect was noticed for mice consumed 40 min catholyte. Over 15 days of experiment, under all kinds of influence, on the background of decrease of mitotic activity and rather vast death of cells, in majority of groups we saw decrease of frequency of the aberrant cells (even lower than the control level), and over 30 days no any appearances of genotoxic activity in mice bone marrow cells was discovered. However, the cytogenetic analysis showed that under an effect of ECFAW an enhance in mitotic activity of the mice bone marrow was observed in the most cases, what, in the background of chromosome aberration increase, indicates the collection of genetic damages in future generations of cells.
There is a point worthy to be added that the 20 min catholyte revealed the genotoxic activity in the similar ways on the gametes of drosophila and on the somatic cells of mice, while for the other kinds of contact-free activated water the genotoxic effects were discovered only in the cells of the bone marrow of mice. The reason of such differences is not yet explicit (Figure 2).
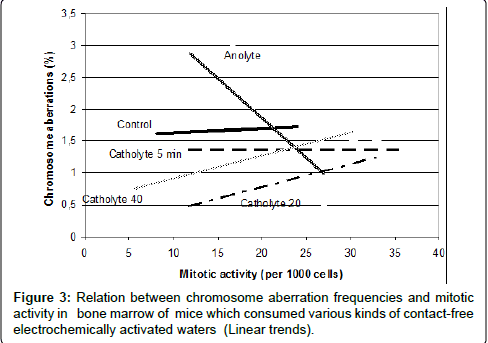
It’s important to note that in this experiment was demonstrated that after different types of contact-free electrochemical activation waters obtained new properties, these properties were realized into different dynamics of relations between the two main biological effects - induction of chromosome aberration and mitotic activity (Figure 3). This analysis once again proved that namely these waters influence to main biological processes in mice organisms and that their influence led to realization of genotoxic effects.
The values of RP for waters, that the mice used to drink, were in direct correlation with frequencies of aberrant cells in bone marrow cells (R=0.42; p=0.032) and frequencies of DNA single-strand breaks (gaps, R=0.58; p=0.021). Even more serious influence on frequency of aberrant cells in bone marrow cells was exerted by concentration of hydrogen peroxide in waters that the animals drank (R=0.79; p=0.025 and R= - 0.83, p=0.012 respectively).
Thus, the results of our experiments have shown that the adaptation of animals to ECFAW was proceeding by way of induction of genetic damages followed by elimination of damaged cells, but in the most cases these processes were accompanied by enhance of mitotic activity.
The results obtained in this series of experiments allowed supposing that similar effects may develop under an impact of ECFAW into human organism, too. In order to have such a possibility for investigation, experiments were carried out on culture of human peripheral blood cells.
3. Cultivation of human blood cells under the condition of cytokinetic block - the method used in this study - allows taking into account all the complex of changes happening during transformation of stable genome of normal cell into an unstable genome being typical for tumor cell, an increase of frequency of cells with genetic damages (micronuclei and bridges), change of mitotic and proliferative activity of cells, their rate of proliferation and symmetry of cellular division, and also the frequency of programmed death of cells apoptosis), et al. [12,13,15]. However, this fragment of researches was carried out not only for analysis of potential cytogenetic effects from ECFAW, but for clarification of possible mechanisms of their occurrence. In order to identify all potential effects of the human genome instability induced by the ECFAW, in this experiment, the physico-chemical properties of activated water in the most varied widely (Table 2).
| Water title | Redox potential, mV | Hydrogen index, рН, conventional units | Conductivity, µS/cm | Light sum of chemiluminescence, *104, conventional units | Chemiluminescence maximum, Ам, conventional units | ||
| average | variation | average | variation | ||||
| Catholytes | -61.7 ± 2.3 | 7.20 ± 0.02 | 25.1 ± 0.00 | 1.58 | 0.04 | 26.5 | 0.07 |
| -39.9 ± 1.6 | 7.20 ± 0.008 | 25.1 ± 0.05 | 2.47 | 0.11 | 50.8 | 0.32 | |
| 9.6 ± 5.2 | 7.07 ± 0.004 | 25.3 ± 0.05 | 3.67 | 0.12 | 71.3 | 0.21 | |
| 113.7 ± 2.7 | 7.00 ± 0.04 | 22.1 ± 0.05 | 2.64 | 0.14 | 57.4 | 0.46 | |
| 149.2 ± 3.2 | 6.81 ± 0.01 | 24.2 ± 0.05 | 4.25 | 0.26 | 74.7 | 0.24 | |
| 206.0 ± 6.7 | 7.19 ± 0.03 | 22 ± 0.05 | 1.72 | 0.17 | 31 | 0.18 | |
| Control without activation | 325.9 ± 7.0 | 6.70 ± 0.016 | 23.0 ± 0.05 | 5.46 | 0.24 | 93 | 0.19 |
| Аnolytes | 346.39 ± 1.4 | 6.40 ± 0.01 | 26.7 ± 0.05 | 2.85 | 0.1 | 51.3 | 0.11 |
| 360.8 ± 6.9 | 6.19 ± 0.006 | 22.2 ± 0.05 | 1.91 | 0.1 | 34.5 | 0.1 | |
Table 2: Characteristic of physicochemical properties of samples of contact-free electrochemically activated water.
These water samples were used to prepare reconstructed media in which human blood cells were cultivated.
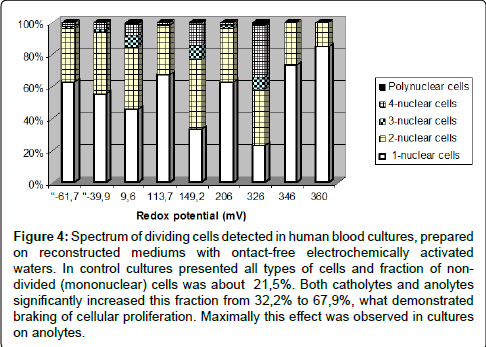
The cytogenetic analysis revealed abundance of cytogenetic effects what proves genotoxic activity of investigated waters and qualitatively support the results of previous experiments on Drosophila melanogaster and mice (Figures 4-6).
Figure 4: Spectrum of dividing cells detected in human blood cultures, prepared on reconstructed mediums with ontact-free electrochemically activated waters. In control cultures presented all types of cells and fraction of nondivided (mononuclear) cells was about 21,5%. Both catholytes and anolytes significantly increased this fraction from 32,2% to 67,9%, what demonstrated braking of cellular proliferation. Maximally this effect was observed in cultures on anolytes.
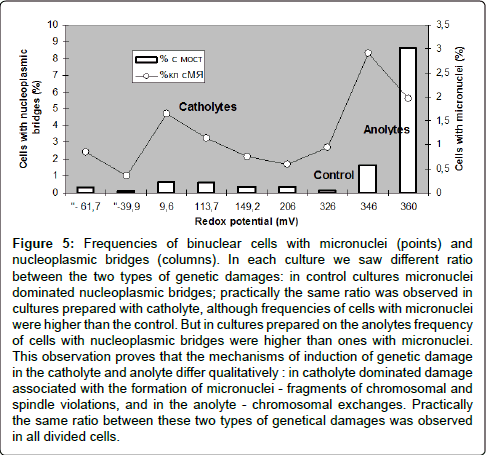
Figure 5: Frequencies of binuclear cells with micronuclei (points) and nucleoplasmic bridges (columns). In each culture we saw different ratio between the two types of genetic damages: in control cultures micronuclei dominated nucleoplasmic bridges; practically the same ratio was observed in cultures prepared with catholyte, although frequencies of cells with micronuclei were higher than the control. But in cultures prepared on the anolytes frequency of cells with nucleoplasmic bridges were higher than ones with micronuclei. This observation proves that the mechanisms of induction of genetic damage in the catholyte and anolyte differ qualitatively : in catholyte dominated damage associated with the formation of micronuclei - fragments of chromosomal and spindle violations, and in the anolyte - chromosomal exchanges. Practically the same ratio between these two types of genetical damages was observed in all divided cells.
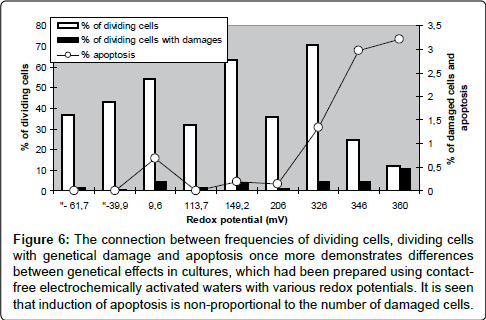
Figure 6: The connection between frequencies of dividing cells, dividing cells with genetical damage and apoptosis once more demonstrates differences between genetical effects in cultures, which had been prepared using contactfree electrochemically activated waters with various redox potentials. It is seen that induction of apoptosis is non-proportional to the number of damaged cells.
In total, contact-free electrochemically activated waters did induce genetical damages in cultures of human blood cells, blocked cellular proliferation and influenced upon systems of apoptosis. It is very important that all of the effects were essentially non-linear. And it was impossible to understand mechanisms of their occurrence without taking into consideration the connection with physical properties of ECFAW.
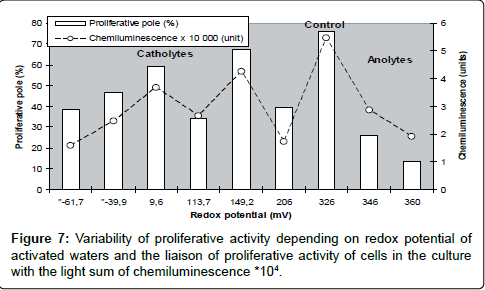
It is well known that proliferative activity is a universal indicator of biological activity of cells both in culture and in organism. As far as RP is commonly considered as the basic physical index qualifying cellular biological activity, before this study we presumed an existence of a common regularity in changes of proliferative activity of cells caused by RP for all the range under study. However, in our experiments the liaison between these indices happened to be essentially non-linear : a direct correlative liaison between RP and the proliferative activity of cells was detected not for all catholytes, the reverse one – only for anolytes, intermediate values of proliferative activity of cells were not correlated with RP, and the maximum proliferative pool was marked in the control, where the culture was growing on non activated water (these data are support with results, presented on Figures 4 and 6). At the same time we observed the conformity of luminol-dependent chemiluminescence and proliferative activity of the cells, what allowed supposing the presence in the medium of a factor (factors) of radical nature and its influence on the proliferative activity? (Figure 7).
First of all, we supposed that the source of free radicals may be H2O2, which presents in, practically, each tap water. By the directives WHO and EC the content H2O2 is not standardized. But in Russia and many other countries this standard exists (for Russia it is 100 μkg/l) [16,17].
In model experiments with distilled water and then with culture medium RPMI -1640, we determined dependence of the chemiluminescence from the concentration of hydrogen peroxide (r=0.98; p=0.000002). Because of this we analyzed correlation between the indices of genotoxic effects in human blood cells cultures and the luminal-dependent chemiluminescence and hydrogen peroxide content, which were 0.67<r<0.82 and p ≤ 0.05.
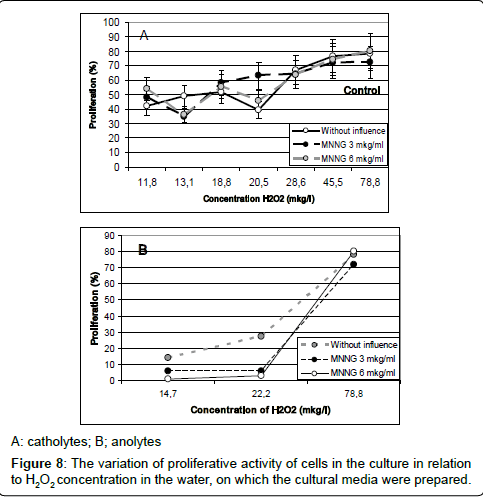
An analysis of liaison between the H2O2 concentration in the kinds of water and the proliferative activity of cells in the cultures, prepared on these waters, is shown in Figure 8.
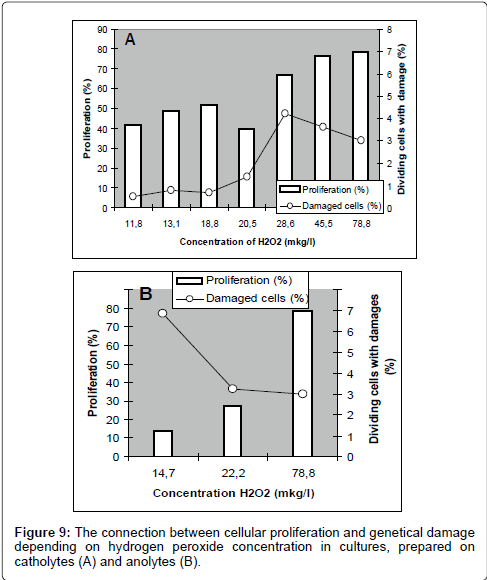
As shown in Figure 8, catholyte-induced proliferation (both without and with various concentrations of MNNG) smoothly describes a linear dependence on the content of hydrogen peroxide in the culture medium. A similar relationship for anolyte was detected. As these figures show, the most intensive proliferation activity was discovered in the culture, prepared with water containing maximum concentration of hydrogen peroxide: it was the control culture. With reduction of hydrogen peroxide concentration in catholytes, the proliferative activity of cells in cultures, prepared on these kinds of water, declined but, the frequency of cells with genetic damages has increased Figure 9.
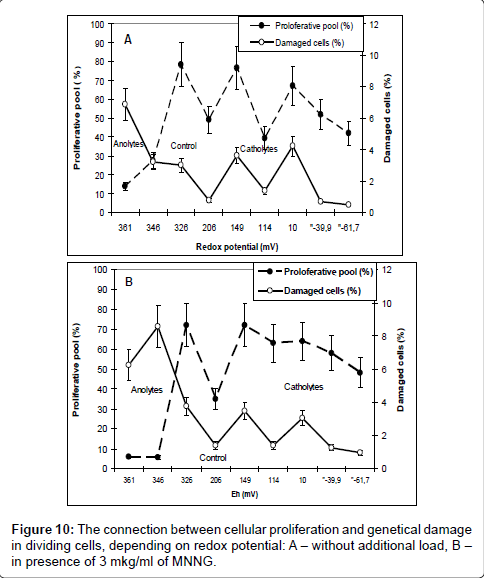
For the end we analyzed the connection between the effects of cellular proliferation and genetic damages in dividing cells depending on redox potential Figure 10.
As it is, the frequencies of genetic damages changed in parallel with cellular proliferation, and, consequently, the level of genetic damage in dividing cells is determined by level of cellular proliferation. This conclusion - so obvious to cytogenetics, unfortunately, is not always taken into account when analyzing the mechanisms of induction of genetic damage. The same results were obtained as a results of several our previous publications [18,19].
So, the results of analysis of genotoxic effects in human blood cells culture showed that at presence of contact-free electrochemically activation water:
- The proliferative activity of cells in reconstructed cultures were prepared on contact-free electrochemically activated waters was associated with RP of water: the maximum proliferation was observed in cultures prepared using control water; at the same time in cultures, prepared on both anolytes and catholytes, cellular proliferation decreased against the control depending on RP;
- The proliferative activity of lymphocytes correlated with luminol-dependent chemiluminescence of waters, which were used for preparation of reconstructed cultures; the maximum of proliferative activity of cells in the culture was observed at the most high value of the chemiluminescence was observed in the control water having the highest concentration of hydrogen peroxide;
- The best situation - with maximal proliferation and minimal level of genetic damages was observed in the cultures prepared on the control water; in the cultures, prepared on contact-free electrochemically activated waters – both anolytes or catholytes - the inhibition of cellular proliferation in parallel with enhance of level of genetic damage relatively to control was observed; these effects were most obviously expressed for cultures prepared on anolytes;
- Summarizing the results, submitted in the present publication, one may to conclude that:
- Drinking waters with contact-free altered physical parameters induce effects of genome instability in biological objects of various levels – from drosophila to human blood cells in vitro;
- The received data argue the necessity of creating a system for assessment of genetic safety of these waters before the equipment for their preparation will be sold uncontrolledly;
- The living test-objects, used in this work, may be used as test-objects in the system of genetic safety of drinking waters received by the method of contact-free electrochemical activation;
- An inhibition of genotoxic effects of drinking waters, received by electrochemical contact-free activation, may be achieved by:
a) Decrease of hydrogen peroxide concentration in the source water or using special schemes of application of these waters (reduction of water consumption, using of fractional schemes, et al), what, though, is also in need of assessment of genetic safety;
b) Reduction of water’s redox potential.
References
- Savostikova N (2008) Hygienic assessment of influence of structural changes in water on its physical-chemical and biological properties, candidate of medical sciences. Ph.D. thesis in Biological Science. Moscow 160 P.
- Lobyshev VI ( 2007) Water as a sensor of weak impacts of physical and chemical nature. Russ.chem.mag 1: 107-114.
- Rakhmanin IuA, Stekhin AA, Iakovlev GV (2007) [Structural and energy changes in water and its biological activity]. Gig Sanit : 34-36.
- Shirahata S, Nishimura T, Kabayama S, Aki D, Teruya K, et al. (2001) Anti-oxidative water improves diabetes. In E. Lindner-Olsson, et al. (Eds.). Animal cell technology: 574-577.
- Osada K, Li YP, Hamasaki T, Abe M, Nakamichi N. et al. (2010) Anti-diabetes effects of Hita Tenryosui water, a natural reduced water. In K. Ikura, et al. (Eds.). Animal cell technology: Basic & applied aspects 15: 307-313.
- Bakhir V, Zadorozhniy Yu G, Leonov BI, Panicheva S, Prilutsky VI (2001) Electrochemical activation: water purification and receiving of useful solutions VNIIIMT SS : 176.
- Abe M, Sato S, Toh K, Hamasaki T, Nakamichi N, et al. (2010) Suppressive effect of ERW on lipid peroxidation and plasma triglyceride level. In M. Kamihira, et al. (Eds.), Animal cell technology: Basic & applied aspects 16: 315-321
- Abol-Enein H, Gheith OA, Barakat N, Nour E, Sharaf AE (2009) Ionized alkaline water: new strategy for management of metabolic acidosis in experimental animals. Ther Apher Dial 13: 220-224.
- Aksyonov SI (2004) Water and its role in regulation of biological processes. Moscow, Izhevsk, Institute of Computer Researches 212.
- Mendelson GI (1992) Dominant lethal mutations with various species of drosophilas as a test for assessment of mutagen effects of environment pollutant: Author’s abstract in Biological science.
- Iurchenko VV, Krivtsova EK, Beliaeva NN, Ingel' FI, Olesinov AA, et al. (2008) [Use of micronuclear test to assess drinking waters]. Gig Sanit : 49-53.
- Fenech M (2000) The in vitro micronucleus technique. Mutat Res 455: 81-95.
- Ingel FI (2006) Prospects of using micronuclei test on human blood lymphocytes, cultured under conditions of cytogenetic block. Part 1. Proliferation of cells. Ecological genetics 4: 7-19.
- Ingel FI (2006) Prospects of using micronuclei test on human blood lymphocytes, cultured under conditions of cytogenetic block. Part 2. Factors of environmental and individual features in the human genome instability system. Auxiliary options of the test. The technique of tests and cytogenetic analysis. Ecological genetics 4: 38-54.
- Smith LE, Nagar S, Kim GJ, Morgan WF (2003) Radiation-induced genomic instability: radiation quality and dose response. Health Phys 85: 23-29.
- Zatsepina V, Ingel FI, Stekhin, Yakovleva GV (2013) Influence of physically activated water on proliferative activity and apoptosis of human blood lymphocytes in vitro. Biological defense and biological safety 3: 19-26.
- SanPi N 2.1.4.1074-01 Drinking water. Hygienic requirements for the quality of waters in the central water supply systems (with amendments of April 7, 2009, February 25 and June 28, 2010, Russian Federation).
- Ingel FI, Krivtsova EK, Yurtseva NA, Antipanova NA, Legostaeva TB (2013) Instability and sensitivity of genome of healthy children in Magnitogorsk. Hygiene and sanitation 3: 20-27.
- Akhal'tseva LV, Moshkov NE, Ingel' FI, Iurtseva NA, Iurchenko VV (2011) [Effect of titanium dioxide nano- and microparticles on the values of the micronucleus test using human blood lymphocytes in culture]. Gig Sanit : 61-63.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 14667
- [From(publication date):
December-2013 - Dec 21, 2024] - Breakdown by view type
- HTML page views : 10240
- PDF downloads : 4427