Research Article Open Access
Electrochemical Studies of Dopamine, Ascorbic Acid and Uric Acid at Lignin Modified Carbon Paste Electrode by Cyclic Voltammetric
Chandrashekar C Vishwanatha, Bahaddurghatta E Kumara Swamy* and K Vasantakumar Pai
Department of P.G .Studies and Research in Industrial Chemistry, Kuvempu University, Jnana Sahyadi, Karnataka, India
- *Corresponding Author:
- Kumara Swamy BE
Department of P.G .Studies and Research in Industrial Chemistry
Kuvempu University, Jnana Sahyadi
Shankaraghatta-577451, Shimoga, Karnataka, India
Tel: +91-8282-256225
Fax: +91-8282-256255
E-mail: kumaraswamy21@yahoo.com
Received Date: February 10, 2015; Accepted Date: March 23, 2015; Published Date: March 26, 2015
Citation: Vishwanatha CC, Swamy BEK, Pai KV (2015) Electrochemical Studies of Dopamine, Ascorbic Acid and Uric Acid at Lignin Modified Carbon Paste Electrode by Cyclic Voltammetric. J Anal Bioanal Tech 6:237. doi: 10.4172/2155-9872.1000237
Copyright: © 2015 Vishwanatha CC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In this study the lignin modified carbon paste electrode was applied for the electrochemical determination of dopamine, ascorbic acid and uric acid at pH 7.2 PBS solution with scan rate 50 mVs-1. The modified carbon paste electrode shows good electrochemical activity towards dopamine and ascorbic acid, where as in uric acid shows decrease in peak current when compared to bare carbon paste electrode. The effect of scan rate and concentration was studied at modified carbon paste electrode. The electrode process was found to adsorption and diffusion controlled and the detection limit was found to be 1.4 × 10-5 M.
Keywords
Dopamine; Ascorbic acid; Uric acid; Lignin; Modified carbon paste electrode
Introduction
Lignin is a natural amorphous polymer contained in most woody plants such as trees and shrubs [1,2]. It is highly viscous liquid called black liquor as well as the main disposal of the cellulose industries. Every year tons of black liquor is rich in lignin and lignin derivatives being burned in thermal generators to generate a fraction of the energy consumed in industry [3,4]. Lignin from various pulping process has been shown to be applicable in electrochemical sensors owing to its residual quinine moieties, which are redox and thus electroactive. In order to intensify the chemical properties of lignin that makes it suitable for electrochemical applications. Various methods of combining these materials with electrical conductors (carbon nanotubes or conducting polymers) have been proposed [5-9].
Dopamine (DA) is an important neurotransmitter molecule of catecholamine’s which is widely distributed in the mammalian central nervous system [10-13], renal, hormonal and cardiovascular systems. DA is also involved in the regulation of congnitive functions. High concentration of DA is found in a particular region of brain called caudate nucleus [50 nmolg-1] and very low concentration of DA is found in extra cellular fluids (0.01-1 mM). The excess level of DA can lead of euphoria whereas depletion in DA level can lead to neurodegenerative disease such as Parkinsonism, Schizophrenia, Huntington`s diseases and senile dementia [14-19]. Consequently various electroemical techniques have been proved to be advantages in the selective and sensitive determination of DA concentration [20]. The fact that DA and other catecholamine’s are easily oxidizable compounds makes their detection possible by electrochemical method based on anodic oxidation [21].
Ascorbic Acid (AA) is a soluble vitamins present in many biological systems and in multi vitamin preparations which are commonly used to supplement inadequate dietary intake and as anti-oxidants. The concentration of AA on food stuffs, beverages and pharmaceutical can be an index of quality since it varies during production and storage stages [22-24], Similarly AA has been used for the prevention and treatment of the common cold, mental illness, infertility and even cancer [25,26]. Now-a-days AA has been more interested in medical, veterinary science, toxicology, diagnosis of certain metabolic disorder and in the determination of nutrition value of foods [27-31].
Uric Acid (UA) is the primary end product of purine [32] metabolism. Through the liver which is present in the blood of urine monitoring UA in the blood or urine is important because it can be used as a powerful indicator for an easily warning sign of kidney diseases. Abnormal UA level in a human body could be caused by several diseases such as Gout hyperuricemia, Lesch-Nyan syndrome, Cardiovascular and chronic renal diseases. There are some electrochemical methods for determine of uric acid [33-37].
In continuation of our work on the modification of carbon paste electrodes we intended for the lignin was modified and applied for the electrochemical investigation for dopamine, ascorbic acid, and uric acid individually. Lignin MCPE shows high sensitivity in anodic and cathodic peak current towards dopamine and ascorbic acid not for the uric acid at pH 7.2 PBS solutions.
Experimental Part
Reagents and chemicals
Dopamine (DA), Ascorbic Acid (AA) and Uric Acid (UA) were obtained from Himedia chemical company and of analytical grade used without further purification. 25×10-4 M DA stock solution was prepared in 0.1 M perchloric acid, 25×10-4 AA was prepared in double-distilled water and 25×10-4 UA was prepared in 1 M NaOH. Graphite powder of 50 mm size was purchased from Loba, silicon oil was purchased from Himedia and Lignin was purchased from Aldrich company. The chemicals for preparation of buffer solution were purchased from Merck. Phosphate buffer (0.2 M pH 7.2) was used as supporting electrolyte.
Apparatus
Cyclic voltammetry (CV) was performed in a model CHI-660c (CH Instrument-660 electrochemical workstation). All experiments were carried out in a conventional electrochemical cell. The electrode system contained a carbon paste working electrode (3.0 mm in diameter), a platinum wire as counter electrode and saturated calomel as reference electrode.
Preparation of bare carbon paste electrode
The carbon paste electrode was prepared by using 70% graphite powder and 30% silicone oil were mixed by hand to produce a homogeneous carbon paste. The paste was then packed into the cavity of a homemade carbon paste electrode and smoothed on a weighing paper. Similarly in the preparation of lignin modified carbon paste same procedure was followed along with different concentration of lignin. The packing is same as that of bare CPE.
Results and Discussion
Effect of lignin as modifier
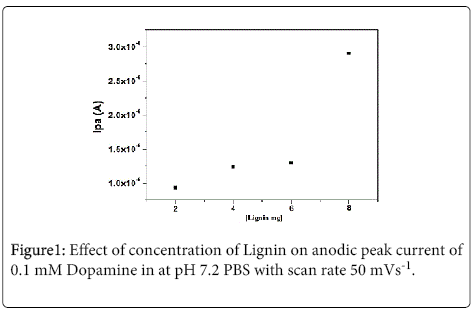
Lignin is used as a modifier in the preparation of carbon paste electrode and it was investigated by cyclic voltammetric technique. The different weight of lignin 2-8 mg was added to the graphite powder along with the silicon oil which acts as a binding agent when the concentration of lignin the anodic and cathodic peak current gradually increases. This is due to lignin molecules contain quinine/ hydroquinone (Q/HQ) groups and its shows antioxidant properties beyond catalytic properties therefore lignin can be used for MCPE and for sensors properties [3,5,6,38-41]. The graph of peak current v/s different concentration of lignin modified carbon paste electrode was plotted as shown in Figure 1. The maximum anodic peak current signal was obtained for 8 mg lignin MCPE. The 4 mg lignin was chosen for the work.
Electrochemical response of Potassium ferrocyanide at Lignin modified carbon paste electrode
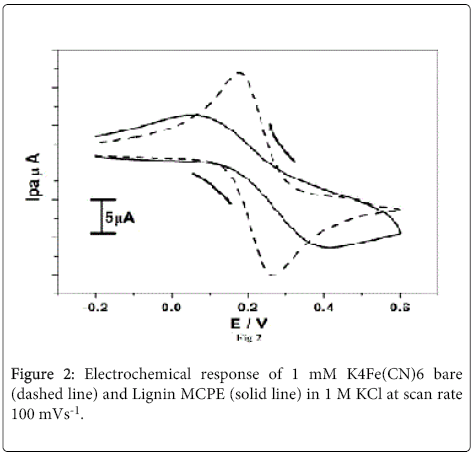
The Figure 2 shows Electrochemical response of 1 mM K4Fe(CN)6 bare (dashed line) and Lignin MCPE (solid line) with supporting electrolyte 1 M KCl at scan rate 100 mVs-1. The bare CPE shows well defined oxidation and reduction peak was found to be 0.26 V and 0.17 V with the maximum peak potential difference (ΔEp) 94 mV. After modification with lignin the oxidation and reduction peak potential found to be 0.41 V and 0.04 V with small peak potential difference (ΔEp) 34 mV and the peak currents decreased rapidly when compared to BCPE. This is due to the anionic character of the oxidized lignin layer leading to the repulsive interaction of the electrode surface and the reduction species [5] which inhibit the electroctalytic effect towards potassium ferricyanide.
Electrochemical response of Dopamine
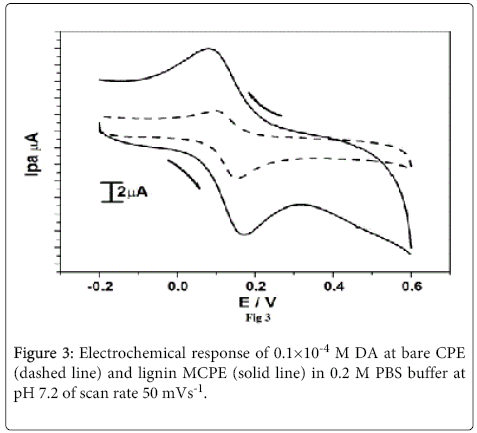
The Figure 3 displays the cyclic voltammograms of bare CPE (dashed line) and lignin MCPE (solid line) in 0.1×10-4 M DA solution at pH 7.2 PBS with scan rate 50 mVs-1. The bare carbon paste electrode shows anodic and cathodic peak potential and currents. After the modification with lignin the current signals were enhanced with decrease in over potential. This modified electrode shows electrocatalytic properties and the sensor properties.
Effect of Dopamine concentration
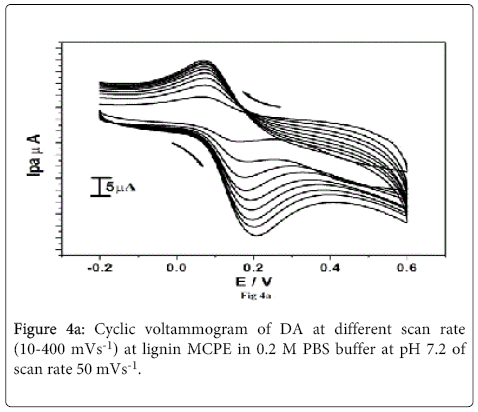
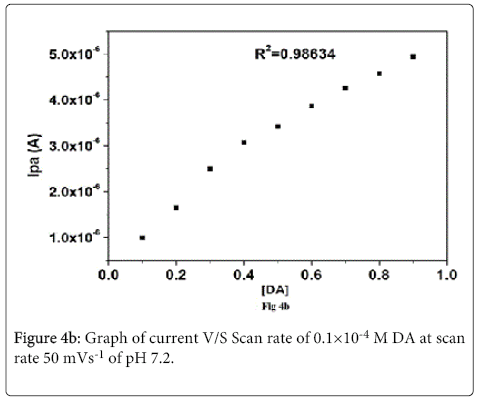
The effect of concentration of DA in the range (0.1-0.8×10-4 M) was studied at lignin MCPE in 0.2 M PBS buffer at pH 7.2 of scan rate 50 mVs-1 From the Figure 4a it is clear that with increase in the concentration of DA the Ipa and Ipc also increases with shifting of Epa towards positive potential and Epc towards negative potential. The plot of Ipa v/s DA concentration Figure 4b shows increase in electrochemical peak current with linear regression equation Ipa(μA)=4.819 (C) μM/L+0.8391(μA) (R2=0.98634). The detection limit for dopamine was found to be 1.4×10-5 M. The detection limit was calculated by using the formula (1) [42] where S is the standard deviation and M is the slope obtained from the calibration plots.
LOD = 3S/M…………… (1)
Effect of scan rate
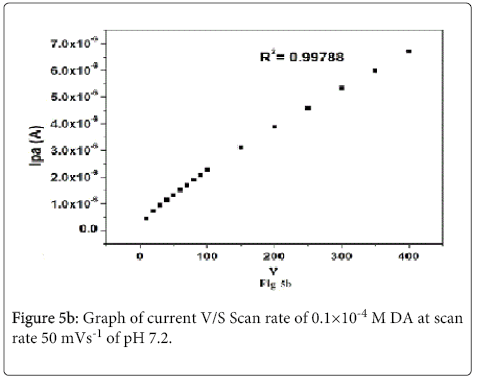
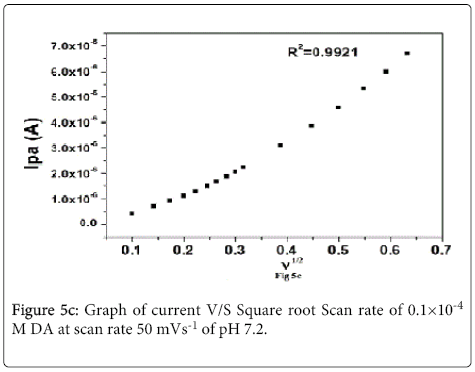
Cyclic voltammograms of 0.1×10-4 M DA with different scan rate at lignin MCPE at pH 7.2 PBS solution as shown in Figure 5a. The anodic and cathodic peak current goes on increasing with increasing the scan rate from (10–400 mVs-1) and the potential remains constant. The plot of Ipa v/s υ shows linear dependence on the scan rate with a linear regression equation Ipa= -5.4171×10-7+0.01585(υ) R2=0.9978 Figure 5b Ipa v/s υ1/2 shows linear regression equation Ipa= -1.2628×10-6+11.91 (υ1/2) R2=0.9978 respectively Figure 5c This indicates that lignin MCPE of dopamine shows adsorption and diffusion controlled process [43-45].
Electrochemical oxidation of AA at lignin MCPE
The Figure 6 shows the cyclic voltammograms of 1×10-4 M AA on the bare CPE (dashed line) and lignin MCPE (solid line) at pH 7.2 PBS solutions with scan rate 50 mVs-1. At bare CPE AA shows well oxidation peak current at 0.31 V with a reduced voltammetric wave. However in lignin MCPE shows for some extent of enhancement in oxidation peak current and the Epa was found to be 0.36 V by shifting the oxidation potential towards positive side when compared to bare CPE. This results show that our modified carbon paste electrode act as a sensor for AA.
Electrochemical oxidation of UA at lignin MCPE
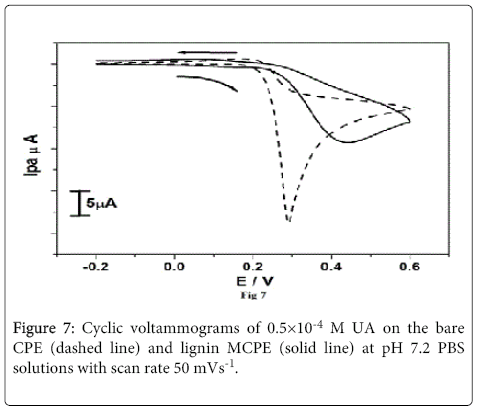
The Figure 7 shows the cyclic voltammograms of 0.5×10-4 M UA on the bare CPE (dashed line) and lignin MCPE (solid line) at pH 7.2 PBS solution with scan rate 50 mVs-1. The oxidation peak potential (Epa) of UA at bare CPE and lignin MCPE was found to be 0.28 V and 0.43 V respectively. After the modification electrode shows decrease in current signal when compared to bare CPE. This indicates that the modified electrode shows lower electrocatalytic activity for UA.
Conclusion
In this study the modified electrode was prepared by adding different quantity of lignin to proportion of 70:30 of graphite powder and silicon oil and was applied for the electrochemical studies for AA, DA, and UA. The Lignin MCPE shows electrochemical sensors for DA and AA. This lignin MCPE is not sensitive towards the diction of UA and this MCPE shows sensors application for DA and AA individually as well as simultaneous. This method can be also been applied for other neurotransmitters.
References
- Gosselink RJA, De Jong E, Abacherli A (2004) Co-ordination network for lignin standardisation, production and applications adapted to market requirements (EUROLIGNIN). Journal of Industrial Crops and Products 20: 121-129.
- Degefua H, Amareb M, Tessemaa M, Admassiea S (2014) Lignin modified glassy carbon electrode for the electrochemical determination of histamine in human urine and wine samples.ElectrochimicaActa 121: 307-314.
- Buoroa RM, Bacila RP, Da Silvaa RP, Da Silvaa LCC, et al. (2013) Lignin-AuNp modified carbon paste electrodes Preparation, characterization, and applications.ElectrochimicaActa 96: 191-198.
- Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin.J Hazard Mater 151: 134-142.
- Milczarek G (2007) Preparation and characterization of a lignin modified electrode. Electroanalysis 19:1411-1414.
- Milczarek G (2009) Lignosulfonate-modified electrodes: electrochemical properties and electrocatalysis of NADH oxidation.Langmuir 25: 10345-10353.
- Milczarek G, (2010) Kraft lignin as dispersing agent for carbon nanotubes. Journal of Electroanalytical Chemistry 638: 178-181.
- Milczarek G, Rebis T (2012) Synthesis and electroanalytical performance of a composite material based on poly (3,4ethylenedioxythiophene doped with lignosulfonate. International Journal of Electrochemistry.
- Klapiszewski A,Wysokowski M, Majchrzak I, Szatkowski T, Nowacka M, et al. (2013) Preparation and Characterization of Multifunctional Chitin/LigninMaterials. Journal of Nanomaterials.
- Lin L, Chen J, Yao H, Chen Y, Zheng Y, et al. (2008) Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (Evans Blue) modified glassy carbon electrode.Bioelectrochemistry 73: 11-17.
- Martin C (1998) The Parkinson's puzzle new developments in our understanding of Parkinson's disease have generated a number of promising new treatments for this disabling condition. Journal of Brazilian Chemical society 34: 40-42.
- Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain.Anal Chem 60: 769A-779A.
- Heinz A, Przuntek H, Winterer G, Pietzcker A(1995) [Clinical aspects and follow-up of dopamine-induced psychoses in continuous dopaminergic therapy and their implications for the dopamine hypothesis of schizophrenic symptoms].Nervenarzt 66: 662-669.
- Thomas T, Mascarenhas R J, Nethravathi C, Rajamathi M, Kumara Swamy B E (2011) Graphite oxide bulk modified carbon paste electrode for the selective detection of dopamine: A voltammetric study. Journal of Electroanalytical Chemistry 659: 113-119.
- Lin X, Zhang Y, Chen W, Wu P (2007) Electrocatalytic oxidation and determination of dopamine in the presence of ascorbic acid and uric acid at a poly (p-nitrobenzenazo resorcinol) modified glassy carbon electrode. Sensors and Actuators B 122: 309-314.
- Alwarappan S, Liu G, Li CZ (2010) Simultaneous detection of dopamine, ascorbic acid, and uric acid at electrochemically pretreated carbon nanotube biosensors.Nanomedicine 6: 52-57.
- Thiagarajan S, Chen SM (2007) Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid.Talanta 74: 212-222.
- Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, et al. (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes.BiosensBioelectron 25: 2366-2369.
- Curulli A (2009) Electrochemical direct determination of catecholamines for the early detection of neurodegenerative diseases.Sensors (Basel) 9: 2437-2445.
- Gilbert O, Kumara Swamy BE, Chandra U, Sherigara BS (2009) Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study. Journal of Electroanalytical Chemistry 636: 80-85.
- Zheng J, Zhou X (2007) Sodium dodecyl sulfate-modified carbon paste electrodes for selective determination of dopamine in the presence of ascorbic acid.Bioelectrochemistry 70: 408-415.
- Babaei A, Zendehdel M, Khalilzadeh B, Taheri A (2008) Simultaneous determination of tryptophan, uric acid and ascorbic acid at iron(III) doped zeolite modified carbon paste electrode.Colloids Surf B Biointerfaces 66: 226-232.
- Fox BA, Cameron AG (1989) In: E. Arnold, Food Science, Nutrition and Health. London 261.
- Yu A M, Chen H.Y (1997) Electrocatalytic oxidation and determination of ascorbic acid at poly(glutamic acid) chemically modified electrode. Analytical ChimicaActa 344: 181-185.
- Dursun Z, Pelit L, Taniguchi I (2009)Voltammetric determination of ascorbic acid and dopamine simultaneously at a single crystal Au(111) electrode.Tubitak Journal of Chemistry 33: 223-231.
- Zhang L, Jiang X (2005) Attachment of gold nanoparticles to glassy carbon electrode and its application for the voltammetric resolution of ascorbic acid and dopamine. Journal of Electroanalytical Chemistry 583: 292-299.
- Shahrokhian S, Karimi M (2004) Voltammetric studies of a cobalt(II)-4-methylsalophen modified carbon-paste electrode and its application for the simultaneous determination of cysteine and ascorbic acid. ElectrochimicaActa 50: 77-84.
- Belli SL, Rechnitz GA (1986) Prototype potentiometric biosensor using intact chemoreceptor structures. Analytical Letters 19: 403-416.
- Yu M, Dovichi NJ (1989) Attomole amino acid determination by capillary zone electrophoresis with thermooptical absorbance detection.Anal Chem 61: 37-40.
- Amini MK, Shahrokhian S, Tangestaninejad S (1999) PVC-Based Mn(III) Porphyrin Membrane-Coated Graphite Electrode for Determination of Histidine.Anal Chem 71: 2502-2505.
- Shahrokhian S (2001) Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode.Anal Chem 73: 5972-5978.
- Ensafi AA, Taei M, Khayamian T (2010) Simultaneous Determination of Ascorbic Acid, Dopamine, and Uric Acid by Differential Pulse Voltammetry using Tiron Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci., 5: 116 -130.
- Mazloum-Ardakani M, Beitollahi H, Ganjipour B, Naeimi H, Nejati M (2009) Electrochemical and catalytic investigations of dopamine and uric acid by modified carbon nanotube paste electrode.Bioelectrochemistry 75: 1-8.
- Shahrokhian S, Zare-Mehrjardi H R (2007) Simultaneous voltammetric determination of uric acid and ascorbic acid using a carbon-paste electrode modified with multi-walled carbon nanotubes/nafionon and cobalt (ii) nitrosalophen. Electroanalysis 19: 2234-2242.
- MazloumArdakani M, Akrami Z, Kazemian H, Zare H R (2006) Electrocatalytic characteristics of uric acid oxidation at graphite zeolite- modified electrode doped with iron (III). Journal of Electroanalytical Chemistry 586: 31-38.
- Gilmartin M A T, Hart J P (1992) Voltammetric and amperometric behaviour of uric acid at bare and surface-modified screen-printed electrodes: studies towards disposable uric acid sensor. Analyst 117: 1299-1303.
- Tatsuma T, Watanabe T (1991) Oxidase/peroxidase bilayer-modified electrodes as sensors for lactate, pyruvate, cholesterol and uric acid. Analytical ChimicaActa 242: 85-89.
- Buoroa RM, Bacila RP, Da Silvaa RP, Da Silvaa LCC, Limaa AWO, et al. (2013) Lignin-AuNpmodified carbon paste electrodes Preparation, characterization, and applications. ElectrochimicaActa 96: 191-198.
- Buoro RM, Silva RP, Bacil RP, Lima AWO, Serrano SHP, et al. (2012) Electrochemical characterization and evaluation of the analytical potentialities of glassy carbon electrodes modified with extracted lignin from black (Kraft) liquor Serrano. ECS Transactions. 43: 119-126.
- Milczarek G (2007) Preparation and characterization of a lignin modified electrode.Electroanalysis 19: 1411-1414.
- Milczarek G (2008) Lignosulfonate-modified electrode for electrocatalytic reduction of acidic nitrite. Electroanalysis 20: 211-214.
- Milczarek G (2009) Preparation, characterization and electrocatalytic properties of an iodine lignin- modified gold electrode. ElectrochimicaActa. 54: 3199-3205.
- Milczarek G (2009) Lignosulfonate-modified electrodes: electrochemical properties and electrocatalysis of NADH oxidation.Langmuir 25: 10345-10353.
- Chen A, Rogers EI, Compton RG (2010) Abrasive stripping voltammetric studies of lignin and lignin model compounds. Electroanalysis 22: 1037-1044.
- Milczarek G (2007) Preparation and Characterization of a Lignin Modified Electrode. Electroanalysis 19: 1411-1414.
- Shankar SS, Kumara Swamy BE, Chandrashekar BN (2012) Electrochemical selective determination of dopamine at TX-100 modified carbon paste electrode: A voltammetric study. Journal of Molecular Liquids 168: 80-86.
- Ardakani MM, Rajabi H, Beitollahi H, Mirjalili BB, Akbari A, et al. (2010) Voltammetric Determination of Dopamine at the Surface of TiO2 Nanoparticles modified Carbon Paste Electrode. International Journal of Electrochemical Science 5: 147-157.
- Chandra U, Kumara Swamy BE, Gilbert O, Sherigara BS (2011) Poly (Naphthol Green B) film based sensor for resolution of dopamine in the presence of uric acid: A voltammetric study. Analytical Methods 3: 2068-2072.
- Suresh R, Giribabu K, ManigandanR, Praveen Kumar S, Munusamy S, et al. (2014) New electrochemical sensor based on Ni-doped V2O5 nanoplates modified glassy carbon electrode for selective determination of dopamine at nanomolar level. Sensors and Actuators B. 202: 440-447.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16356
- [From(publication date):
April-2015 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 11588
- PDF downloads : 4768