Electrochemical Response of Dopamine in Presence of Uric Acid at Pregabalin Modified Carbon Paste Electrode: A Cyclic Voltammetric Study
Received: 11-Dec-2015 / Accepted Date: 08-Jan-2016 / Published Date: 15-Jan-2016 DOI: 10.4172/2155-9872.1000297
Abstract
The electrochemical analysis of dopamine and uric acid was performed at Pregabalin modified carbon paste electrode by cyclic voltammetry in 0.2M phosphate buffer solution of pH 7.5 at scan rate 100 mV/s. The Pregabalin modified carbon paste electrode showed an increase in anodic peak current as compared to the bare carbon paste electrode. The pregabalin modified carbon paste electrode showed excellent electrocatalytic activity for selective determination of dopamine and uric acid by differential pulse voltammetry technique. Several elctrochemical parameters like effect of pH, Scan rate, different concentrations of dopamine and uric acid were studied at Pregabalin modified carbon paste electrode. The modified Pregabalin carbon paste electrode behaves like selective electrochemical sensor for detection of dopamine and uric acid.
Keywords: Dopamine; Pregabalin; Modified carbon paste electrode; Uric acid; Cyclic voltammetry; Differential pulse voltammetry
302489Abbrevations
DA: Dopamine; UA: Uric acid; CV: Cyclic voltammetry; DPV: Differential Pulse Voltammetry
Introduction
Drug is a chemical substance used in the treatment, prevention and diagnosis of disease and it is used to enhance physical or mental wellbeing. Anticonvulsants are commonly known as anti-epileptic drugs and they are used in the treatment of epileptic seizures. Pregabalin is an anti-epileptic drug and is used for the treatment of diabetic neuropathy and post-herpetic neuralgia [1]. Preclinical and clinical studies have shown the effectiveness of pregabalin in managing the neuropathic pain. Clinical studies have also shown the efficacy and dose dependent effects of pregabalin either as monotherapy or in combination with analgesics in relieving pain and related symptoms [2,3].
Neurotransmitters are important biological key molecules. Dopamine is a unique neurotransmitter compound extensively distributed in the brain for message transfer in the mammalian central nervous system. 3,4-Dihydroxyphenyl ethylamine, is commonly known as dopamine, which plays a very important role in the function of central nervous, endocrine function and cognition systems. It affects brain processes that control movement, emotional response, and ability to experience pleasure and pain. High level concentration of dopamine causes euphoria. Any fluctuation in level of dopamine is very effective toward brain tissues, whereas low level causes many neurological disorders such as Parkinson’s disease, schizophrenia, HIV infection [4,5] and Huntington’s disease, Senile dementia [6-8].
Uric Acid (UA) (2,6,8-trihydroxypurine) is electroactive biomolecule, a white crystalline powder, soluble in water and is one of the important final end products of purine metabolism in the human body. In healthy persons the concentration of uric acid in urine is around 2mM and in the blood it is in the range of 120mM to 450mM. Normally, healthy human beings excrete about 400 to 700 mg through urine per day [9]. Abnormal levels of uric acid are symptoms of several physiological disorders [10]. High levels of uric acid cause hyperuricemia, excess serum accumulation of uric acid in the blood can lead to arthritis (gout) [11]. The low concentration of uric acid leads to hyperuricemia and other diseases such as Lesch-Nyhan syndrome and heavy hepatitis [12]. Uric acid is also a marker for the renal failure. Its quantitative determination in body fluids is necessary for the treatment of diseases.
Dopamine and uric acid are the two significant biomolecules for physiological processes in human metabolism. Their irregular levels lead some disorders, it is a great important task to design simple and sensitive methods for the determination of their concentrations. There are a number of analytical methods were developed for the detection of dopamine [13-16]. Electrochemical methods have been widely used to quantify the dopamine detection with several advantages like simple design, sensible and cost effective, fast response as compared to conventional analytical methods [17]. Dopamine, uric acid usually coexist in physiological samples, dopamine normally present at low concentration along with uric acid which is at higher concentration. The simultaneous determination of these compounds is a special interest in the development of electrochemical sensors [18]. The successful route to overcome the problems of selectivity is to modify the carbon paste electrode surface, because the modified electrode could decrease the over voltage, improve the velocity of mass transfer effectively and enhances the selectivity of the analyte [19]. The modification can be done by using organic and inorganic substances and biomoecules [20,21].
The modified carbon paste electrodes are gaining prominence because of their biocompatibility and good electron transfer. The present work describes the fabrication of the modified carbon paste electrode by modifying the carbon paste electrode using drug pregabalin for the determination of dopamine and uric acid using cyclic voltammetry and differential pulse voltammetric techniques. This modified electrode shows better selectivity for the determination of dopamine in presence of uric acid. No literature was found for the determination of dopamine by using pregabalin modified carbon paste electrode (Scheme 1).
Experimental
Reagents and chemicals
Pregabalin was received from Srini Pharmaceuticals Ltd. (AP, India) Dopamine (DA) was obtained from Himedia chemical company with analytical grade used without further purification. Dopamine stock solution was prepared in 0.1M Perchloric acid, potassium ferrocyanide and KCl were prepared in double distilled water. Graphite powder of 50 mm size was purchased from Loba and silicon oil was purchased from Himedia. The chemicals for preparation of buffer solution were purchased from Merck. Phosphate buffer (0.2M pH 7.5) was used as supporting electrolyte.
Instrumentation
Cyclic voltammetric experiments were performed using a model CHI-660c (CH Instrument-660 electrochemical workstation). All experiments were carried out with a conventional three electrode cell. The electrode system contained a bare carbon paste electrode (BCPE) pregabalin modified carbon paste electrode (MCPE) as working electrode, platinum wire as a counter electrode and saturated calomel as a reference electrode.
Preparation of bare carbon paste electrode
The bare carbon paste electrode was prepared by graphite powder and silicon oil at the ratio of 70:30 (w/w) in an agate mortar by hand mixing to get homogenous paste. The prepared carbon paste was tightly packed into the cavity of a homemade carbon paste electrode then polished the surface by rubbing on a weighing paper.
Preparation of modified carbon paste electrode
The modified carbon paste electrode was prepared by using 5 mg of Pregabalin, graphite powder and silicon oil at a ratio of 70:30 (w/w) in an agate mortar by hand mixing to get homogenous paste. The modified carbon paste was tightly packed into the cavity of a homemade carbon paste electrode then polished the surface by rubbing on a weighing paper.
Results and Discussion
Influence of modifier concentration on the surface of carbon paste electrode
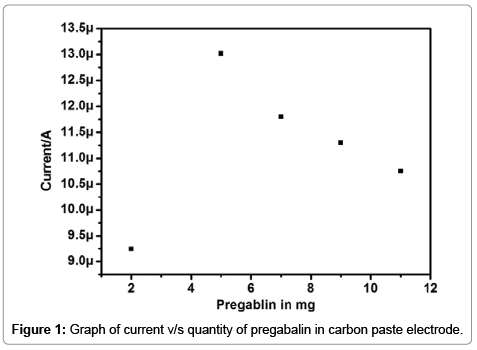
The electrochemical response of the carbon paste electrode is mainly depends upon the properties and concentration of the modifying species [17,22-26], In our study pregabalin was used as a modifier, Pregabalin modified carbon paste electrode was prepared by adding a different ratio of pregabalin to the carbon paste electrode. The concentration of pregabalin varied from 2 to 11 mg by increasing the concentration of pregabalin in the modification of electrode the anodic peak current (Ipa) and cathodic peak current (Ipc) goes on increasing from 2 mg to 5 mg with negligible change in redox peak potentials as shown in Figure 1 further increase in the quantity of pregabalin both anodic and cathodic peak current was decreased, better and acceptable voltammogram was obtained with 5 mg Pregabalin modified carbon paste electrode, Hence 5 mg Pregabalin was chosen to fabricate modified carbon paste electrode and modified pregabalin electrode was used for further investigation of dopamine.
Cyclic voltammetric studies of K4Fe(CN)6 at Pregabalin MCPE
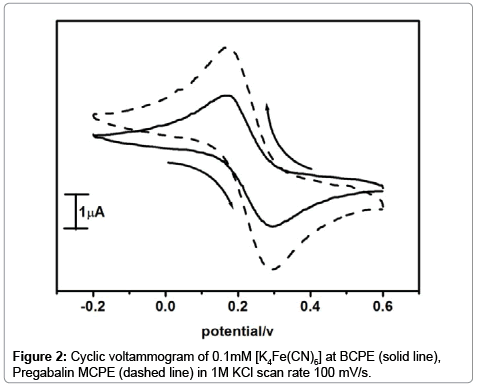
Potassium ferro cyanide is always used as an electrochemical query to evaluate the electrochemical properties of a modified carbon paste electrode. The Cyclic voltammogram of potassium ferrocyanide at Pregabalin modified carbon paste electrode in 0.1mM [K4Fe(CN)6] and 1M KCl as a supporting electrolyte with scan rate 100 mV/s as shown in Figure 2. The cyclic voltammogram of potassium ferrocyanide at the bare carbon paste electrode shows low current signal, The voltammetric response was improved at the modified Pregabalin carbon paste electrode which is revealed by the enrichment of both anodic and cathodic peak current (dashed line) as compared to the bare carbon paste electrode (solid line). The electrochemical surface area of carbon paste electrode and pregabalin modified carbon paste electrode were calculated using Randles-Sevick’s equation was found to be 0.0240 cm2 and 0.0280 cm2 respectively.
Electrochemical responses of Dopamine at pregabalin modified carbon paste electrode
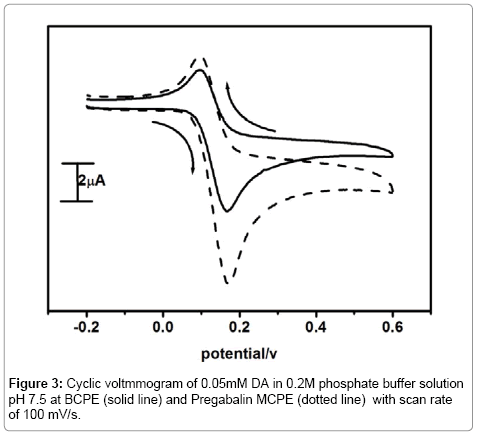
Figure 3 shows the cyclic volatammograms obtained for the electrochemical response of dopamine at Pregabalin modified carbon paste electrode (dashed line) and bare carbon paste electrode (solid line) in 0.2M PBS at pH 7.5 containing 0.05mM DA scan rate 100 mV/s. The cyclic voltammogram obtained at the bare carbon paste electrode exhibited poor current signal. However, the voltammertric response of dopamine at pregabalin modified carbon paste electrode showed a good increment in redox peak current as compared to the bare carbon paste electrode. The ΔEp was found to be 52 mV. The significantly enhanced redox peak current shows the enhanced electrochemical property of pregabalin modified carbon paste electrode towards the dopamine detection.
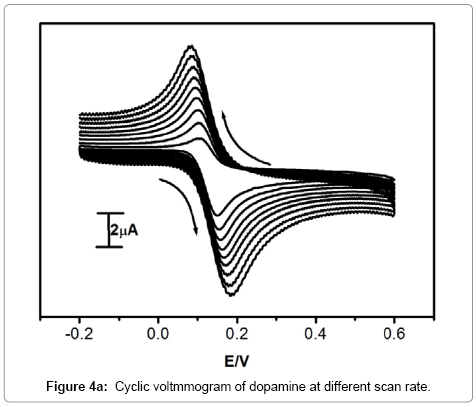
Effect of scan rate at Pregabalin modified carbon paste electrode
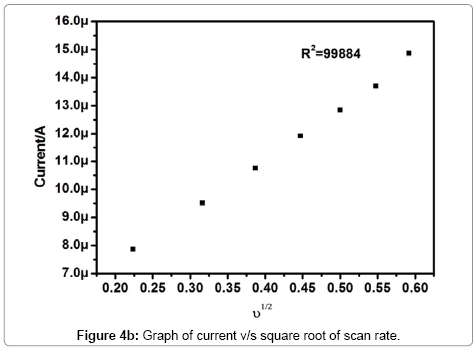
According to Randles-Sevick’s equation, peak current was directly proportional to scan rate. The different scan rate study exhibits a reflective effect on the oxidation peak current of dopamine. The scan rate observation was used to understand the electrode process. The Pregabalin modified carbon paste electrode showed an increase in redox peak current with an increase in scan rate from 50 to 350 mV/s, It indicates that direct electron transfer between dopamine and modified carbon paste electrode. The cyclic voltammograms for dopamine at different scan rates on Pregabalin modified carbon paste electrode at pH 7.5 was shown in Figure 4a. The graph of anodic peak current (Ipa) versus square root of scan rate was plotted as shown in Figure 4b. The graph showed a linear relationship of anodic peak current and scan rate with correlation coefficient 0.9944, this indicates that the electrode process was diffusion controlled [23,27-29].
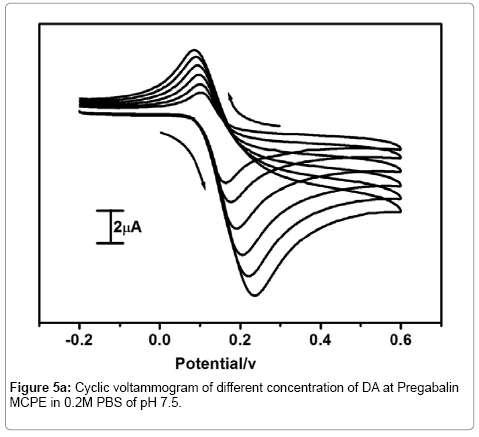
Effect of Dopamine concentration at pregabalin modified carbon paste electrode
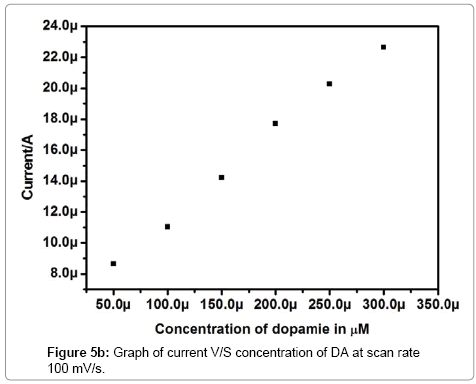
Figure 5a shows that the electrochemical investigation of different concentration of dopamine at Pregabalin modified carbon paste electrode. The redox peak current of dopamine was increased linearly with increasing the concentration of dopamine from 50 to 300 μM. The Figure 5b shows the graph of anodic peak current (Ipa) versus concentration of dopamine. The result shows gradually increase in anodic peak current and cathodic peak current with slightly shifting of anodic peak potential (Epa) towards positive direction and cathodic peak potential (Epc) towards the negative direction with an increase in dopamine concentration.
Influence of pH on Pregabalin modified carbon paste electrode
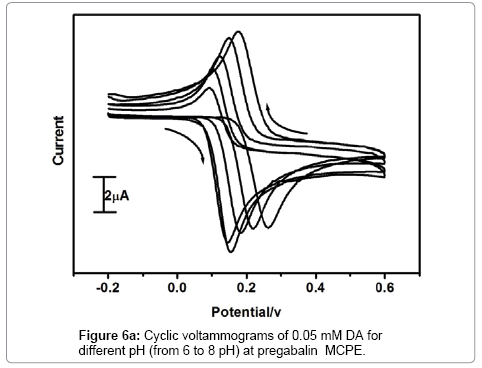
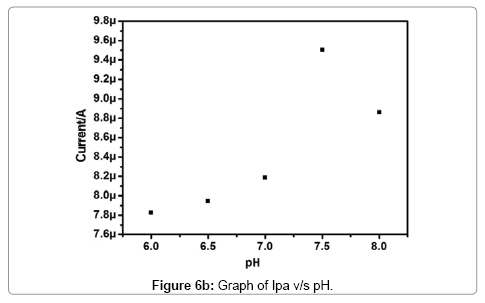
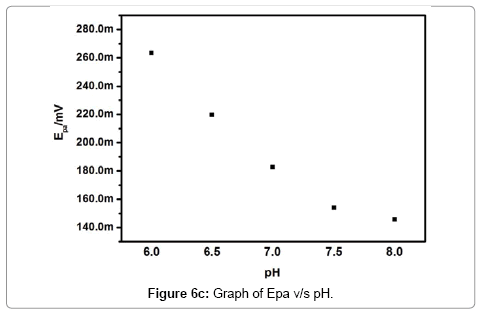
The effect of pH on the electrochemical response of Pregabalin modified carbon paste electrode towards dopamine was investigated. The Figure 6a demonstrates the pH dependence of dopamine electrochemical response at Pregabalin modified carbon paste electrode with scan rate 100 mV/s. The anodic peak current increase with increasing pH as shown in Figure 6b. The anodic peak current increases with an increment of pH value up to 7.5, then it showed a decrease in current signal. The pH 7.5 leads to the highest peak current. Therefore, pH 7.5 was selected for all successive electrochemical analysis of dopamine and uric acid. The anodic peak potential of dopamine shifted from 263 to 145 mV with an increase in the pH 6-8. The potential diagram Figure 6c was constructed by plotting the graph of anodic peak potential (Epa) versus pH of the solution. The graph shows good linearity with a slope of 60 mV/pH this behavior is nearly obeyed the Nernst equation for equal number of electron and proton transfer reaction [30].
Simultaneous determination of Dopamine in presence of uric acid
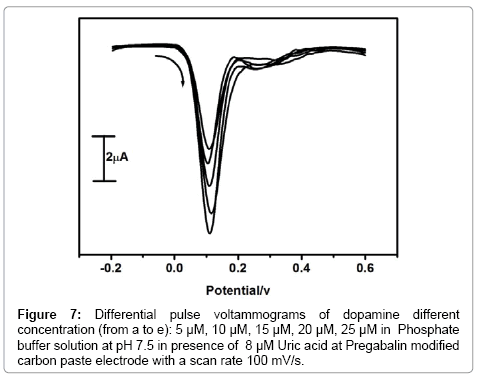
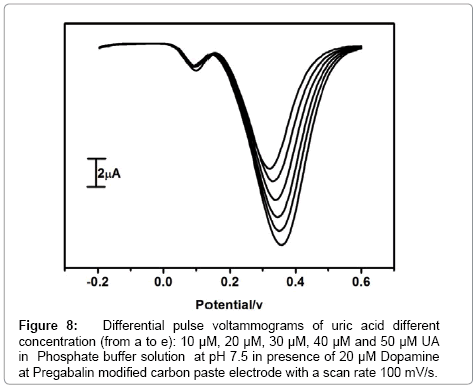
Selectivity is a very important aspect of modified carbon paste electrode so commonly co-existing species like dopamine and uric acid was chosen to evaluate the selectivity of modified carbon paste electrode. Differential Pulse Voltammetry offers a much interest due to their high current sensitivity and better resolution so for simultaneous determination of dopamine and uric acid was carried out by using differential Pulse Voltammetric technique. The resolution of dopamine and uric acid in the mixture of solution was investigated at Pregabalin modified carbon paste electrode by varying the concentration of one species and keeping the concentration of other species constant. The pregabalin modified carbon paste electrode has achieved the separation of two analytes in the mixture. The Figure 7 shows that the peak current of dopamine is proportional to the concentration of dopamine. The peak current of dopamine was increased linearly with increasing the concentration of dopamine from 5 μM to 25 μM, while keeping the concentration of uric acid fixed (8 μM). The change in concentration of dopamine did not show any substantial influence on the peak current of uric acid. The Figure 8 shows the oxidation peak current of uric acid increased linearly with an increase in the concentration of uric acid form 10 μM to 50 μM while keeping the other analyte concentration constant. The change in concentration of uric acid has no significant influence on the peak current of dopamine. The above results confirm that the electro oxidation peaks of dopamine and uric acid at Pregabalin modified carbon paste electrode peaks are well separated from each other. Therefore, it is possible to determine dopamine and uric acid in the mixture samples using Pregabalin modified carbon paste electrode by differential pulse voltammetry technique. The prepared pregabalin modified carbon paste electrode has detection limit of 2.0 × 10-6 M and 3.7 × 10-6 M for dopamine and uric acid respectively. The detection limit was calculated by using the formula (1) [31] where S is the standard deviation and M is the slope obtained from the calibration plot (Table 1).
| Analyte | Electrode | Method | Detection limit (μM) | References |
|---|---|---|---|---|
| DA | Ag/Ag2S-CNT-Nafion | DPV | 4.7 | [32] |
| DA | SWCNT/GCE | DPV | 7.0 | [33] |
| DA | Fc-MCPE | CV | 9.4 | [34] |
| DA | P/MCPE | DPV | 2.0 | Present Work |
| UA | α-CD/CNT/CPE | DPV | 5.0 | [35] |
| UA | CNT/PEDOT | DPV | 10.0 | [36] |
| UA | P/MCPE | DPV | 3.7 | Present Work |
Table 1: The Detection limit for DA and UA previous work and present work.
LOD=3S/M (1)
Conclusion
The present work demonstrates that the modified pregabalin carbon paste electrode can be used for the electrochemical determination of dopamine in the presence of uric acid. The pregabalin is a drug used as the modifier to check the sensor properties of dopamine. The modified Pregabalin carbon paste electrode shows an excellent electrocatalytic activity for the oxidation of dopamine and uric acid. The anodic peak current was enhanced dramatically as compared to bare carbon paste electrode. The proposed methods were successfully applied for the simultaneous determination of electrocatalytic oxidation of dopamine and uric acid using cyclic voltammetry and differential pulse voltammetric methods, both individually and simultaneously with low detection limit, good sensitivity and selectivity.
References
- Wang J, Martinez T, Yaniv DR, McCornick L (1990) Characterization of the microdistribution of conductive and insulating regions of carbon paste electrodes with scanning tunneling microscopy. J Electroanal Chem 286: 265-272.
- Rice ME, Galus Z, Adams RN (1983) Graphite paste electrodes: Effects of paste composition and surface states on electron-transfer rates. J Electroanal Chem 143: 89-102.
- Tassone DM, Boyce E, Guyer J, Nuzum D (2007) Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther 29: 26-48.
- Yeong-Tarng S, Chi-Ming W, Rong-Hsien L, Tzong-Liu W, Chien-Hsin Y, et al. (2012) Effects of pH on Electrocatalytic Activity of Carbon Nanotubes in Poly(acrylic acid) Composites Used for Electrochemical Selective Detection of Uric Acid and Dopamine in the Presence of Ascorbic Acid. Int J Electrochem Sci 7: 8761-8778.
- Lin L, Chen J, Yao H, Chen Y, Zheng Y, et al. (2008) Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (Evans Blue) modified glassy carbon electrode. Bioelectrochemistry 73: 11-17.
- Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60: 769A-779A.
- Kim YR, Bong S, Kang YJ, Yang Y, Mahajan RK, et al. (2010) Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens Bioelectron 25: 2366-2369.
- Curulli A (2009) Electrochemical direct determination of catecholamines for the early detection of neurodegenerative diseases. Sensors (Basel) 9: 2437-2445.
- Grabowska I, Chudy M, Dybko A, Brzoz Z (2010) Simultaneous determination of ascorbic acid, adrenaline and uric acid at a hematoxylin multi-wall carbon nanotube modified glassy carbon electrode. Sensors Actuators B: 666-672.
- Heinig M, Johnson RJ (2006) Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 73: 1059-1064.
- Manjunatha JE, Swamy BEK, Mamatha GP, Gilbert O, Sherigara BS (2011) Poly(maleic acid) Modified Carbon Paste Electrode for Simultaneous Detection of Dopamine in the Presence of Uric Acid : A Cyclic Voltammetric Study. Anal Bioanal Electrochem 3: 146-159.
- Wightman RM, May LJ , Michael AC (1988) Detection of Dopamine Dynamics in the Brain. Analytical Chemistry 60: 769A-793A.
- Cannazza G, Di Stefano A, Mosciatti B, Braghiroli D, Baraldi M, et al. (2005) Detection of levodopa, dopamine and its metabolites in rat striatum dialysates following peripheral administration of L-DOPA prodrugs by mean of HPLC-EC. J Pharm Biomed Anal 36: 1079-1084.
- Nagaraja P, Murthy KCS, Rangappa KS, Gowda NMM (1998) Spectrophotometric methods for the determination of certain catecholamine derivatives in pharmaceutical preparations. Talanta 46: 39-44.
- Teixeira MFS, Marcolino-Junior LM, Fatibello-Filho O, Dockal ER, Bergamini MF (2007) An electrochemical sensor for l-dopa based on oxovanadium-salen thin film electrode applied flow injection system. Sens Actuators B 122: 549-555.
- Hansson C, Agrup G, Rorsman H, Rosengren AM, Rosengren E, et al. (1979) Analysis of cysteinyldopas, dopa, dopamine, noradrenaline and adrenaline in serum and urine using high-performance liquid chromatography and electrochemical detection. J Chromatogr 162: 7-22.
- Manjunatha JG, Swamy BEK , Mamatha GP, Chandra U, Niranjana E, et al. (2009) Cyclic Voltammetric Studies of Dopamine at Lamotrigine and TX-100 Modified Carbon Paste Electrode. Int J Electrochem Sci 4: 187-196.
- O'Neill RD (1994) Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo. A review. Analyst 119: 767-779.
- Guadalupe AR, Abruna HD (1985) Electroanalysis with chemically modified electrodes. Anal Chem 57: 142-149.
- Shibahara H, Ando A, Suzuki S, Uematsu N, Nishimura D (2011) Oxycodone and pregabalin using transdermal fentanyl patch provided relief of symptoms for postherpetic neuropathic pain in a patient with non-small cell lung cancer. Gan To Kagaku Ryoho 38: 1675-1677.
- Shibahara H, Okubo K, Takeshita N, Nishimura D (2012) Medical treatment including pregabalin and radiation therapy provided remarkable relief for neuropathic pain by brachial plexus invasion in a patient with esophageal cancer. Gan To Kagaku Ryoho 39: 277-280.
- Sherigara BS, Kutner W, D’Souza F (2003) Electrocatalytic Properties and Sensor Applications of Fullerenes and Carbon Nanotubes. Electroanalysis 15: 753-772.
- Gilbert O, Swamy BEK, Chandra U, Sherigara BS (2009) Electrocatalytic Oxidation of Dopamine and Ascorbic Acid at Poly (Eriochrome Black-T) Modified Carbon Paste Electrode. Int J Electrochem Sci 4: 582-591.
- Gilbert O, Chandra U, Swamy BEK, Pandurangachar M, Nagaraj C, et al. (2008) Poly (Alanine) Modified Carbon Paste Electrode for Simultaneous Detection of Dopamine and Ascorbic Acid. Int J Electrochem Sci 3: 1186-1195.
- Manjunatha JG, Swamy BEK, Deepa R, Krishna V, Mamatha GP, et al. (2009) Electrochemical studies of Dopamine at Eperisone and Cetyl Trimethyl Ammonium Bromide Surfactant modified Carbon paste electrode: A Cyclic Voltammetric Study. Int J Electrochem Sci 4: 662- 671.
- Aslanoglu M, Abbasoglu S, Karabulut S, Kutluay A (2007) Electrochemical Determination of Dopamine in the Presence of Ascorbic Acid Using a Poly(3-Acetylthiophene) Modified Glassy Carbon Electrode. Acta Chim Slov 54: 834-839.
- Chandra U, Swamy BEK, Gilbert O, Pandurangachar M, Reddy S, et al. (2010) Poly(amaranth) film based sensor for resolution of dopamine in the presence of uric acid: A voltammetric study. Chin Chem Lett 21: 1490-1492.
- Shankar SS, Swamy BEK, Chandra U, Manjunatha JG, Sherigara BS (2009) Simultaneous Determination of Dopamine, Uric Acid and Ascorbic Acid with CTAB Modified Carbon Paste Electrode. Int J Electrochem Sci 4: 592-601.
- Chandra U, Swamy BEK, Gilbert O, Sherigara BS (2010) Voltammetric resolution of dopamine in the presence of ascorbic acid and uric acid at poly (calmagite) film coated carbon paste electrode. Electrochim Acta 55: 7166-7174.
- Mahanthesha KR, Swamy BEK, Chandra U, Bodke YD, Pai KV, et al. (2009) Cyclic Voltammetric Investigations of Alizarin at Carbon Paste Electrode using Surfactants. Int J Electrochem Sci 4: 1237-1247.
- Chandra U, Swamy BEK, Gilbert O, Pandurangachar M , Sherigara BS (2009) Voltammetric Resolution of Dopamine in presence of Ascorbic Acid at Polyvinyl Alcohol Modified Carbon Paste Electrode. Int J Electrochem Sci 4: 1479-1488.
- Chih YK, Yang MC (2014) Simultaneous detection of dopamine and ascorbic acid using silver/silver sulfide modified carbon nanotube electrodes. J Taiwan Inst Chem Eng 45: 833-839.
- Goyal RN, Singh SP (2008) Simultaneous voltammetric determination of dopamine and adenosine using a single walled carbon nanotube-Modified glassy carbon electrode. Carbon 46: 1556-1562.
- Ali Kamyabi M, Aghajanloo F (2009) Electrocatalytic Response of Dopamine at a Carbon Paste Electrode Modified with Ferrocene. Croat Chem Acta 82: 599-606.
- Ghoreishi SM, Behpour M, Fard MHM (2012) Electrochemical methods for simultaneous determination of trace amounts of dopamine and uric acid using a carbon paste electrode incorporated with multi-wall carbon nanotubes and modified with cyclodextrine. J solid state electrochem 16: 179-189.
- Lin KC, Tsai TH, Chen SM (2010) Performing enzyme-free H2O2 biosensor and simultaneous determination for AA, DA and UA by MWCNT-PEDOT film. Biosens Bioelectron 26: 608-614.
Citation: Tanuja SB, Swamy BEK, Pai KV (2016) Electrochemical Response of Dopamine in Presence of Uric Acid at Pregabalin Modified Carbon Paste Electrode: A Cyclic Voltammetric Study. J Anal Bioanal Tech 7:297. DOI: 10.4172/2155-9872.1000297
Copyright: © 2016 Tanuja SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 12613
- [From(publication date): 2-2016 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 11600
- PDF downloads: 1013