Research Article Open Access
Electrochemical Investigation of Paracetamol at Poly(Glycine) Modified Carbon Paste Electrode: A Voltametric Study
Chethan M kuskur1, Kumara Swamy BE1* and Jayadevappa H2
1Department of P.G. Studies and Research in Industrial Chemistry, Kuvempu University, Jnana Sahyadri, Shankaraghatta, Shimoga, Karnataka, India
2Department of Chemistry, Sahyadri Science College, Shimoga, Karnataka, India
- *Corresponding Author:
- Kumara Swamy BE
Department of P.G. Studies and Research in Industrial Chemistry
Kuvempu University, Jnana Sahyadri
Shankaraghatta, Shimoga
Karnataka 577451, India
Tel: +91-8282-256225
Fax: +91-8282-256255
E-mail: kumaraswamy21@yahoo.com
Received date: July 02, 2015; Accepted date: July 22, 2015; Published date: July 29, 2015
Citation: Kuskur CM, Kumara Swamy BE, Jayadevappa H (2015) Electrochemical Investigation of Paracetamol at Poly(Glycine) Modified Carbon Paste Electrode: A Voltametric Study. J Anal Bioanal Tech 6:260. doi: 10.4172/2155-9872.1000260
Copyright: © 2015 kuskur CM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The carbon paste electrode was modified by electropolymerisation of 1 mM Glycine in 0.2 M Acetate buffer solution (ABS) at pH-5. The voltammetric response of Paracetamol (PA) at Poly(Glycine) Modified carbon paste electrode (MCPE) shows excellent electrocatalytic activity when compared to bare carbon paste electrode (BCPE) at sweep rate of 100 mV/s-1. From the study of scan rate variation the electrode process was found to be diffusion controlled. The concentration effect of paracetamol was studied. The simulations determination of PA, DA and AA in their sample mixture was analyzed by using both cyclic voltammetric and differential pulse voltammetric techniques.
Keywords
Dopamine; Paracetamol; Ascorbic acid; Glycine; Cyclic voltammetry; Electropolymerisation; Carbon paste electrode
Introduction
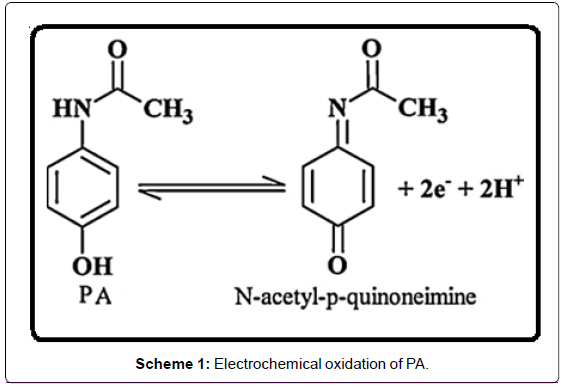
Paracetamol, (PA) N-acetyl-p-aminophenol or acetaminophen, is one of the most commonly used drugs in the world. It is the preferred alternative analgesic and antipyretic to aspirin, particularly for patients who cannot tolerate aspirin [1,2]. Paracetamol rapidly gets absorbed and distributed after oral administration and is easily excreted in urine [3]. Generally it is highly effective for the release of pain associated with artralgia, neuralgia and headache and even to patients from gastric symptoms. In most of countries it is used as a substitute for aspirin [4]. Paracetamol is easily and completely metabolized, normally it does not show any Harmful side-effects. However, the over dosage causes the accumulation of toxic metabolites leading to kidney damage, liver disorders, skin rashes ,inflammation of the pancreas and may lead to death [1,5]. Therefore, it is very important to establish a simple, fast, sensitive and accurate detection method for paracetamol. Many methods have been described for the determination of paracetamol, such as liquid chromatography [6], liquid chromatography-tandem mass spectrometry [7], spectrophotometry [8], spectrofluorometry [9], FT-IR Raman spectrometry [10], micellar electrokinetic chromatography [11], automatic sequential injection analysis [12] and Chemiluminescence [13]. However, these methods suffer from some disadvantages, such as high costs, tedious extraction process, long analysis times and requirement for the complicated sample pretreatment, and most of these above procedures require large instruments and skilled operators, which make them unsuitable for routine analysis. Chemically modified electrodes (CMEs) have the advantages of fast response, time saving, inexpensive instrument, simple operation, high sensitivity, good selectivity and in situ determination, and they are widely used for the trace determination of various analyte. Since paracetamol is an electroactive compound which can be electrochemically oxidized (Scheme 1), CMEs can be applied for paracetamol determination, for instance, polyaniline microfilms coated platinum electrode [14] and carbon paste electrode modified with nanogold and glutamic acid [15,16] are the examples of electrode surface modification.
Dopamine (DA) is one of the most important catecholamine neurotransmitters in mammalian central nervous system [17-21]. The loss of DA in the human body may result in some serious diseases such as Parkinson diseases [22]. Similarly ascorbic acid (AA) has been used for the prevention and treatment of common cold, mental illness, infertility and cancer [23]. Both DA and AA are compounds that can be determined by electrochemical methods based on anodic oxidation. However, a major problem is that the oxidation potentials for AA and DA occur almost in the same potential at unmodified electrodes, which result in overlapped voltammetric responses, making their discrimination highly difficult [24]. Most of the studies on these compounds demonstrated the possibility for the separate determination of either AA [25-27] or DA [28-30] by eliminating the other using different membranes or selecting particular potentials. It was reported that stearic acid [31], Nafion [32], polypyrrole [33], and micellar [34] could selectively detect DA in the presence of AA based on the cationic permeability of the polymer. However, it is most important to develop a sensor, which can determine both AA and DA simultaneously Some efforts have been taken to fabricate modified electrodes for the simultaneous determination of DA and AA, such as poly (neutral red) modified electrode [35], poly(phenosafranine) electrode [36], poly(N,N-dimethylaniline)-modified electrode [37,38] are the examples of electrode surface modification.
Polymer modified electrodes (PMEs) have received great attention in recent years, as the polymer film have good stability, reproducibility, more active sites, homogeneity in electrochemical deposition and strong adherence to the electrode surface [39,40]. The aim of the present study is to construct a stable as well as sensitive electrochemical sensor based on Poly(Glycine) MCPE and to extend the same for the simultaneous studies of PA and to observe the interference behavior on dopamine (DA) and ascorbic acid (AA) by using cyclic voltammetric technique and the results were compared with other available reports.
Experimental Part
Materials and methods
Cyclic voltammetric experiments were performed on a model CH660c (CH instrument). All the electrochemical experiments were carried out in a three electrode cell system, which contained a bare carbon paste electrode (BCPE)/Poly(Glycine) film coated MCPE as the working electrode, a platinum wire and saturated calomel electrode as counter and reference electrode.
Reagents and chemicals
Paracetamol, dopamine hydrochloride (DA), Ascorbic acid (AA), was obtained from Sigma-Aldrich. Graphite powder of 50 mm size was purchased from Loba and silicon oil was purchased from Himedia. All chemicals are of analytical grade quality and were used as supplied without further purification. 25 mM Paracetamol and 25 × 10-4 M AA was prepared in double-distilled water, 25 mM DA was prepared in 0.1 M Perchloric acid (HClO4). Phosphate buffer (0.2 M) was prepared by mixing appropriate quantity of aqueous sodium dihydrogen phosphate monohydrate and aqueous disodium hydrogen phosphate.
Preparation of bare carbon paste electrode
Bare carbon paste electrodes (BCPE) were made with silicon oil (30%), and graphite powder (70%). The two components were thoroughly mixed in an agate mortar for about 30 minutes. The BCPE was packed into a homemade Teflon cavity having a current collector and was polished on a weighing paper.
Preparation of poly(Glycine) modified carbon paste electrode
The poly(glycine) modified carbon paste electrode was prepared by electrochemical polymerization of glycine at the surface of carbon paste electrode were carried out by using cyclic voltammetric method in 0.2 M acetate buffer solution at pH-5 containing 1 mM glycine solution. After polymerization the electrode was thoroughly washed with distilled water to remove unreacted glycine until used.
Results and Discussion
Electropolymerisation of glycine at carbon paste electrode
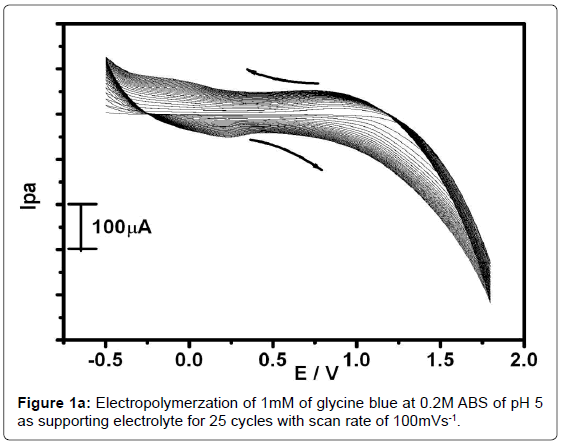
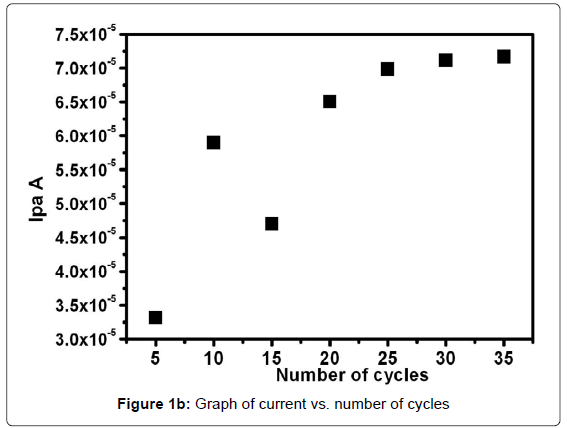
Figure 1a shows the growth of polymer film of 1 mM aqueous glycine solution on the surface of carbon paste electrode in 0.2 M ABS of pH 5 as supporting electrolyte. Electropolymerzation was achieved by the formation of film that grew between -0.5 V to 1.8 V at the sweep rate of 100 mVs1 for 25 cycles using cyclic voltametric technique [41]. During the process of multiple cycles the voltammogram has gradually descended with increase of cyclic time increasing. This indicates that the poly(glycine) film was formed and deposited on the surface of carbon paste electrode [42]. Figure 1b shows the 5 to 20 cycle electropolymerised electrode shows variation in current towards the 0.1 mM paracetamol and there after 25 to 35 cycles the peak current response becomes almost constant. This may be due to a complete surface coverage of the electrode with poly(Glycine) with increased scanning cycle. However, after 25 cycles, poly(glycine) covers the electrode surface completely and the active area doesn’t change significantly. Therefore the current will be higher than that of 5 to 20 cycle electropolymerised electrode. Therefore 25 cycle electropolymerised electrode was used as standard one for further studies of electrochemical processes.
Electrochemical response of paracetamol at poly(glycine) MCPE
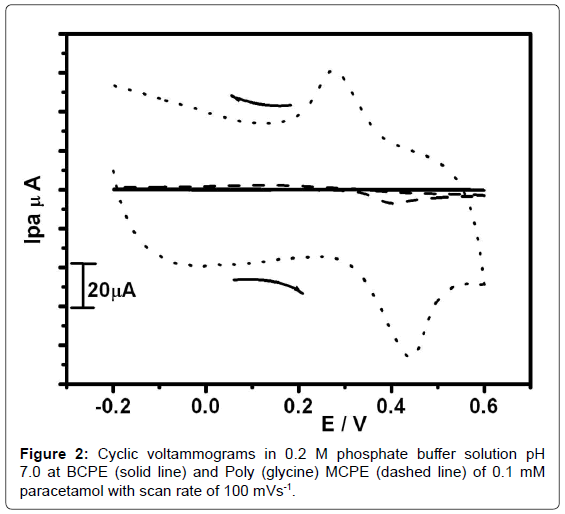
The Figure 2 shows the cyclic voltammograms obtained for the electrochemical response of PA at Poly(Glycine) MCPE (dotted line) and BCPE (dashed line) and the absence of analyte (solid line) in 0.2 M PBS pH 7 containing 0.1 mM PA at sweep rate 100 mV/s-1. From Figure 3 the BCPE (dashed line) PA shows an irreversible behavior with an anodic peak potential of 404 mV. In poly(Glycine) MCPE shows significantly enhanced peak current and more reversible electron transfer process to PA with slight shift in redox peak potentials. A welldefined redox wave of PA was observed with anodic and cathodic peak potential at 414 mV and 313 mV respectively. It reveals that in BCPE only oxidation occurs and in MCPE both oxidation and reduction take place. In solid line shows no any other peaks due to presence of only PBS.This shows the modified CPE has fast electron kinetics and also due to large specific area and good conductivity and a large capacitive current [43]. This indicates that our modified shows electrocatalytic activity towards Paracetamol (Table 1).
| Sl.No | Electrode | Detection limit | Techniques | Reference |
|---|---|---|---|---|
| 1 | MCPE/PR | 0.53 (μM) | DPV | [47] |
| 2 | PolyDAN-RB4/GCE | 0.083 ± 0.003 (μM) | DPV | [48] |
| 3 | Bi2O3/GCE | 0.2 (μM) | DPV | [49] |
| 4 | f-MWCNTs/GCE | 0.039 (μM) | DPV | [50] |
| 5 | PEDOT/GCE | 1.13 (μM) | DPV | [51] |
| 6 | CF/GCE | 0.036 (μM) | CV | [52] |
| 7 | Poly(Glycine)/MCPE | 0.14 (nM) | CV | Present work |
Table 1: Comparison of poly (glycine) MCPE with other working electrodes.
Effect of scan rate
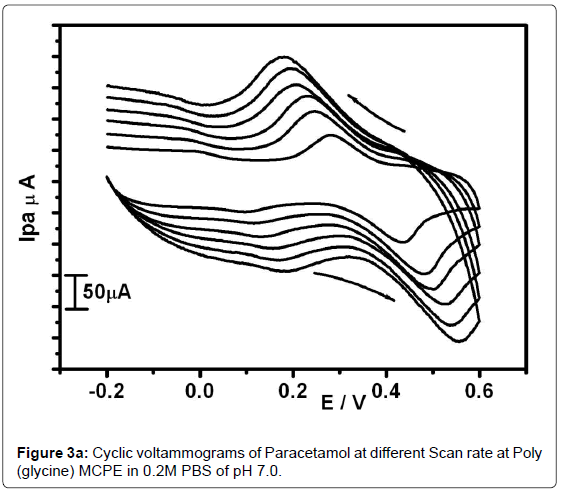
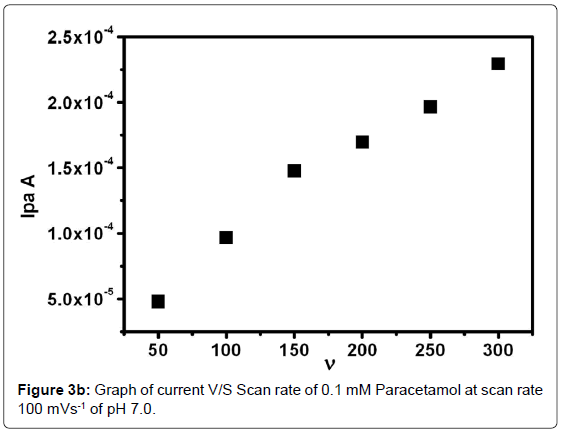
The effect of scan rate was studied by using 0.1 mM PA solution in PBS at pH -7 in an electrochemical cell. Cyclic voltammetric behavior of the PA was studied by varying the sweep rate from 50 to 300 mVs-1. As in the Figure 3a the cyclic voltammograms for poly(glycine) MCPE shows that by increasing the scan rate the Ipa and Ipc also increase and the anodic peak potential shifted towards the positive side. The graph of Ipa versus scan rate (υ) was plotted Figure 3b. The resulted graph showed excellent linearity with correlation co-efficient was found to be 0.98787.This revealed that the electrode process is diffusion controlled.
Effect of concentration
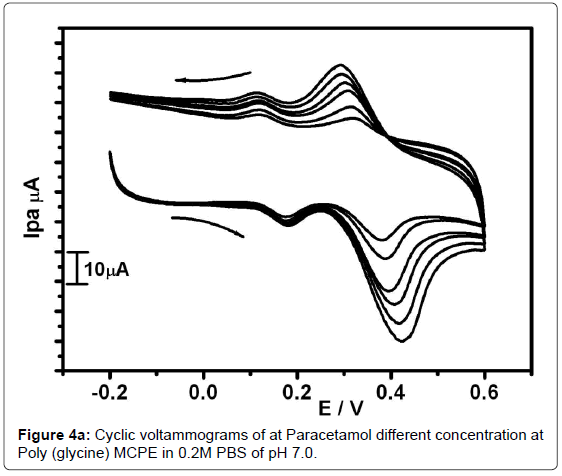
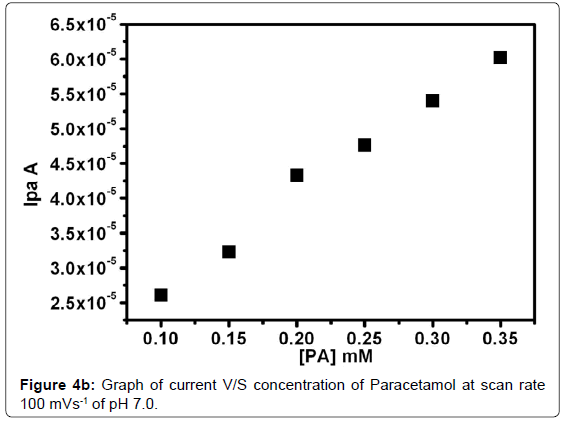
The Figure 4a shows the cyclic voltammograms at Poly(glycine) MCPE for 0.1 mM DA and PA with different concentration (0.1-0.35 mM) in 0.2 M PBS of pH -7 at sweep rate100 mVs-1. The anodic current of PA was increase with increase in concentration while the anodic current of DA remains constant due to its constant concentration. Therefore Poly(Glycine) MCPE shows good selectivity and sensitivity in the electrochemical studies of PA in the presence of DA. The plot Ipa verses concentration of PA gives correlation co-efficient value of 0.9933 as in Figure 4b. This revealed that good linearity. The detection limit of PA at poly(Glycine) MCPE was 0.14 nM. The detection limit was calculated by using the formula (1) [44] where S is the standard deviation and M is the slope obtained from the calibration plot.
LOD = 3S/M (1)
Simultaneous determination PA, DA and AA
The main aim is the simultaneous determination of PA, DA and AA in a 0.2 M phosphate buffer solution of pH 7. The Figure 5 shows the cyclic voltammograms obtained for the electrochemical response of a solution containing 0.1 mM PA, 0.1 mM DA and 2 mM AA in 0.2 M phosphate buffer solution (pH 7) at the bare CPE (solid line) and the Poly(glycine) MCPE (dashed line).The solid line shows one broad peak for both analytes, indicating that the oxidation potentials of PA, DA and AA are amalgamated and indistinguishable. While three distinct anodic peaks and two cathodic peaks were obtained Poly(glycine) MCPE (dashed line). The observations clearly indicate that bare failed to separate each voltammetric signal of PA, DA and AA. The anodic peak potentials were located at 0.417 V, 0.204 V and 0.030 V respectively, with a potential difference PA-DA was 0.213 V and DA-AA was 0.174 V. Additionally, the difference between the oxidation potentials facilitated the sensitive and accurate detection of the three compounds simultaneously even for AA at a higher concentration [45].
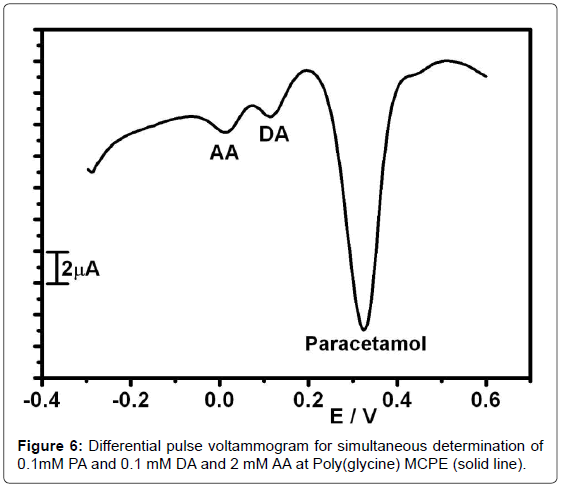
Differential pulse voltammetric technique was used for the determination of 0.1 mM PA, 0.1 mM DA and 2 mM AA at Poly(glycine) MCPE because to get higher current sensitivity and better resolution. The simultaneous study was carried out in the potential range 200 to 600 mV in Figure 6. The anodic peak potentials for PA, DA and AA were at 0.323 V, 0.116 V, and 0.013 V respectively [46-50].
Conclusion
In the present work, the electropolymerisation of glycine on the carbon paste electrode produced a stable polymeric film. This study has shown that the poly(glycine) MCPE exhibits highly electrocatalytic activity towards the oxidation of paracetamol. The effect of scan rate and concentration shows. The electrode process was found to be diffusion controlled. The modified electrode displays the higher selectivity and sensitivity in voltammetric measurements of, PA DA and AA in their mixture solutions and is also evidenced by DPV technique. Therefore this method can also be applied for other drug.
References
- Espinosa Bosch M, Ruiz Sánchez AJ, Sánchez Rojas F, Bosch Ojeda C (2006) Determination of paracetamol: historical evolution. J Pharm Biomed Anal 42: 291-321.
- de Carvalho RM, Freire RS, Rath S, Kubota LT (2004) Effects of EDTA on signal stability during electrochemical detection of acetaminophen. J Pharm Biomed Anal 34: 871-878.
- Parojcic J, Karljikovic-Rajic K, Duric Z, Jovanovic M, Ibric S (2003) Development of the second-order derivative UV spectrophotometric method for direct determination of paracetamol in urine intended for biopharmaceutical characterisation of drug products. Biopharm Drug Dispos 24: 309-314.
- Zen JM, Ting YS (1997) Simultaneous determination of caffeine and acetaminophen in drug formulations by square-wave voltammetry using a chemically modified electrode. Anal Chim Acta 342: 175-180.
- Prabakar RJS, Narayanan SS (2007) Amperometric determination of paracetomol by a surface modified cobalt hexacyanoferrate graphite wax composite electrode. Talanta 72: 1818-1827.
- Monser L, Darghouth F (2002) Simultaneous LC determination of paracetamol and related compounds in pharmaceutical formulations using a carbon-based column. J Pharm Biomed Anal 27: 851-860.
- Li H, Zhang C, Wang J, Jiang Y, Fawcett JP, et al. (2010) Simultaneous quantitation of paracetamol, caffeine, pseudoephedrine, chlorpheniramine and cloperastine in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 51: 716-722.
- Sirajuddin, Khaskheli AR, Shah A, Bhanger MI, Niaz A, et al. (2007) Simpler spectrophotometric assay of paracetamol in tablets and urine samples. Spectrochim Acta A Mol Biomol Spectrosc 68: 747-751.
- Vilchez JL, Blanc R, Avidad R, Navalón A (1995) Spectrofluorimetric determination of paracetamol in pharmaceuticals and biological fluids. J Pharm Biomed Anal 13: 1119-1125.
- Ramos ML, Tyson JF, Curran DJ (1998) Determination of acetaminophen by flow injection with on-line chemical derivatization: Investigations using visible and FTIR spectrophotometry. Anal Chim Acta 364: 107-116.
- Németh T, Jankovics P, Németh-Palotás J, Koszegi-Szalai H (2008) Determination of paracetamol and its main impurity 4-aminophenol in analgesic preparations by micellar electrokinetic chromatography. J Pharm Biomed Anal 47: 746-749.
- Gutes A, Calvo D, Cespedes F, del Valle M (2007) Automatic sequential injection analysis electronic tongue with integrated reference electrode for the determination of ascorbic acid, uric acid and Paracetamol. Microchim Acta 157: 1-6.
- Easwaramoorthy D, Yu Y-C, Huang H-J (2001) Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal Chim Acta 439: 95-100.
- Kozlovskaja S, Baltrunas G, Malinauskas A (2009) Response of hydrogen peroxide, ascorbic acid, and paracetamol at a platinum electrode coated with microfilms of polyaniline. Microchim Acta 166: 229-234.
- Zhang Y, Luo L, Ding Y, Liu X, Qian Z (2010) A highly sensitive method for determination of paracetamol by adsorptive stripping voltammetry using a carbon paste electrode modified with nanogold and glutamic acid. Microchim Acta 171: 133-138.
- Yin H, Shang K, Meng X, Shiyun Ai (2011) Voltammetric sensing of paracetamol, dopamine and 4-aminophenol at a glassy carbon electrode coated with gold nanoparticles and an organophillic layered double hydroxide. Microchim Acta 175: 39-46.
- Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brainII. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437-1448.
- Shankar SS, Swamy BEK, Pandurangachar M, Chandra U, Chandrasekhar BN, et al. (2010) Electrocatalytic Oxidation of Dopamine on Acrylamide Modified Carbon Paste Electrode : A Voltammetric Study. Int J Electrochem Sci 5: 944-954.
- Naik RR, Niranjana E, Swamy BEK, Sherigara BS, Jayadevappa H (2008) Surfactant Induced Iron (II) Phthalocyanine Modified Carbon Paste Electrode for Simultaneous Detection of Ascorbic Acid, Dopamine And Uric Acid. Int J Electrochem Sci 3: 1574-1583.
- Gilbert O, Chandra U, Swamy BEK, Pandurangachar M, Nagaraj C, et al. (2008) Int J Electrochem Sci 3: 1186-1195.
- Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60: 769A-779A.
- Hayash K, Iwaski Y, Kurita R, Sunagawa K, Niwa O (2003) On-line microfluidic sensor integrated with a micro array electrode and enzyme-modified pre-reactor for the real-time monitoring of blood catecholamine. Electrochem Commun 5: 1037-1042.
- O'Neill RD (1994) Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo. A review. Analyst 119: 767-779.
- Grossman M, Glosser G, Kalmanson J, Morris J, Stern MB, et al. (2001) Dopamine supports sentence comprehension in Parkinson's Disease. J Neurol Sci 184: 123-130.
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569: 1-9.
- Nalini B, Narayanan SS (2000) Amperometric determination of ascorbic acid based on electrocatalytic oxidation using a ruthenium (III) diphenyldithiocarbamate-modified carbon paste electrode. Anal Chim Acta 405: 93-97.
- Muñoz RA, Matos RC, Angnes L (2001) Gold electrodes from compact discs modified with platinum for amperometric determination of ascorbic acid in pharmaceutical formulations. Talanta 55: 855-860.
- Malinauskas A, Garjonyt R, Mazeikien R, Jureviciūt I (2004) Electrochemical response of ascorbic acid at conducting and electrogenerated polymer modified electrodes for electroanalytical applications: a review. Talanta 64: 121-129.
- Pandey PC, Upadhyay BC (2005) Studies on differential sensing of dopamine at the surface of chemically sensitized ormosil-modified electrodes. Talanta 67: 997-1006.
- Doménech A, García H, Doménech-Carbó MT, Galletero MS (2002) 2,4,6-triphenylpyrylium ion encapsulated into zeolite Y as a selective electrode for the electrochemical determination of dopamine in the presence of ascorbic acid. Anal Chem 74: 562-569.
- Zhang M, Gong K, Zhang H, Mao L (2005) Layer-by-layer assembled carbon nanotubes for selective determination of dopamine in the presence of ascorbic acid. Biosens Bioelectron 20: 1270-1276.
- Gelbert MB, Curran DJ (1986) Alternating current voltammetry of dopamine and ascorbic acid at carbon paste and stearic acid modified carbon paste electrodes. Anal Chem 58: 1028-1032.
- Zhou DM, Ju HX, Chen HY (1996) Catalytic oxidation of dopamine at a microdisk platinum electrode modified by electrodeposition of nickel hexacyanoferrate and Nafion®. J Electroanal Chem 408: 219-223.
- Pihel K, Walker QD, Wightman RM (1996) Overoxidized polypyrrole-coated carbon fiber microelectrodes for dopamine measurements with fast-scan cyclic voltammetry. Anal Chem 68: 2084-2089.
- Wen XL, Jia YH, Liu ZL (1999) Micellar effects on the electrochemistry of dopamine and its selective detection in the presence of ascorbic acid. Talanta 50: 1027-1033.
- Sun YX, Ye BX, Zhang WM, Zhou XY (1998) Simultaneous determination of dopamine and ascorbic acid at poly(neutral red) modified electrodes. Anal Chim Acta 363: 75-80.
- Selvaraju T, Ramaraj R (2003) Simultaneous determination of ascorbic acid, dopamine and serotonin at poly(phenosafranine) modified electrode. Electrochem Commun 5: 667-672.
- Manjunatha JG, Swamy BEK, Deraman M, Mamatha GP (2012) Simultaneous voltammetric measurement of ascorbic acid and dopamine at poly (vanillin) modified carbon paste electrode: A cyclic voltammetric study. Der Pharma Chemica 4: 2489-2497.
- Ohnuki Y, Ohsaka T, Matsuda H, Oyama N (1983) Permselectivity of films prepared by electrochemical oxidation of phenol and amino-aromatic compounds. J Electroanal Chem Interfacial Electrochem 158: 55-67.
- Volkov A, Tourillon G, Lacaze PC, Dubois JE (1980) Electrochemical polymerization of aromatic amines: IR, XPS and PMT study of thin film formation on a Pt electrode. J Electroanal Chem Interfacial Electrochem 115: 279-291.
- Gilbert O, Swamy BEK, Chandra U, Sherigara BS (2009) Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study. J Electroanal Chem 636: 80-85.
- Hong Y, Yuanyuan S, Xinhua L, Yuhai T, Liying H (2007) Electrochemical characterization of poly(eriochrome black T) modified glassy carbon electrode and its application to simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim Acta 52: 6165-6171.
- Reddy S, Swamy BEK, Vasan HN, Jayadevappa H (2012) ZnO and ZnO/polyglycine modified carbon paste electrode for electrochemical investigation of dopamine. Analytical Methods 4: 778-2783.
- Kutluay A, Aslanoglu M (2013) Modification of electrodes using conductive porous layers to confer selectivity for the voltammetric detection of paracetamol in the presence of ascorbic acid, dopamine and uric acid. Sensors and Actuators B 185: 398-404.
- Kumary VA, Divya J, Nancy TEM, Sreevalsan K (2013) Voltammetric Detection of Paracetamol at Cobalt Ferrite Nanoparticles Modified Glassy Carbon Electrode. Int J Electrochem Sci 8: 6610-6619.
- Thomas T, Mascarenhas RJ, Cotta F, Guha KS, Swamy BE, et al. (2013) Poly(Patton and Reeder's reagent) modified carbon paste electrode for the sensitive detection of acetaminophen in biological fluid and pharmaceutical formulations. Colloids Surf B Biointerfaces 101: 91-96.
- Chandra P, Son NX, Noh HB, Goyal RN, Shim YB (2013) Investigation on the downregulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. Biosens Bioelectron 39: 139-144.
- Zidan M, Tee TW, Abdullah AH, Zainal Z, Kheng GJ (2011) Electrochemical Oxidation of Paracetamol Mediated by Nanoparticles Bismuth Oxide Modified Glassy Carbon Electrode. Int J Electrochem Sci 6: 279-288.
- Umasankar Y, Unnikrishnan B, Chen SM, Ting TW (2012) Effective Determination of Acetaminophen Present in Pharmaceutical Drug Using Functionalized Multi-Walled Carbon Nanotube Film. Int J Electrochem Sci 7: 484-498.
- Mehretie S, Admassie S, Hunde T, Tessema M, Solomon T (2011) Simultaneous determination of N-acetyl-p-aminophenol and p-aminophenol with poly(3,4-ethylenedioxythiophene) modified glassy carbon electrode. Talanta 85: 1376-1382.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15792
- [From(publication date):
August-2015 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 11042
- PDF downloads : 4750