Research Article Open Access
Electrochemical Determination of Folic Acid at Sodium Alpha Olefin Sulphonate Modified Carbon Paste Electrode: A Voltammetric Study
TS Sunil Kumar Naik, BE Kumara Swamy*, CC Vishwanath and Mohan Kumar
Department of PG Studies and Research in Industrial Chemistry, Kuvempu University, JnanaSahyadri, Shankaraghatta, Shivamoga, Karnataka, India
- *Corresponding Author:
- Kumara Swamy BE
Department of PG Studies and Research in Industrial Chemistry
Kuvempu University, JnanaSahyadri
Shankaraghatta-577451
Shivamoga, Karnataka, India
Tel: +91-8282-256225
Fax: +91-8282-256255
E-mail: kumaraswamy21@yahoo.com
Received date: June 16, 2015; Accepted date: August 22, 2015; Published date: August 29, 2015
Citation: Sunil Kumar Naik TS, Kumara Swamy BE, Vishwanath CC, Kumar M (2015) Electrochemical Determination of Folic Acid at Sodium Alpha Olefin Sulphonate Modified Carbon Paste Electrode: A Voltammetric Study. J Anal Bioanal Tech 6:272. doi:10.4172/2155-9872.1000272
Copyright: © 2015 Sunil Kumar Naik TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credied.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sodium alpha olefin sulphonate (SAOS) was used for the modification of carbon paste electrode (CPE) to determine the electrochemical behavior of folic acid (FA) in 0.2M phosphate buffer solution (PBS) at pH 7.4 with the scan rate of 50 mVs-1. The effects of scan rate, concentration and simultaneous determination of FA at modified carbon paste electrode (MCPE) were studied. The effect of interference of dopamine was carried out and real sample analysis of FA was studied at MCPE. From the scan rate and concentration shows that, the overall electrode process was found to be diffusion-controlled at SAOSMCPE and detection limit was found to be 28.8 μM. The modified electrode (SAOSMCPE) exhibits good electrocatalytic activity towards the determination of folic acid when compared to BCPE. The same method can also be applied for other drug analysis.
Keywords
Folic acid; Sodium alpha olefin sulphonate; Modified carbon paste electrode; Cyclic voltammetry
Introduction
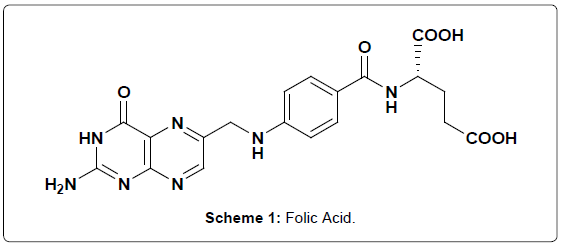
Vitamins are a group of organic compounds, essential in small amount for the normal functioning of the body and regulate the metabolic activity [1]. Deficiency of vitamins may result in often painful and potentially harmful diseases. As a result vitamins play an important role in our body. Folic acid (FA) (vitamin BC, vitamin M or vitamin B9) is a water soluble vitamin and was first discovered in Spinach [2]. During metabolism FA involved in single carbon transfer reactions and it is the precursor of the active tetrahydrofolic acid coenzyme [3]. FA is a nutrient of great importance, especially for women planning for pregnancy. To reduce significantly the incidence and reoccurrence of neural tube defects periconceptual supplementation of folic acid has been demonstrated [4]. Moreover FA is usually employed in the treatment or prevention of megaloblastic anemia during pregnancy [5]. The US Food and Drug Administration introduced mandatory reinforcement of cereal-grain products with folic acid at a concentration of 140 mg/100 g in January 1998 [6]. The Department of Health in the UK proposed fortification of flour with folic acid at 240 mg/100 g [7]. Deficiency of FA gives rise to the macrocytic anemia [8]. Hence FA determination is often required in pharmaceutical, clinical and food samples. There are several methods were reported for the determination of FA including liquid chromatography/tandem mass spectrometry (LC/MS/MS) [9], High Performance Liquid Chromatography (HPLC) [10], Capillary Electrophoresis [11], Microemulsion Electrokinetic Chromatography (MEEKC) [12] and Enzyme linked Immunosorbent assay (ELISAs) [13] and electrochemical methods [14]. Among these techniques electrochemical method is significant one because of its convenience and low cost [15]. FA (Scheme 1) is one of the electroactive species and accordingly we employed electrochemical method for the determination and some of the electrochemical methods for other electro-active species have been reported [16-20].
Dopamine (Scheme 2) is one of the most significant catecholamine, functioning as a neurotransmitter in the central nervous system and deficiency of DA leads to the Parkinson’s disease [21-22]. Changes in DA concentration in biological sample indicate the possibility of abnormalities or diseases in human being. Therefore, the determination of DA plays an important role. DA possesses high electrochemical activity and has been widely studied by electroanalytical techniques to significantly advantage towards biosciences [23-25]. Dopamine has been determined using various electrochemical methods [26]. One of the most significant methods is the determination of analytes by using modified carbon paste electrode through the voltammetic technique, which has the ability to eliminate the interfering substances from DA. The modification can be done by adding different types of modifiers [27-32].
In this study, the surfactant (sodium alpha olefin sulphonate (Scheme 3)) is used as a modifier for the electrochemical determination of FA and DA. The term surfactant is derived from surface active agent and is a compound that contains a hydrophilic (attracted to water) and a hydrophobic (repelled by water) segments. Because of their unique molecular structures, surfactant has been extensively used in the fields of electrochemistry and electroanalytical chemistry [33-36] for various purposes. To improve the detection limits of some biomolecules Hu’s group [37-39] has introduced surfactants to electroanalytical chemistry.
In present work, a simple and sensitive voltammetric method was presented for the electrochemical determination of folic acid at SAOS modified carbon paste electrode. The modified electrode showed an excellent electrocatalytic activity for the oxidation of FA in presence of dopamine.
Experimental
Reagents and chemicals
Chemicals used in present work were Folic acid (FA) from Merck chemicals. Stock solution of FA was prepared in 0.1M NaOH. Disodium hydrogen phosphate (Na2HPO4), Sodium dihydrogen orthophosphate (NaH2PO4) and Sodium alpha olefin sulphonate were purchased from Himedia chemicals. Spectrally pure graphite powder (particle size <50 mm) from Merck and high viscous paraffin oil (density=0.88 gcm-3) from Fluka were used for the preparation of the carbon paste electrode. Phosphate buffer (0.2M pH 7.4) was used as optimum measurements. All chemicals used in this experiments were analytical grade and used without any further purification.
Apparatus
Cyclic voltammetric (CV) measurements were performed with a model CHI-660c (CH Instrument-660 electrochemical workstation). All electrochemical experiments were performed in a standard three electrode cell. The bare and sodium alpha olefin sulphonate modified carbon paste electrode (SAOSMCPE) was used as working electrode, Platinum electrode as counter electrode and saturated Calomel electrode (SCE) as the reference electrode. All potentials reported were versus the SCE.
Preparation of bare and modified carbon paste electrode (SAOSMCPE)
The bare CPE was prepared by hand mixing graphite powder and silicone oil in the ratio 70:30 (w/w) for about 30 minutes in an agate mortar to produce a homogeneous mixture. The paste was then packed into the homemade cavity. Sodium alpha olefin sulphonate modified carbon paste electrode (SAOSMCPE) was prepared by immobilizing 15 μL of sodium alpha olefin sulphonate (SAOS) on to the surface of bare carbon paste electrode for 5 minutes.
Results and Discussion
Effect of sodium alpha olefin sulphonate (SAOS) on the surface of CPE
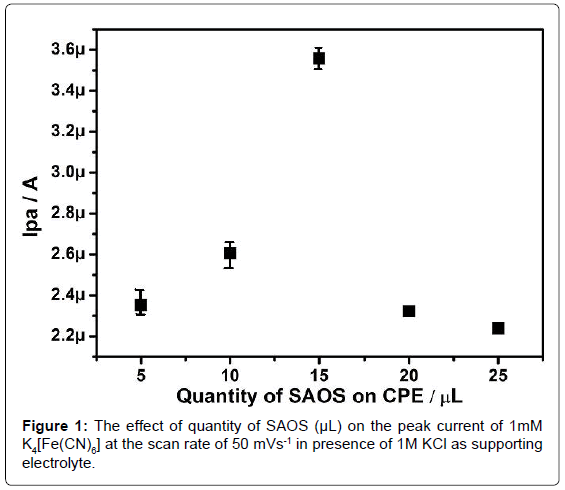
In optimization of modified CPE towards the K4[Fe(CN)6], the bare CPE was immobilized on to the surface of the carbon paste electrode in the different concentrations (5, 10, 15, 20, 25 and 30 μL) of SAOS and their electrochemical response towards 1mM K4[Fe(CN)6] in presence of 1M KCl as supporting electrolyte was studied. The obtained result illustrates the increase in the quantity of SAOS the corresponding anodic peak current increases upto 15 μL and afterwards the current goes on decreasing (Figure 1), as a result the 15 μL of SAOS immobilized modified CPE was preferred for further electrochemical investigation.
Electrocatalytic behavior of SAOSMCPE at potassium ferrocyanide
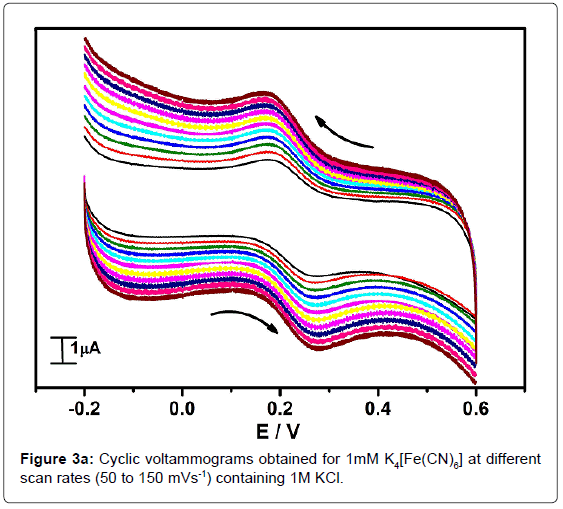
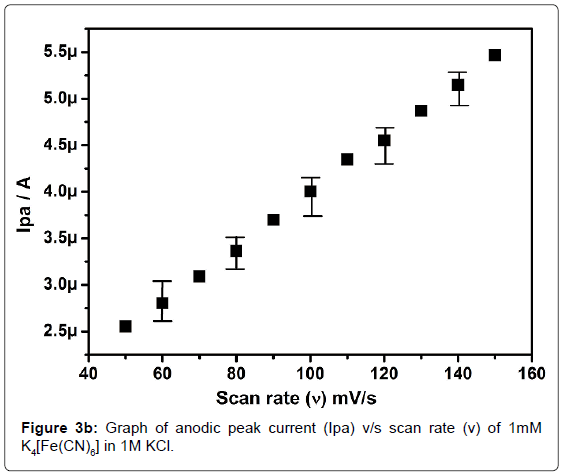
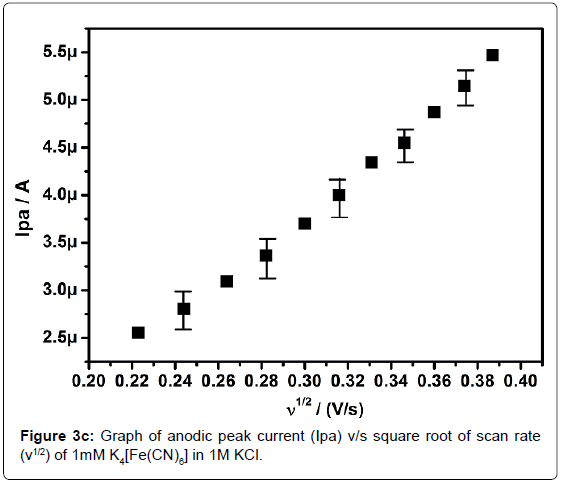
In order to standardize the fabricated SAOSMCPE, potassium ferrocyanide was used for the electrochemical parameters. Figure 2 shows the cyclic voltammograms of 1mM K4[Fe(CN)6] at bare (dotted line) and SAOSMCPE (solid line) in presence of 1M KCl as supporting electrolyte with a scan rate of 50 mVs-1. Comparatively SAOSMCPE shows increase in redox peak currents of 1mM K4[Fe(CN)6] than BCPE. The probable mechanism is the SAOS surfactant molecules diffuses into the carbon paste electrode along with the potassium ferrocyanide and causes increase in the redox peak signals [40]. The difference in redox peak potential (ΔEp) for BCPE was found to be 0.114 V and 0.084 V for SAOS modified carbon paste electrode. The ΔEp is a function of the rate of electron transfer, hence lower the ΔEp value higher will be the electron transfer rate [41]. The proposed modified electrode shows lower ΔEp value than the BCPE and exhibits great enhancement in the redox peak current. Figure 3a shows the cyclic voltammograms of potassium ferrocyanide at different scan rates. The redox peak current of potassium ferrocyanide goes on increases with increase in scan rate. The plot of Ipa versus scan rate shows good linearity with correlation coefficient value R2=0.99958 as shown in Figure 3b. The plot of Ipa v/s square root of scan rate also shows good linearity with the correlation coefficient value R2=0.9965 as shown in Figure 3c. From the scan rate study the electrode process was found to be both adsorption and diffusion controlled. Based on the above observation SAOSMCPE shows favorable electrocatalytic behavior and it might be used as a chemically modified electrode to explore electroanalytical application.
Electrochemical response of folic acid at SAOSMCPE
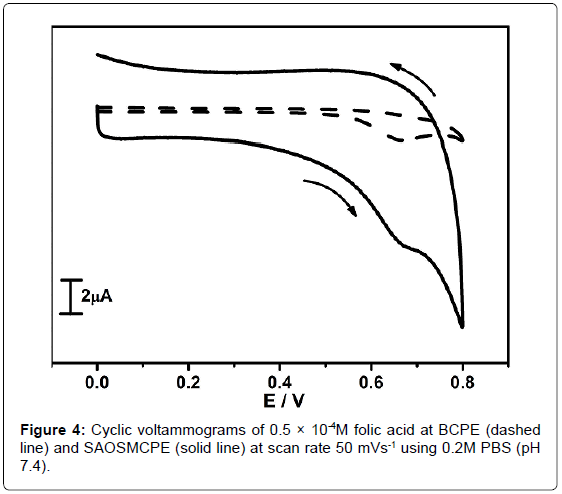
The electrochemical response of folic acid (FA) was studied at SAOS modified carbon paste electrode. The Figure 4 shows the cyclic voltammograms of 0.5 × 10-4M folic acid at BCPE (dotted line) and SAOSMCPE (solid line) in 0.2M PBS at pH 7.4 with the scan rate of 50 mVs-1. Comparatively SAOSMCPE shows great enhancement in the anodic peak current (Ipa) than BCPE. Thus remarkable improvement in electrochemical sensitivity towards folic acid at SAOSMCPE gives an evidence for the catalytic effect of proposed electrode.
Effect of scan rate on SAOSMCPE
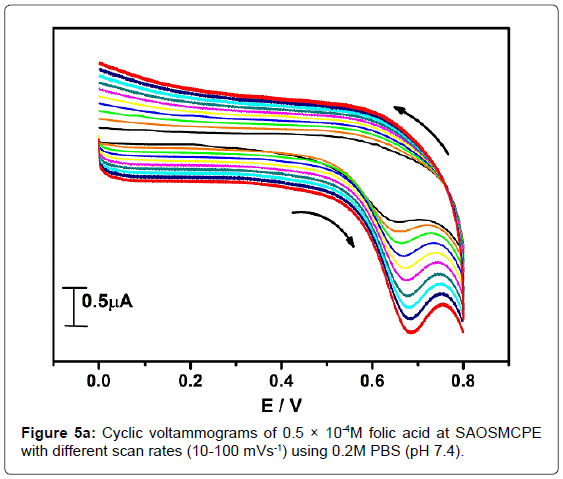
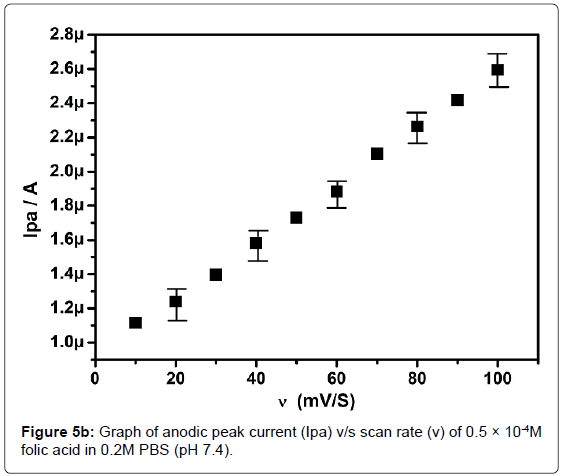
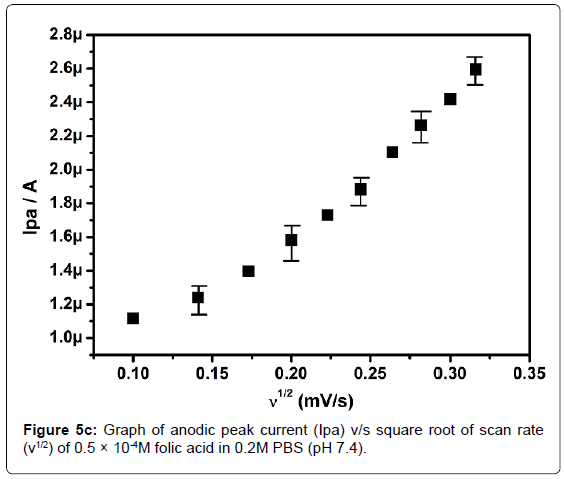
In order to investigate the kinetics of electrode reaction, effect of scan rate (ν) was studied at SAOSMCPE. Figure 5a shows the cyclic voltammograms of 0.5 × 10-4M folic acid at different scan rate in 0.2M phosphate buffer solution (pH 7.4). The result shows that, with the increase in the scan rate (10-100 mVs-1) the anodic peak current (Ipa) of folic acid goes on increases. The plot of anodic peak current (Ipa) v/s scan rate (ν) shows good linearity with correlation coefficient value R2=0.99916 as shown in Figure 5b. Along with the plot of anodic peak current (Ipa) versus square root of scan rate (ν1/2) was also studied and correlation coefficient value was found to be R2=0.9846 indicating the modified electrode process was both adsorption and diffusion controlled electrode reactions (Figure 5c).
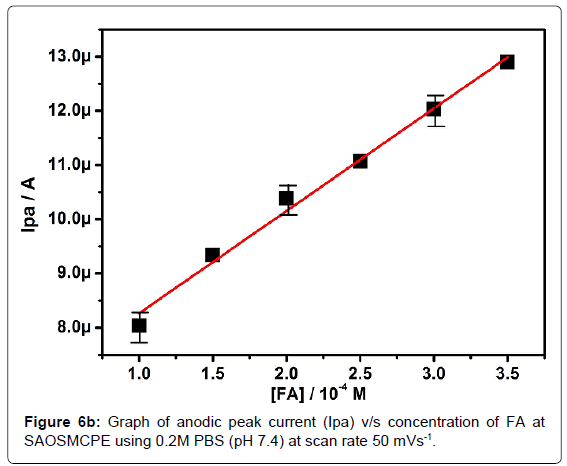
Effect of folic acid concentration at SAOSMCPE
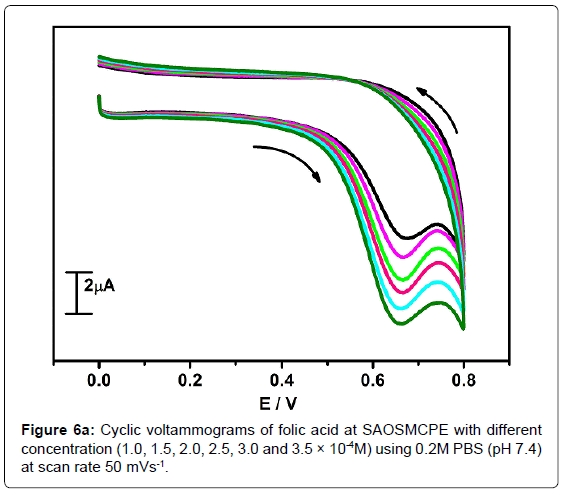
The electrochemical oxidation of folic acid at different concentration using SAOSMCPE was studied. Figure 6a shows the cyclic voltammograms obtained for the folic acid at different concentration in presence of 0.2M PBS (pH 7.4) with the scan rate of 50 mVs-1. With the increase in the concentration (1.0-3.5 × 10-4M) the anodic peak current (Ipa) of folic acid goes on increasing. The plot of anodic peak current (Ipa) versus concentration (Figure 6b) shows good linearity with the correlation coefficient value R2=0.9958. The limit of detection (LOD) was calculated by using the formula (1) [42-45] and it was found to be 2.88 × 10-5M and the limit of quantification (LOQ) was calculated by using the formula (2) and it was found to be 9.6 × 10-5M.
LOD=3S/M (1)
LOQ=10S/M (2)
Where, S is the standard deviation and M is the slope
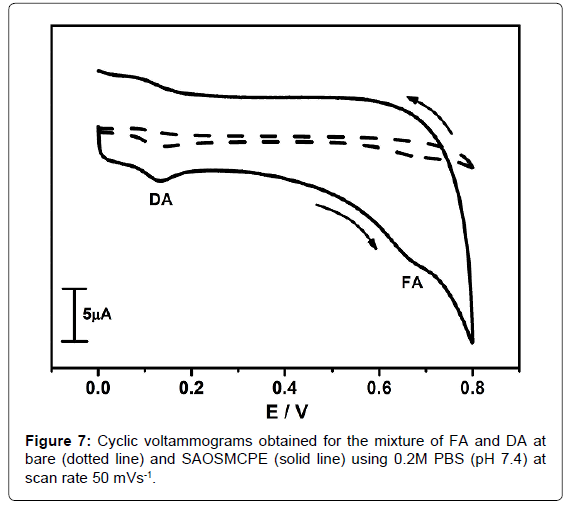
Simultaneous electrochemical determination of FA and DA at SAOSMCPE
The cyclic voltammetry was employed for the simultaneous electrochemical determination of FA and DA. The Figure 7 shows the cyclic voltammograms obtained for the mixture of 0.5 × 10-4M FA and 0.5 × 10-4M DA in the presence of 0.2M PBS (pH 7.4) at the scan rate of 50 mVs-1. The Dotted line shows cyclic voltammograms obtained for the mixture of FA and DA at BCPE. The solid line shows cyclic voltammogram obtained for the mixture of FA and DA at SAOSMCPE. The modified electrode shows great enhancement in peak current (Ip) than BCPE and showing good voltammetric separation for both DA and FA and proved its electrocatalytic behaviour in simultaneous determination. Hence the proposed modified electrode (SAOSMCPE) has the ability for the simultaneous determination of FA and DA.
Determination of folic acid in tablet
To investigate the capability of the modified electrode for the determination of the folic acid in the tablet. By using standard addition method, the recovery test was obtained for the voltammetric signals and the observed results were shown in Table 1.
| Sample | Content | Added in mL | Found | Recovery |
|---|---|---|---|---|
| Tablet | 5 mg | 3.5 | 8.038 ± 0.414 | 92.2% |
| Tablet | 5 mg | 4.0 | 1.039 ± 0.031 | 99.2% |
Table 1: Voltammetric signals and their results.
Conclusion
For the electrochemical determination of folic acid, an anionic surfactant sodium alpha olefin sulphonate was used as the modifier. The modified electrode exhibits good electrocatalytic response towards the determination of folic acid. The electrode process of modified electrode was investigated and found to be both adsorption and diffusion controlled and it shows good detection limit. To know the capability of the modified electrode folic acid was determined in the tablet. Finally, it concludes that the proposed electrode (SAOSMCPE) shows very good electrocatalytic properties and can be applied to the electrochemical determination of folic acid.
References
- Ball GFM (2008) Vitamins-Their Role in the Human Body. John Wiley & Sons, UK.
- Mitchell HK, Snell EE, Williams RJ (1988) Journal of the American Chemical Society, Vol. 63, 1941: The concentration of "folic acid" by Herschel K. Mitchell, Esmond E. Snell, and Roger J. Williams. Nutr Rev 46: 324-325.
- Blakley RL (1969) The Biochemistry of Folic Acid and Related Pteridines. North-Holland Publishing Company, New York, USA.
- Gujska E, Kuncewicz A (2005) Determination of folate in some cereals and commercial cereal-grain products consumed in Poland using trienzyme extraction and high-performance liquid chromatography methods. European Food Research and Technology 221: 208-213.
- Al-Shammary FJ, Al-Rashood KA, Mian NA, Mian MS (1990) Analytical Profile of Folic Acid. Anal Profiles Drug Sub 19: 221-259.
- Rockville MD (1996) Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Food and Drug Administration 61: 8781-8797.
- Wright AJA, Finglas PM, Southon S (2001) Proposed mandatory fortification of the UK diet with folic acid: have potential risks been underestimated. Trends Food Sci Technol 12: 313-321.
- Chong WM (1990) Cardiology: A Socratic Approach. Orient Longman Pvt Ltd, India.
- Nelson BC, Sharpless KE, Sander LC (2006) Quantitative determination of folic acid in multivitamin/multielement tablets using liquid chromatography/tandem mass spectrometry. J Chromatogr A 1135: 203-211.
- Rodríguez-Bernaldo de Quirós A, Castro de Ron C, López-Hernández J, Lage-Yusty MA (2004) Determination of folates in seaweeds by high-performance liquid chromatography. J Chromatogr A 1032: 135-139.
- Zhao S, Yuan H, Xie C, Xiao D (2006) Determination of folic acid by capillary electrophoresis with chemiluminescence detection. J Chromatogr A 1107: 290-293.
- Aurora-Prado MS, Silva CA, Tavares MF, Altria KD (2004) Determination of folic acid in tablets by microemulsion electrokinetic chromatography. J Chromatogr A 1051: 291-296.
- Hoegger D, Morier P, Vollet C, Heini D, Reymond F, et al. (2007) Disposable microfluidic ELISA for the rapid determination of folic acid content in food products. Anal Bioanal Chem 387: 267-275.
- Kumar M, Swamy KBE (2014) ZnO Modified Carbon Paste Electrode for the Detection of Folic Acid and Paracetamol: A Cyclic Voltammetric Study. Journal of Chemical Engineering and Research 2: 121-126.
- Alvarez JMF, Garcia AC, Oridieres AJM, Blanco PT (1987) Adsorptive stripping voltammetric behaviour of folic acid. J Electroanal Chem 225: 241-253.
- Prasad BB, Madhuri R, Tiwari MP, Sharma PS (2010) Electrochemical sensor for folic acid based on a hyperbranched molecularly imprinted polymer-immobilized sol-gel-modified pencil graphite electrode. Sens Actuators B Chem 146: 321-330.
- Wei SH, Zhao FQ, Xu ZY, Zeng BZ (2006) Voltammetric Determination of Folic Acid with a Multi-Walled Carbon Nanotube-Modified Gold Electrode. Microchim Acta 152: 285-290.
- Xiao F, Ruan C, Liu L, Yan R, Zhao F, et al. (2008) Single-walled carbon nanotube-ionic liquid paste electrode for the sensitive voltammetric determination of folic acid. Sens Actuators B Chem 134: 895-901.
- Ensafi AA, Karimi-Maleh H (2010) Modified multiwall carbon nanotubes paste electrode as a sensor for simultaneous determination of 6-thioguanine and folic acid using ferrocenedicarboxylic acid as a mediator. J Electroanal Chem 640: 75-83.
- Beitollahi H, Sheikhshoaie I (2011) Electrocatalytic oxidation and determination of epinephrine in the presence of uric acid and folic acid at multi walled carbon nanotubes/molybdenum(VI) complex modified carbon paste electrode. Analytical Methods 3: 1810-1814.
- Smith TE (1992) Text Book of Biochemistry with Clinical Correlations. Devlin TM (Ed), Wiley/Liss, New York, USA 929.
- O'Neill RD (1994) Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo. A review. Analyst 119: 767-779.
- Junter GA (1988) Electrochemical Detection Techniques in the Applied Biosciences. Halsted, New York.
- Wang Q, Dong D, Li N (2001) Electrochemical response of dopamine at a penicillamine self-assembled gold electrode. Bioelectrochemistry 54: 169-175.
- Chicharroa M, Sancheza A, Zapardiel A, Rubianesc MD, Rivasc G (2004) Capillary electrophoresis of neurotransmitters with amperometric detection at melanin-type polymer-modified carbon electrodes. Anal Chim Acta 523: 185-191.
- Xue KH, Tao FF, Xu W (2005) Selective determination of dopamine in the presence of ascorbic acid at the carbon atom wire modified electrode. J Electroanal Chem 578: 323-239.
- Gilbert O, Chandra U, Swamy BEK, Pandurangachar M, Nagaraj C, et al. (2008) Poly (Alanine) Modified Carbon Paste Electrode for Simultaneous Detection of Dopamine and Ascorbic Acid. International Journal of Electrochemical Sciences 3: 1186-1195.
- Raj CR, Tokuda K, Ohsaka T (2001) Electroanalytical applications of cationic self-assembled monolayers: square-wave voltammetric determination of dopamine and ascorbate. Bioelectrochemistry 53: 183-191.
- Ganesh PS, Swamy BEK (2015) Voltammetric Resolution of Dopamine in Presence of Ascorbic Acid and Uric Acid at Poly (Brilliant Blue) Modified Carbon Paste Electrode. J Anal Bioanal Tech 5: 229.
- Downard AJ, Roddick AD, Bond AM (1995) Covalent modification of carbon electrodes for voltammetric differentiation of dopamine and ascorbic acid. Anal Chim Acta 317: 303-310.
- Zhang P, Wu FH, Zhao GC, Wei XW (2005) Selective response of dopamine in the presence of ascorbic acid at multi-walled carbon nanotube modified gold electrode. Bioelectrochemistry 67: 109-114.
- Lokesh SV, Sherigara BS, Jayadev, Mahesh HM, Mascarenhas JR (2008) Electrochemical Reactivity of C60 Modified Carbon Paste Electrode by Physical Vapor Deposition Method. International Journal of Electrochemical Sciences 3: 578-587.
- Hu C, Hu S (2004) Electrochemical characterization of cetyltrimethyl ammonium bromide modified carbon paste electrode and the application in the immobilization of DNA. Electrochim Acta 49: 405-412.
- Rusling JF (1991) Controlling electrochemical catalysis with surfactant microstructures. Acc Chem Res 24: 75-81.
- Kumar M, Swamy BEK, Reddy S, Sathisha TV, Manjanna J (2013) Synthesis of ZnO and its surfactant based electrode for the simultaneous detection of dopamine and ascorbic acid. Analytical Methods 5: 735- 740.
- Hu SS, Wu KB, Yi HC, Cui DF (2002) Voltammetric behavior and determination of estrogens at Nafion-modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Anal Chim Acta 464: 209-216.
- Hu S, Yan Y, Zhao Z (1991) Determination of progesterone based on the enhancement effect of surfactants in linear sweep polarography. Anal Chim Acta 248: 103-108.
- Yi H, Wu K, Hu S, Cui D (2001) Adsorption stripping voltammetry of phenol at Nafion-modified glassy carbon electrode in the presence of surfactants. Talanta 55: 1205-1210.
- Zhang S, Wu K, Hu S (2002) Voltammetric determination of diethylstilbestrol at carbon paste electrode using cetylpyridine bromide as medium. Talanta 58: 747-754.
- Niranjana E, Naik RR, Swamy BEK, Sherigara BS, Jayadevappa H (2007) Studies on Adsorption of Triton X-100 at Carbon Paste and Ceresin Wax Carbon Paste Electrodes and the Enhancement Effect in Dopamine Oxidation by Cyclic Voltammetry. International Journal of Electrochemical Sciences 2: 923-934.
- Gilbert O, Swamy BEK, Chandra U, Sherigara BS (2009) Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study. J Electroanal Chem 636: 80-85.
- Shankar SS, Swamy BEK, Pandurangachar M, Chandra U, Chandrashekar BN, et al. (2010) Electrocatalytic Oxidation of Dopamine on Acrylamide Modified Carbon Paste Electrode : A Voltammetric Study. International Journal of Electrochemical Sciences 5: 944-954.
- Chandra U, Swamy BEK, Mahanthesha KR, Vishwanath CC, Sherigara BS (2013) Poly(malachite green) film based carbon paste electrode sensor for the voltammetric investigation of dopamine. Chemical Sensors 3.
- Chitravathi S, Swamy BEK, Mamatha GP, Sherigara BS (2013) Determination of salbutamol sulfate by Alcian blue modified carbon paste electrode: A cyclic voltammetric study. Chemical Sensors 3.
- Mahanthesha KR, Swamy BEK, Chandra U, Sathish Reddy, Pai KV (2014) Sodium dodecyl sulphate/polyglycine/phthalamide/carbon paste electrode based voltammetric sensors for detection of dopamine in the presence of ascorbic acid and uric acid. Chemical Sensors 4.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16688
- [From(publication date):
December-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12000
- PDF downloads : 4688