Research Article Open Access
Graphene and Polyaniline Composite Modified Glassy Carbon Electrode for Electrochemical Determination of Doripenem and Meropenem Metabolites
Sreedhar NY*, Sivaprasad M, Swarupa Ch, Dhananjayulu M and Jayapal MRElectroanalytical Lab, Department of Chemistry, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India
- *Corresponding Author:
- Sreedhar NY
Electroanalytical Lab, Department of Chemistry
Sri Venkateswara University, Tirupati-517502
Andhra Pradesh, India
Tel: +91-9440087124
E-mail: sreedharny.chem@gmail.com
Received date: April 16, 2014; Accepted date: May 28, 2014; Published date: May 30, 2014
Citation: Sivaprasad M, Swarupa Ch, Dhananjayulu M, Jayapal MR, Sreedhar NY (2014) Graphene and Polyaniline Composite Modified Glassy Carbon Electrode for Electrochemical Determination of Doripenem and Meropenem Metabolites. J Anal Bioanal Tech 5:192 doi: 10.4172/2155-9872.1000192
Copyright: © 2014 Sivaprasad M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The theme of this study was the preparation, characterization, and application of a polyaniline (PAN) and graphene composite modified glassy carbon electrode (PAN/Gr/GCE) for the voltammetric determination of doripenem (DPM) and meropenem metabolites (MPM) in human urine and serum samples. The morphological study of the PAN/ Gr/GCE composite was examined by scanning electron microscopy (SEM) and cyclic voltammetry (CV). The electrochemical behaviour of DPM and MPM at the PAN/Gr/GCE were investigated using cyclic voltammetry in Ag/ AgCl/KCl supporting electrolyte at pH 2.0-10.0 in phosphate buffer solution. The linear dependence of current versus concentration was reached in a wide concentration range from 2.5×10-7 M to 3.5×10-4 M using cyclic voltammetry and differential pulse voltammetric methods. In acidic media a non-reversible diffusion controlled reduction involving two protons and two electrons occurs at carbon and nitrogen double bond (C=N) in the metabolites. The best electroanalytical performances of this composite electrode were achieved with the detection limits 3.6×10-9 M and 1.75×10-9 M for DPM and MPM respectively. The simplicity of preparation, high sensitivity and stability of this composite electrode should open novel avenues and applications for fabricating robust sensors for detection of DPM and MPM in human urine and serum samples.
Keywords
Doripenem; Meropenem metabolites; Polyaniline; Graphene; Cyclic voltammetry; Differential pulse voltammetry
Introduction
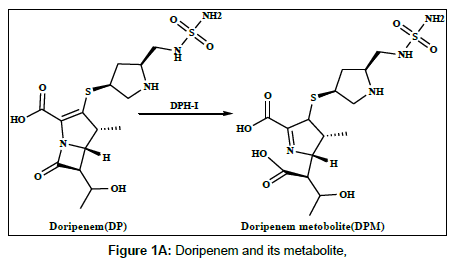
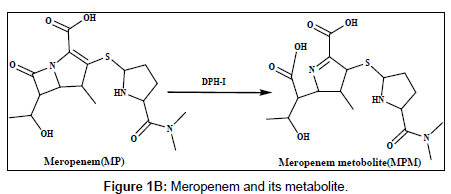
Doripenem (DP) and meropenem (MP) are new parenteral carbapenems (Figures 1a and 1b). Both are similarly to ertapenem and biapenem contains a 1-β-methyl group that prevents degradation caused by renal dehydropeptidase-I (DPH-I) and therefore does not need to be protected with an inhibitor of this enzyme [1,2]. DP and MP were broad spectrum of antimicrobial activity against both gram-positive and gram-negative bacteria, including pseudomonas aeruginosa [3-5].The catalytic effect of citrate, phosphate and acetate buffers on the degradation of imipenem has been studied [6]. Several methods have been reported in the literature for the determination of doripenem and meropenem and its metabolites in biological fluids and pharmaceutical formulation using different techniques, including spectrophotometry, high performance liquid chromatography (HPLC) and liquid chromatography methods [7-11].
The characteristics and application of conducting polymers, such as polyaniline (PAN) polypyrrole, and polythiophene have attracted great fundamental interest in recent years [12-14]. Among these polymers, PAN is considered as one of the most promising electrode materials due to its high capacitive characteristics, and lower cost than many other conducting polymers [15]. The PAN modified electrode has proven to have potential applications in electrochemical sensors [16,17]. In order to ease this limitation, the combination of PAN with other nanomaterials, such as carbon material, might be one better selection for modification of electrodes.
Seeing that a latest member of carbon nanomaterials, such as graphene (Gr), a two-dimensional monolayer of sp2 bonded carbon atoms tightly packed into a planar honeycomb lattice and it has been proven as an excellent support material due to its high surface area to volume ratio, remarkable mechanical stiffness and excellent electrical conductivity, which is very beneficial in designing electrochemical sensors [18]. Recently, the graphene-polyaniline nanocomposite has been successfully prepared by in situ electrochemical polymerization process. However, the poor solubility of graphene in both polar and apolar solvents has greatly limited the preparation and application of graphene based composites [19]. The graphene and polyaniline modified electrode has already been used for determination of 4-amino phenol and chemiluminescence of luminal [20,21].
Harrison et al. [22] studied the metabolism of meropenem and detected only one metabolite in the urine, the ring-open lactam. Iolanda Cirillo et al. [23] reported the metabolism of doripenem in urine. In this work, the electrochemical behaviour of the metabolites of DPM and MPM has been studied, the reduction mechanism has been suggested on the modified electrode and differential pulse voltammetry method have been offered and applied to the determination of DPM and MPM in human urine and serum samples [24].
In the present study, graphene (Gr) was firstly fabricated on a glassy carbon electrode (GCE), then polyaniline by in situ electrochemical polymerized on the graphene modified electrode. Nevertheless, to the best of our knowledge, there is no electroanalytical report concerning the DP and MP metabolites at the graphene (Gr) and polyaniline (PAN) modified electrode.
Materials and Methods
Chemicals and reagents
Doripenem, meropenem metabolites obtained from Aurobindo pharmaceuticals, Hyderabad, AP. N,N′-dimethylformamide (DMF), polyaniline and grapheme (Gr) were obtained commercially from sigma Aldrich (Mumbai, India). Double distilled water was used to prepare all the experimental solutions. Phosphate buffer was prepared using potassium dihydrogen phosphate. All reagents were used as analytical reagent grade.
Apparatus
Electrochemical studies were carried out by Autolab PG STAT101 supplied by Metrohm Autolab B.V. Netherlands. A three electrode system comprising of a glassy carbon electrode modified with polyaniline (PAN) and graphene (Gr) composites as a working electrode. Graphene (Gr) and polyaniline (PAN) obtained from Aldrich. Saturated Ag/AgCl/KCl as a reference electrode and Pt wire as a counter electrode obtained from local scientific labs. Electrode surface morphology study was carried out by SEM instrument model OXFORD INCA PENTA FETX3 CARL ZEISS from Japan. An Elico LI-120 pH meter supplied by Elico LTD, Hyderabad, India was used to determine the pH of the buffer solution.
Preparation of samples
An aliquot containing 2.5×10-7 M of DPM and MPM was placed into a 25 ml calibrated flask and 5 ml of buffer solution, pH 6.0 was added. The solution was diluted to the mark with water and mixed well. The solution was transferred into electrolytic cell. After deoxygenating for 10 min with a stream of pure nitrogen up to 2 ml of untreated urine and serum samples containing 2.5×10-7 M of DPM and MPM was placed each separately into a 25 ml volumetric flask and diluted with water to the mark. To this 0.5 ml of the solution with 5 ml of pH 6.0 phosphate buffer solution was diluted with water to 25 ml into a volumetric flask. The voltammograms were recorded according to the above recommended procedure.
Preparation of PAN/Gr/GCE
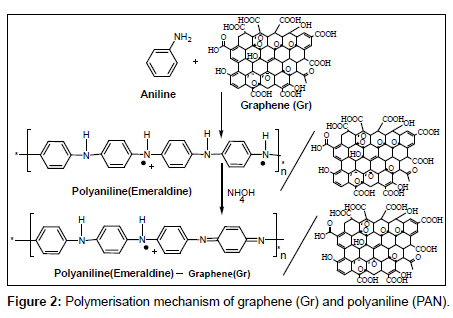
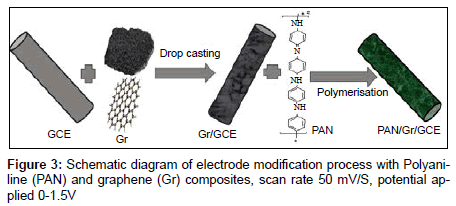
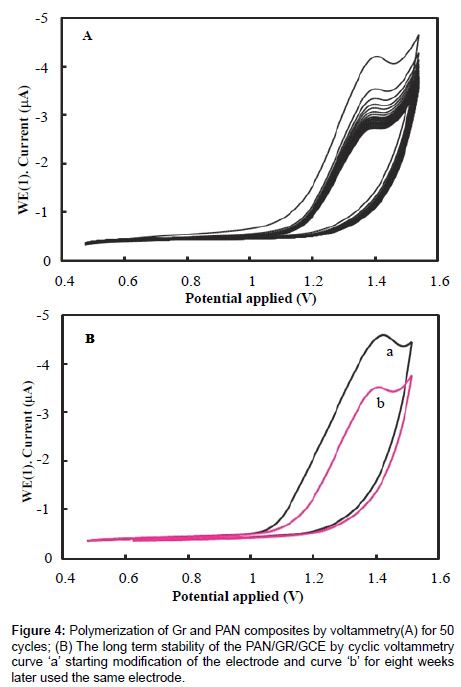
Before modification, a bare glassy carbon electrode (GCE) with 3 mm in diameter was polished with abrasive paper and rinsed with ethanol and redistilled water to remove the trace remainder. Then, the electrode was cycled in 1 mM K3Fe(CN)6 solution between −0.3 and 0.5 V (vs SCE) at a scan rate of 100 mV s−1 until a pair reversible CV peaks were obtained, indicating that the surface of glassy carbon electrode was cleaned. The electrode was again rinsed with redistilled water and cleaned in an ultrasonic bath. A given amount of graphene powder was dispersed in double distilled water with the help of ultra-sonication for one hour, to obtain a homogeneous, well-distributed black solution. A 5 μL graphene solution was dropped onto the cleaned glassy carbon electrode and dried under air, to obtain a graphene modified glassy carbon electrode, denoted as Gr/GCE. The polyaniline (PAN) film was in situ polymerized on the Gr/GCE by cyclic voltammetry between 0.20 V to 1.50 V at 50 mVs−1 for fifty cycles in the solution of 0.25 mol/L H2SO4 and 0.1 mol/L aniline. The modified Gr/GCE was obtained and it was denoted as PAN/Gr/GCE. Figures 2 and 3 have shown the polymerization and schematic diagram of electrode modification with PAN and Gr composites. Figure 4 shows the typical cyclic voltammogram for 50 cycles of polymerisation of graphene and polyaniline composite doping on glassy carbon electrode.
Results
Characterization of PAN/Gr/GCE
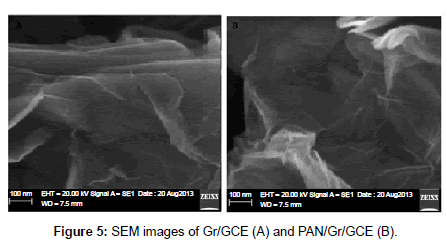
The thinning out and bonding of the PAN and Gr composites are most important subject in producing the PAN/Gr composite materials. SEM microscopy was used to gain insight into the surface characteristics of the PAN/Gr/GCE composite. Figure 5 shows the SEM image of the PAN/Gr/GCE composite electrode and it can be seen that PAN and Gr was dispersed and distributed surrounded the glassy carbon electrode. Cyclic voltammetry (CV) is one of the most versatile electrochemical techniques used in the study of electroactive behaviour and the characterization of sensors. In order to determine the electroactive surface area of the PAN/Gr/GCE composite electrode, the electrochemical behaviour of potassium ferrocyanide K3[Fe(CN)6] in 1 M KNO3 supporting electrolyte was studied using cyclic voltammetry recorded at different scan rates. According to the Randles–Sevcik Equation:
Ip = 2.69×105 AD1/2n3/2v1/2C
where ‘A’ represents the area of the electrode (cm2), ‘n’ the number of electrons participating in the reaction, and ‘n’ is equal to 1, ‘D’ is the diffusion coefficient of the molecule in solution, ‘C’ the concentration of the probe molecule in the solution and ‘v’ is the scan rate (Vs−1). The apparent diffusion coefficient of K3[Fe(CN)6] was determined to be 5.33×10−6 cm2s−1. By comparison with the theoretical diffusion coefficient value of 6.7×10−6 cm2s−1 based on the literature data, the value of the active electrode area was found to be 0.252 cm2 vs. the value of the electrode geometric area of 0.235 cm2.
Cyclic voltammetric study
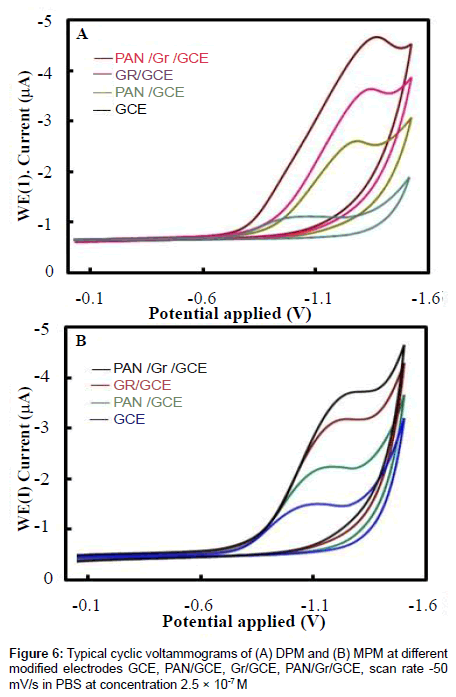
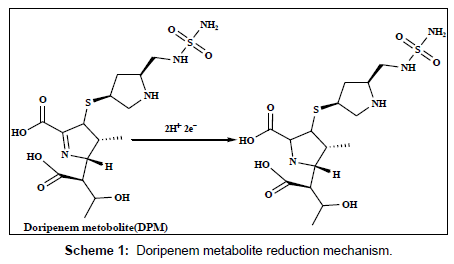
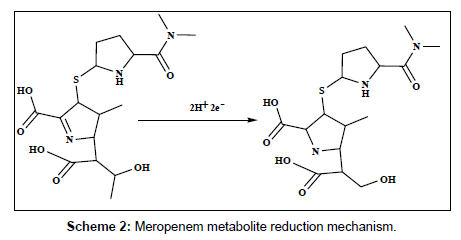
The electrochemical behavior of 2.5×10-7 M of DPM and MPM at the bare GCE, PAN/GCE, Gr/GCE and Gr/PAN/GCE was investigated in phosphate buffer of pH 6.0 using cyclic voltammetry and the results were shown in Figure 6. It can be seen an irreversible reduction peak of DPM and MPM appears at the GCE with a potential range of-1.15 V to 1.25 V and a current is -1.3 μA to -1.6 μA. The response on the PAN/GCE was a small bulge at -2.60 μA, then at Gr/GCE and PAN/ Gr/GCE the peak current increases more, here the peak current observed -3.55 μA to -4.85 μA the suggesting that the Gr film improved the accumulation of the metabolites. The CV curve of PAN/Gr/GCE shifted to more negative current at pH 6.0. There is no corresponding oxidation peaks observed at the reverse scan, indicating that the electrochemical reduction of DPM and MPM were totally irreversible reaction under the above experimental conditions. The suggested reduction mechanisms of the metabolites are shown in scheme 1and scheme 2.
This occurrence should be attributed to the reduction medium of deposition and the surface area was significantly increased in the resulting nanocomposite, suggesting the effect of PAN/Gr/GCE composite provides efficient results for the electrochemical reaction of DPM and MPM with enhanced voltammetric response. In sequence about the mechanism of electrochemical reactions can be determined from the relationship between scan rate and peak current. Therefore,
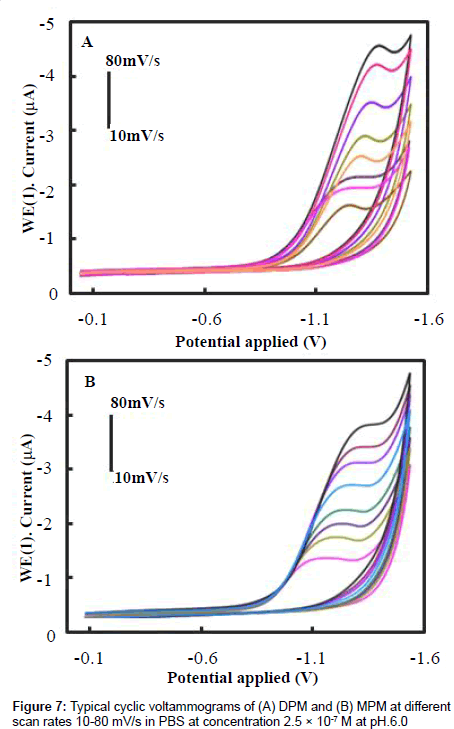
we studied the effect of different scan rates on the electrochemical reduction of DPM and MPM. Figures 7A and 7B show the CV curves of 2.5×10-7 M DPM and MPM at different scan rates from 0.01 to 0.10 V/s using the PAN/Gr/GCE. Both compounds reduction peak currents increased with scan rate, indicating that the conductivity of the surface of the electrode gradually increased with scan rate. The linear relationship between scan rate and peak current suggests that the reduction of DPM and MPM is a typical adsorption controlled process, which can be used to quantitatively analyze the DPM and MPM present at the electrode surface. To reduce the effects of background current and still achieve high sensitivity, 50 mV/s was selected as the scan rate for subsequent experiments.
Effect of pH
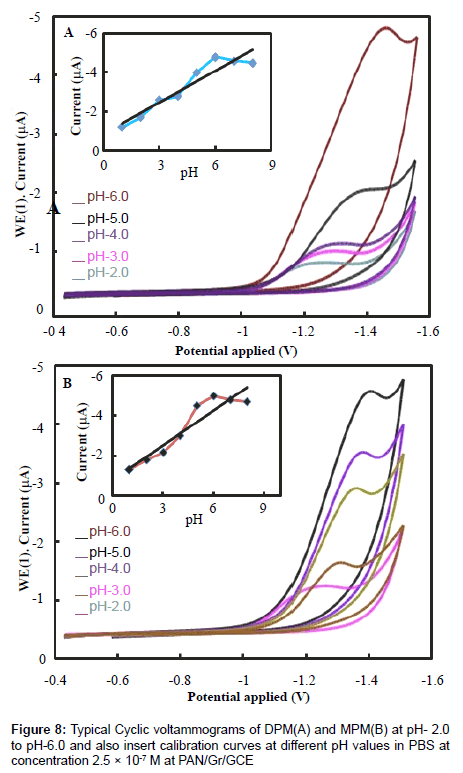
Solution pH also affects the performance of peak current and reduction mechanism, so we examined the influence of pH on the electrochemical response of the electrodes. The relationship between chemical reduction efficiency and pH of the solution was obtained by CV, the results are shown in Figure 8. The reduction peak current increased from pH 2.0 to 6.0, with the increasing pH, the peak current reaching a maximum at pH 6.0. Further increasing the pH of the buffer solution caused the reduction peak current to decrease. Furthermore, within the pH range of 2.0 to 6.0, the reduction peak potential increased gradually, whereas from 6.0 to 8.0 it decreased gradually, indicating a low electron transfer rate [22].
Reproducibility and stability
The stability of the PAN/Gr/GCE was also investigated and shown in Figure 4A. The PAN/Gr/GCE exhibits good measurement stability by consecutive 50 cycles of CV test, which a little decrease of peak current was observed with increase of scanning cycle, but the reduction peak current retained 99% of its initial current after it was tested by consecutive CV scan. This indicates that the PAN/Gr/GCE film did not peel off from the surface of GCE through 50 times of CV cycles. The long term stability of the electrode was investigated for the CV measurement, after storage in the air, for eight weeks at room temperature and the result shows that about 5% of its initial current was loss. The elevated stability of the film can be owing to the GCE was used as substrate which can improve the interaction force between GCE and electro active PAN, so the film can be strongly coated on the GCE; another reason is that graphene has good stability properties for immobilization of electro active PAN. The long term stability of the PAN/Gr/GCE by cyclic voltammograms, scanning curve ‘a’ for starting modification and curve ‘b’ for eight weeks later used the same electrode (Figure 4B).
Linear regression and detection limits
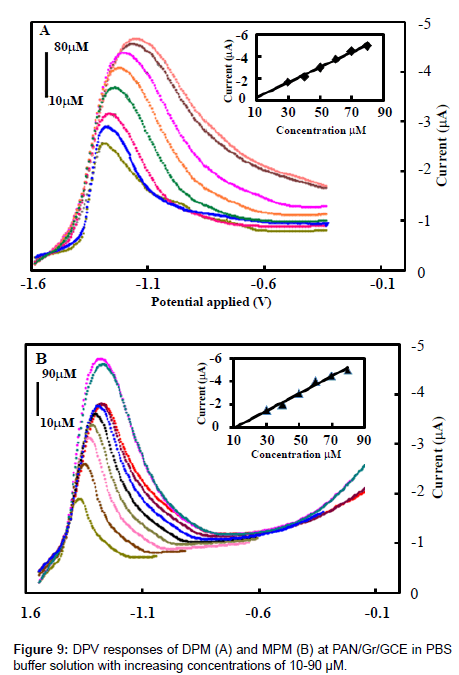
The electrochemical behavior of the PAN/Gr/GCE sensor was quantitatively analyzed by differential pulse voltammetry under optimal conditions for aggregation using different concentrations of DPM and MPM. The results of these experiments are shown in Figure 9. When the concentration of DPM and MPM were increased from 10 μm to 90μm the reduction peak current increased linearly. The linear regression equation for this region was: I (10−7A)=−2.562×10−7C (mol/L)–3.454×10−6 with a correlation coefficient of 0.992 and a limit of detections(S/N=3) are 3.6×10−9mol/L and 1.75×10−9 M of DPM and MPM respectively. Thus, PAN/Gr/GCE has good electrocatalytic activity and adsorption capacity towards high concentrations of DPM and MPM, so it could be used to detect high concentrations of the metabolites of DP and MP.
Recovery study of DPM and MPM
Three human urine and serum samples were prepared as described in the experimental part. The three human urine and serum samples were spiked with appropriate amounts of standard DPM and MPM until the final concentrations are ranging from 2.5×10−7 to 1.2×10-4 M respectively. The recovery study was investigated by comparing the current response for the three human urine samples against the spiked concentrations of standard DPM and MPM in PBS at pH 6.0. Recoveries are obtained from 96.50% to 99.65% for both human urine and serum samples, shown in Table 1 it indicates that the applicability of the method for determination DPM and MPM in spiked samples.
| Human urine samples | ||||||
|---|---|---|---|---|---|---|
| Added(M) | Found(M) | *Recovery (%) | RSD% | |||
| DPM/MPM | DPM | MPM | DPM | MPM | DPM | MPM |
| 2.5 × 10−7 | 2.48 × 10−7 | 2.43 × 10−7 | 99.65 | 98.80 | 2.45 | 3.52 |
| 4.5 × 10-5 | 4.42 ×10-5 | 4.35 × 10-5 | 98.20 | 97.75 | 2.85 | 3.74 |
| 1.2 × 10-4 | 0.97 × 10-4 | 0.93 × 10-4 | 97.30 | 96.70 | 3.10 | 4.10 |
| Human serum samples | ||||||
| 2.5 × 10−7 | 2.45 × 10−7 | 2.40 ×10−7 | 98.45 | 98.30 | 2.52 | 3.63 |
| 4.5 ×10-5 | 4.35 × 10-5 | 4.25 ×10-5 | 97.67 | 97.60 | 3.10 | 3.85 |
| 1.2 ×10-4 | 0.94 ×10-4 | 0.87 ×10-4 | 96.90 | 96.50 | 3.60 | 4.25 |
*Average of five determinations
Table 1: Determination of DPM and MPM.
Analytical applications
The high sensitivity of the developed reduction peak (Ep,c=-1.25V and -1.10V) at the GCE modified with PAN and Gr at pH 6.0, suggests possible application of the developed DPV method for analysis of DPM and MPM. Under the optimum experimental conditions is pH 6.0, pulse amplitude 50 mV, pulse width 30 ms and scan rate -50 mV s−1. DPM and MPM show that the peak current increased linearly with increasing the drug concentration in the range from 2.5×10−7to 1.2×10-4M by DPV method. A lower limit of detections (LOD) were found to be 3.5×10−9M and 1.75×10−9 M for DPM and MPM by use of the formula LOD=3α/a (where ‘α’ is the standard deviation of residuals and ‘a’ is the slope of the calibration plot). The LOQ was found to be 4.2×10−6 M and 2.4×10−6 M for DPM and MPM respectively. The relative standard deviation (RSD) of DPM and MPM were 2.45% and 3.52% in human urine samples and the RSD of DPM and MPM in human serum samples were 2.52% and 4.25%. The peak potentials and peak heights of the given compounds at concentration 2.5×10−7M was compared at GCE modified electrode shown in Figure 9.
Conclusion
The studies have shown that the peak potentials, detection limits at modified glassy carbon electrode (PAN/Gr/GCE) for the determination of DPM and MPM are −1.25 mV and -1.10 mV, and 3.5×10−9 M and 1.75×10−9 M respectively and the peak potentials, detection limits at bare glassy carbon electrode are -1.12 mV and -1.0 mV and 5.3×10-8 M and 6.2×10−7 M respectively. The lower value shows that the modified glassy carbon electrode is superior to bare glassy carbon electrode. Further, it is believed that this alternative approach of determining DPM and MPM by differential pulse voltammetry is convenient, faster and accurate. It is thus finer to on hand spectrophotometric and chromatographic methods which are pricey and time consuming. Thus it can be said that this sensor (PAN/Gr/GCE) is a useful addition in the field of analytical chemistry for the determination of drugs and their metabolites.
Acknowledgements
We are indebted to the university grants commission (UGC- BSR-RFMS), India, for the generous funding.
References
- Walsh F (2007) Doripenem: A new carbapenem antibiotic a review of comparative antimicrobial and bactericidal activities. Ther Clin Risk Manag 3: 789-794.
- Livermore DM (2009) Doripenem: antimicrobial profile and clinical potential. Diagn Microbiol Infect Dis 63: 455-458.
- Goldstein EJ, Citron DM (2009) Activity of a novel carbapenem, doripenem, against anaerobic pathogens. Diagn Microbiol Infect Dis 63: 447-454.
- Psathas PA, Kuzmission A, Ikeda K, Yasuo S (2008) Stability of doripenem in vitro in representative infusion solutions and infusion bags. Clin Ther 30: 2075-2087.
- Snydman DR, Jacobus NV, McDermott LA (2008) In vitro activities of doripenem, a new broad-spectrum carbapenem, against recently collected clinical anaerobic isolates, with emphasis on the Bacteroides fragilis group. Antimicrob Agents Chemother 52: 4492-4496.
- Li SJ, Xing Y, Wang GF (2012) A graphene-based electrochemical sensor for sensitive and selective determination of hydroquinone. Microchim Acta 176: 163-168.
- Mendez R, Alemany T, Villacorta JM (1991) Catalytic effect of buffers on degradation of imipenem (N-Formimidoylthienamycin) in Aqueous Solution. Chem Pharm Bull 39: 1998-2002.
- Cielecka-Piontek J, Jelinska A (2010) The UV-derivative spectrophotometry for the determination of doripenem in the presence of its degradation products. Spectrochim Acta A Mol Biomol Spectrosc 77: 554-557.
- Sutherland C, Nicolau DP (2007) Development of an HPLC method for the determination of doripenem in human and mouse serum. J Chromatogr B Analyt Technol Biomed Life Sci 853: 123-126.
- Ikeda K, Ikawa K, Morikawa N, Kameda K, Urakawa N, et al. (2008) Quantification of doripenem in human plasma and peritoneal fluid by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci 867: 20-25.
- Dailly E, Bouquie R, Deslandes G, Jolliet P, Le Floch R (2011) A liquid chromatography assay for a quantification of doripenem, ertapenem, imipenem, meropenem concentrations in human plasma, Application to a clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 879: 1137-1142.
- Wang P, Liu M, Kan JQ (2009) Amperometric phenol biosensor based on polyaniline. Sensors and Actuators B 140: 577-584.
- Santos LM, Ghilane J, Fave C, Lacaze PC (2008) Electrografting polyaniline on carbon through the electroreduction of diazonium salts and the electrochemical polymerization of aniline. J Phys Chem C 112: 16103-16109.
- Tang Q, Wu J, Sun X, Li Q, Lin J (2009) Shape and size control of oriented polyaniline microstructure by a self-assembly method. Langmuir 25: 5253-5257.
- Dai TY, Jia YJ (2011) Supramolecular hydrogels of polyaniline-poly(styrene sulfonate) prepared in concentrated solutions. Polymer 52: 2550-2558.
- Zhou M, Zhai Y, Dong S (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81: 5603-5613.
- Tang L, Wang Y, Li Y, Feng H, Lu J, et al. (2009) Preparation, Structure, and Electrochemical Properties of Reduced Graphene Sheet Films. Adv Funct Mater 19: 2782-2789.
- Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, et al. (2006) Graphene-based composite materials. Nature 442: 282-286.
- Liu S, Xing X, Yu J, Lian W, Li J, et al. (2012) A novel label-free electrochemical aptasensor based on graphene-polyaniline composite film for dopamine determination. Biosens Bioelectron 36: 186-191.
- Bai H, Li C, Shi G (2011) Functional composite materials based on chemically converted graphene. Adv Mater 23: 1089-1115.
- Dong YP, Zhang J, Ding Y, Chu XF, Chen J (2013) Electrogenerated chemiluminescence of luminol at a polyaniline/graphene modified electrode in neutral solution. Electrochimica Acta 91: 240-245.
- Harrison MP, Haworth SJ, Moss SR, Wilkinson DM, Featherstone A (1993) The disposition and metabolic fate of 14C-meropenem in man. Xenobiotica 23: 1311-1323.
- Cirillo I, Mannens G, Janssen C, Vermeir M, Cuyckens F, et al. (2008) Disposition, metabolism, and excretion of [14C]doripenem after a single 500-milligram intravenous infusion in healthy men. Antimicrob Agents Chemother 52: 3478-3483.
- Ohmori T, Suzuki A, Niwa T, Ushikoshi H, Shirai K, et al. (2011) Simultaneous determination of eight ß-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879: 1038-1042.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 19612
- [From(publication date):
July-2014 - Nov 27, 2024] - Breakdown by view type
- HTML page views : 14956
- PDF downloads : 4656