Review Article Open Access
EGFR Transactivation is Regulated by Neurotensin Receptors in Cancer
Moody TW*Department of Health and Human Services, National Cancer Institute, Center for Cancer Research, Bethesda, MD, USA
- Corresponding Author:
- Terry W Moody, PhD
Department of Health and Human Services
National Cancer Institute, Center for Cancer Research
9609 Medical Center Doctor, Rm. 2W340
Bethesda, MD 20892, USA
Tel: +240-276-7785
E-mail: moodyt@mail.nih.gov
Received Date: March 07, 2017; Accepted Date: March 17, 2017; Published Date: March 18, 2017
Citation: Moody TW (2017) EGFR Transactivation is Regulated by Neurotensin Receptors in Cancer. Arch Sci 1:105.
Copyright: © 2017 Moody TW. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Archives of Science
Abstract
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase (RTK) which regulates the proliferation of cancer cells especially non-small cell lung cancer (NSCLC). NSCLC growth is inhibited by EGFR tyrosine kinase inhibitors (TKI) such as erlotinib or gefitinib. Gefitinib is used to treat NSCLC patients who have EGFR mutations. EGFR tyrosine phosphorylation is regulated by G protein-coupled receptors (GPCR) such as the neurotensin (NTS) receptor. EGFR transactivation caused by NTS addition to NSCLC cells is inhibited by SR48692 (NTSR1 antagonist) or gefitinib. SR48692 and gefitinib are synergistic at inhibiting NSCLC proliferation. The results indicate that GPCR antagonists can potentiate the effects of TKI in cancer.
Keywords
Neurotensin; NTSR1 antagonist; SR48692; Epidermal growth factor receptor; Gefitinib; Non-small cell lung cancer
Abbreviations
CNS: Central Nervous System; EGF: Epidermal Growth Factor; GPCR: G Protein-Coupled Receptor; HB: Heparin Binding; MMP: Matrix Metalloprotease; MAPK: Mitogen Activated Protein Kinase; NTS: Neurotensin; NSCLC: Non-small Cell Lung Cancer; PM: Plasma Membrane; PI: Phosphatidyl Inositol; PK: Protein Kinase; ROS: Reactive Oxygen Species; RTK: Receptor Tyrosine Kinase; SCLC: Small Cell Lung Cancer; TGF: Transforming Growth Factor; TM: Transmembrane; TKI: Tyrosine Kinase Inhibitor
Introduction
High concentrations of receptor tyrosine kinases (RTK) such as the Epidermal Growth Factor Receptor (EGFR, erbB1 ) are present in certain cancers such as non-small cell lung cancer (NSCLC) [1]. After binding ligands such as EGF, heparin binding (HB-EGF), transforming growth factor (TGF α) or amphiregulin, the EGFR can form homodimers with itself or heterodimers with other receptor tyrosine kinases (RTK) such as HER2 (erbB2) [2]. This increases tyrosine phosphorylation of protein substrates such as the mitogen activated protein kinase (MAPK) or phosphatidylinositol-3 kinase (PI3K) leading to increased cancer cellular proliferation and survival [3].
NSCLC, which kills approximately 130,000 citizens annually in the USA, is traditionally treated with combination chemotherapy; however, the 5-year survival rate is only 16% [1]. Approximately 13% of the NSCLC patients have mutated EGFR due to exon 19 deletions or exon 21 mutations such as L858R [4]. The mutated EGFR has increased tyrosine kinase activity resulting in the tyrosine phosphorylation of the EGFR. The patients with mutated EGFR can be treated with tyrosine kinase inhibitors (TKI) such as gefitinib or erlotinib, but after a year secondary EGFR mutations can occur such as T790 M resulting in TKI resistance [4]. There is a need to increase the sensitivity of NSCLC patients to TKI.
The phosphorylation of the EGFR is regulated by G protein-coupled receptors (GPCR) for neurotensin (NTS) within minutes after addition of ligand to NSCLC cells [5]. The expression of RTK such as ErbB1, ErbB2 or ErbB3 is increased by NTS days after addition to NSCLC cells [6]. The tyrosine phosphorylation of the EGFR caused by NTS addition to NSCLC cells is impaired by the NTSR1 antagonist SR48692 and the TKI gefitinib. In this communication, the mechanism by which NTS causes EGFR transactivation is reviewed.
NTS ligand
Neurotensin (NTS) is a 13 amino acid peptide which is biologically active in the central nervous system (CNS). When released from hypothalamic brain neurons, NTS causes analgesia, hypothermia and modulates dopamine signaling in the CNS [7]. NTS may be a neuromodulator in the CNS whereby it is released from brain neurons and activates receptors in adjacent cells. In cancer, NTS is an autocrine growth factor. NTS is abundant in small cell lung cancer (SCLC) [8] and medullary thyroid carcinoma [9]. NTS is secreted from SCLC and binds with high affinity to SCLC cells [10]. The action of NTS is mediated by NTSR1 in cancer cells and NTS stimulates the growth of SCLC cells. SR48692 is a non-peptide NTSR1 antagonist [11] which inhibits the proliferation of pancreatic, prostate and SCLC cells in vitro and in vivo [12-14].
NTS is synthesized as a 170 amino acid precursor protein (prepro- NTS) which lacks biological activity [15]. A signal protease cleaves prepro-NTS to pro-NTS (147 amino acids) which is inactive. A proprotein convertase enzyme and carboxypeptidase cleaves pro-NTS to NTS (13 amino acids) which is biologically active and Neuromedin N (5 amino acids). Table 1 show that the C-terminal hexapeptide of NTS (NTS8-13) is biologically active. Degradation of NTS at the Arg8- Arg9 or Pro10-Tyr11 amide bonds by endopeptidases leads to inactive products [16]. NTS is secreted from the SCLC cells when the cellular cAMP is elevated [8]. The secreted NTS binds to cell surface receptors causing an autocrine SCLC proliferation.
| Ligand | IC50, nM | % Proliferation |
|---|---|---|
| NTS | 4+1 | 176+19** |
| Ac-NTS8-13 | 7+1 | 157+16* |
| NTS8-13 | 10+3 | 143+18* |
| SR48692 | 205+31 | 48+7* |
| NTS1-8 | >2000 | 103+11 |
| Levocabastine | >2000 | 102+12 |
| Gefitinib | >2000 | 62+8* |
| None | ND | 100+7 |
| SR4869+Gefitinib | ND | 23+5** |
Table 1: Effect of ligands on NSCLC cells The IC50+S.E. of 3 determinations to inhibit specific 125I-NTS binding to NSCLC cells are indicated. The % mean colony number+S.E. of 3 determinations is indicated using NSCLC cells; P<0.05*, P<0.01** by ANOVA. The ligand structures are shown below: (1) NTS: Pyr-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu. (2) SR48692: 2{[1-(7-chloro-4-quinolinyl)-5-(2,6)-dimethoxyphenyl]-1H-pyrazole-3-ylcarbonyl} amino)tricycle[3,3,1,13]decane-2-carboxylic acid. (3) Gefitinib N-(3-chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy) quinazoline-4-amine.
NTS receptors
NTS binds with high affinity (Kd=4 nM) to NSCLC cells which have 1500 receptors/cell [5]. Table 1 shows that Ac-NTS8-13, NTS8-13 and SR48692 inhibit specific 125I-NTS binding with high affinity (IC50=7, 10 and 205 nM, respectively); acetylation of the N-terminal of NTS8-13 increases its potency. In contrast, NTS1-8 and levocabastine (NTSR2 agonist) were inactive with IC50 values greater than 2000 nM. Reubi et al. [17] found a high density of specific (125I-Tyr3) NTS binding sites in Ewing’s sarcoma and medullary thyroid cancers using autoradiographic techniques. In NSCLC, NTS and NTSR1 immunoreactivity are present in approximately 60% of lung adenocarcinoma biopsy specimens [18]. NTSR1 is present in numerous cancers.
The human NTSR1 contains 418 amino acids and 7 transmembrane (TM) domains. Asn at positions 4, 37 and 41 of the NTSR1 extracellular N-terminal can be N-glycosylated whereas Cys at positions 381 and 383 of the NTSR1 intracellular C-terminal can be Spalmitoylated. SR48692 binds to a deep protein binding pocket anchored by TM6 and TM7 and numerous amino acids are essential for high affinity SR48692 binding e.g. Tyr319, Arg323, Phe326, Tyr346, Thr349, Phe353 and Tyr354 [19]. In contrast, the NT8-13-NTSR1 complex has been crystalized and NT8-13 sits on top of the NTSR1 binding pocket and binds with high affinity to Asn360, Pro361 and Tyr364 [20]. The greatest number of contacts between NTS8-13 and NTSR1 occur at extracellular loops 2 and 3 and TM domains 6 and 7. When NTSR1 is occupied by SR48692, NTS cannot bind and cause signal transduction.
NTS signal transduction
When activated the NTSR1 interacts with a G protein (Gq) causing phosphatidylinositol (PI) turnover in a phospholipase C dependent manner [21]. PI-4,5-bisphosphate is metabolized to inositol-1,4,5- trisphosphate (IP3) and diacylglycerol (DAG) which elevates cytosolic Ca2+ [22] and activates protein kinase (PK)C, respectively [23]. The PKC can cause phosphorylation of ERK, PKD, focal adhesion kinase and Src [24-27]. In a ligand independent mechanism, Src can directly phosphorylate the EGFR at Tyr845 [28]. In the triple membrane passing signal pathway, NTSR1 activation causes shedding of EGFR ligands from the plasma membrane (PM). Matrix metalloproteases (MMP) of the disintegrin and metalloproteinase (ADAM) family cleave inactive precursors e.g. the 160 amino acid prepro-TGFα in the PM into biologically active TGFα (50 amino acids) which is secreted into the extracellular fluids. The EGFR has a 621 amino acid extracellular N-terminal and subdomains II and IV are structural in nature and enriched in Cys amino acids. Subdomains I and III bind EGF, TGFα, amphiregulin or HB-EGF with high affinity. The EGFR has a single TM domain (24 amino acids) and an intracellular tyrosine kinase domain (541 amino acids) and C-terminal. The EGFR kinase domain binds ATP at Lys721 and transfers the phosphate to tyrosine amino acids on proteins such as PI-3-kinase (K), Phospholipase C and the EGFR. When gefitinib blocks the catalytic site of the EGFR, ATP cannot bind and cause the phosphorylation of Tyr992, Tyr1045, Tyr1068, Tyr1086, Tyr1148 or Tyr1173 of the EGFR. The EGFR interacts with the adapter proteins GRB2 and SHC. This activates the SOS protein leading to the metabolism of GTP by RAS. RAS activates the kinase RAF leading to the activation of MEK which phosphorylates ERK. Phosphorylated ERK enters the nucleus and increases expression of the nuclear oncogenes c-fos and c-jun after 1 hour. NTS addition to glioblastoma cells up-regulates c-myc but down regulates miR-29b-1 and miR-129-3p [29]. The c-fos and c-jun form heterodimers and increase expression of growth factor genes such as neurotensin/ neuromedin N [30].
RTK transactivation
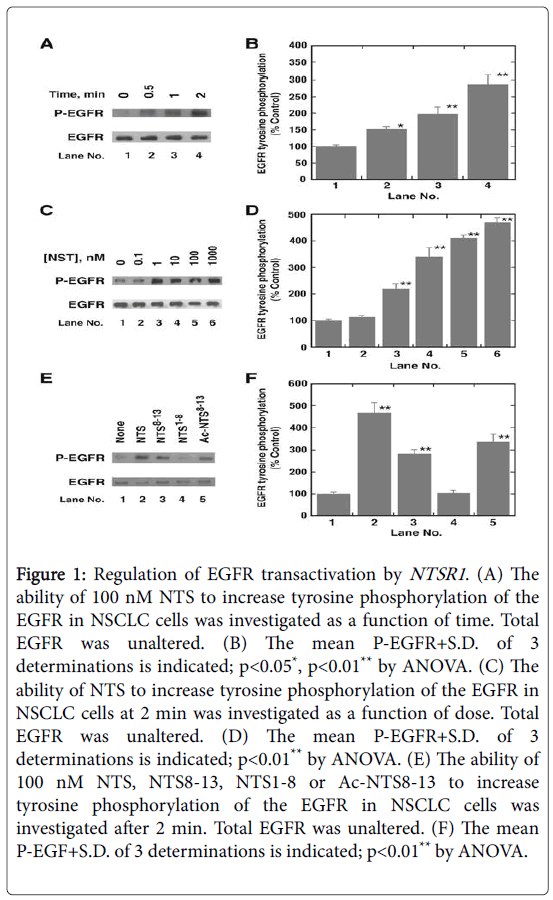
NTS analogs were added to NCI-H1299 NSCLC cells and the tyrosine phosphorylation of the EGFR determined by Western blot. (Figure 1A and 1B) shows that addition of NTS to NSCLC cells increases P-EGFR significantly after 0.5 min and the effect is maximal after 2 min. In contrast, NTS has no effect on total EGFR after 2 min. The effect of NTS on P-EGFR is dose-dependent and half-maximal tyrosine phosphorylation of the EGFR occurs using 5 nM NTS (Figure 1C and 1D). Structure-activity studies showed that NTS, Ac-NTS8-13, NTS8-13 but not NTS1-8 increase phosphorylation of the EGFR (Figure 1E and 1F). Addition of NTS to NSCLC cells for 2 min increased tyrosine phosphorylation of the EGFR 3-fold which was inhibited by SR48692, siRNA for NTSR1 and gefitinib [5]. Addition of JMV449, a NT8-13 analog, to NSCLC cells for 48 hours increased expression of EGFR, HER2 and HER3 approximately 2-fold [6]. Also, JMV449 increased P-EGFR, P-HER2 and P-HER3 which was reversed by SR48692. JMV449 increased MMP1 resulting in elevated HB-EGF and Neuregulin-1 which activate the EGFR and HER3, respectively.
Figure 1: Regulation of EGFR transactivation by NTSR1. (A) The ability of 100 nM NTS to increase tyrosine phosphorylation of the EGFR in NSCLC cells was investigated as a function of time. Total EGFR was unaltered. (B) The mean P-EGFR+S.D. of 3 determinations is indicated; p<0.05*, p<0.01** by ANOVA. (C) The ability of NTS to increase tyrosine phosphorylation of the EGFR in NSCLC cells at 2 min was investigated as a function of dose. Total EGFR was unaltered. (D) The mean P-EGFR+S.D. of 3 determinations is indicated; p<0.01** by ANOVA. (E) The ability of 100 nM NTS, NTS8-13, NTS1-8 or Ac-NTS8-13 to increase tyrosine phosphorylation of the EGFR in NSCLC cells was investigated after 2 min. Total EGFR was unaltered. (F) The mean P-EGF+S.D. of 3 determinations is indicated; p<0.01** by ANOVA.
Reactive oxygen species (ROS) are essential for NTS to cause EGFR transactivation. GPCR may cause p-47phox phosphorylation leading to the activation of NADPH oxidase increasing ROS [31]. The ROS may oxidize protein tyrosine phosphatases reducing catalytic activity resulting in a transient increase in EGFR tyrosine phosphorylation [28]. The transactivation of the EGFR regulated by NTSR1 is impaired by Tiron (superoxide scavenger) and diphenyleneiodonium (NADPH oxidase inhibitor) [5].
NTS causes EGFR transactivation in numerous cancers including prostate cancer, colon cancer and foregut neuroendocrine tumors [23,32,33]. Gastric cancer patients whose tumors had high levels on NTSR1 immunoreactivity had poor patient survival [34]. Addition of NTS to NSCLC cells caused tyrosine phosphorylation of the EGFR, Src and β-catenin in a PKC-dependent manner [5]. When β-catenin is phosphorylated, it dissociates from E-Cadherin and enters the nucleus altering gene expression of the NTSR1 . In hepatocellular carcinoma, NTS/NTSR1 co-expression is activated the Wnt/β-catenin signaling pathway enhancing epithelial to mesenchymal transitions promoting tumor metastasis [35].
Proliferation
The NTSR1 regulates the proliferation of NSCLC cells. Addition of NTS stimulated the proliferation of NSCLC cells whereas SR48692 inhibits the proliferation of NSCLC cells in a cytostatic manner (Table 1). SR48692 potentiated the cytotoxicity of gefitinib in a synergistic manner [5]. Breast cancer tumors grew faster in nude mice if they had high levels of NTS [36]. Tumors with high levels of NTS had elevated EGFR, HER2 and HER3 and their phosphorylated derivatives. The high levels of P-EGFR, P-HER2 and P-HER3 were reversed if the mice were treated with SR48692. The breast tumors with elevated NTS had increased MMP9 resulting in increased secretion of HB-EGF and Neuregulin-2. Approximately 43% of the biopsy specimens from NSCLC patients had immunostaining for NTSR1 . The survival of NSCLC patients who had high NTSR1 levels was significantly reduced relative to NSCLC patients whose tumor had low levels of NTSR1 [6]. It remains to be determined if SR48692 will potentiate the effects of TKI in NSCLC patients. The NTSR1 gene is present in many colon cancer biopsy specimens. Methylation of the NTSR1 gene is associated with increased patient survival [37]. NTSR1 expression is reduced by histone deacetylase inhibitors in colorectal cancer cell lines resulting in decreased proliferation [38]. These results indicate that NTSR1 levels can be regulated by epigenetic mechanisms.
Conclusion
Gefitinib and erlotinib are used currently to treat NSCLC patients who have failed chemotherapy and have EGFR mutations. Due to low potency TKI are not used in patients with wild type EGFR. The potency of gefitinib can be increased in cells with wild type EGFR using GPCR antagonists such as SR48692 [5]. The NTSR1 is an excellent molecular target in cancer and its expression in NSCLC, breast and gastric tumors is associated with poor patient survival.
There are multiple GPCR in cancer cells which can regulate RTK transactivation. Peptide GPCR include angiotensin, bombesin, bradykinin, cholecystokinin, endothelin, pituitary adenylate cyclase activating peptide and substance P, all of which interact with Gq and cause PI turnover [39]. RTK which can be transactivated include erbB1, erbB2, erbB3, platelet derived growth factor receptor and Trk. There are numerous GPCR antagonists which may potentiate the ability of TKI to inhibit the growth of cancer cells.
Acknowledgements
The author thanks Dr. R. Jensen for helpful discussion. This research is supported by the NCI intramural program of the NIH.
References
- Kaufman J, Horn L, Carbone D (2011) Molecular biology of lung cancer. In Cancer: principles and practice of Oncology. Pp. 789-798.
- George AJ, Hannan RD, Thomas WG (2013) Unravelling the molecular complexity of GPCR-mediated EGFR transactivation using functional genomics approaches.FEBS J 280: 5258-5268.
- Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141: 1117-1134.
- Lopes GL, Vattimo EFQ, Junior GDC (2015) Identifying activating mutations in the egfr gene: prognostic and therapeutic implications in non-small cell lung cancer. J Bras Pneumol 41: 365-375.
- Moody TW, Chen DC, Mantey SA, Moreno P, Jensen RT (2014) SR48692 inhibits non-small cell lung cancer proliferation in an EGF receptor-dependent manner. Life Sci 100: 25-34.
- Younes M, Wu Z, Dupouy S, Lupo AM, Mourra N, et al. (2014) Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib.Oncotarget 5: 8252-8269.
- Carraway R, Leeman SE (1978) The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalamus. J BiolChem 248: 6854-6861.
- Moody TW, Carney DN, Korman LY, Gazdar A, Minna JD (1985) Neurotensin is produced by and secreted from classic small cell lung cancer cells. Life Sci 36: 1727-1736.
- Zeytinoglu FN, Gabel RF, Tashjian AH, Hammer RA, Leeman SE (1980) Characterization of neurotensin production in medullary thyroid cells.ProcNatlAcadSci USA 77: 3741-3745.
- Allen AE, Carney DN, Moody TW, (1988) Neurotensin binds with high affinity to small cell lung cancer cells. Peptides 9: 57-61.
- Gulley D, Canton M, Boigegrain R, Jean G, Molimard JD, et al. (1993) Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor.ProcNatlAcadSci USA 90: 65-69.
- Moody TW, Chiles J, Casibang M, Moody E, Chan D, et al. (2001) SR48692 is a neurotensin receptor antagonist which inhibits the growth of small cell lung cancer cells.Peptides 22: 109-115.
- Valerie NC, Casarez EV, DaSilva JO, Dunlap-Brown ME, Parsons SJ, et al. (2011) Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation.Cancer Res 71: 6817-6826.
- Wang JL, Li NN, Li HN, Cui L, Wang P (2011) Pancreatic cancer bears overexpression of neurotensin and neurotensin receptor subtype-1 and SR48692 counteracts neurotensin induced cell proliferation in human pancreatic ductal carcinoma cell line PANC-1.Neuropeptides 45: 151-156.
- Kitabgi P (2010) Neurotensin and neuromedin N are differentially processed from a common precursor by prohormoneconvertases in tissues and cell lines.Results Probl Cell Differ 50: 85-96.
- Moody TW,Mayr CA, Gillespie TJ, Davis TP (1998) Neurotensin is metabolized by endogenous proteases in prostate cancer cell lines.Peptides 19: 253-258.
- Reubi JC, Waser B, Schaer JC, Laissue JA (1999) Neurotensin receptors in human neoplasm: high incidence in ewing’s sarcoma. Int J Cancer 82: 213-218.
- Alfano M, Souaze F, Dupouy S, Camilleri-Broet S, Younes M, et al. (2010) Neurotensin receptor 1 determines the outcome of non-small cell lung cancer.Clin Cancer Res 16: 4401-4410.
- Barroso S, Richard F, Nicolas-Etheve D, Reversat JL, Bernassau JM, et al. (2000) Identification of residues involved in neurotensin binding and modeling of the agonist binding site in neurotensin receptor 1.J BiolChem 277: 328-366.
- White JF, Noinaj N, Shibata Y, Love J, Kloss B, et al. (2012) Structure of the agonist-bound neurotensin receptor. Nature 490: 508-513.
- Dupouy S, Mourra N, Doan WK, Gompel A, Alifano M, et al. (2011) The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie 93: 1369-1378.
- Staley J, Fiskum G, Davis TP, Moody TW (1989) Neurotensin elevates cytosolic calcium in small cell lung cancer cells. Peptides 10: 1217-1221.
- Muller KM, Tveteraas IH, Aastum M, Odegard J, Dawood M, et al. (2011) Role of protein kinase C and epidermal growth factor receptor signaling in growth stimulation by neurotensin in colon carcinoma cells. BMC Cancer 11: 421-432.
- Lee LF, Guan J, Qiu Y, Kung HJ (2001) Neuropeptide induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src and focal adhesion kinase. Mol Cell Biol 21: 8385-8397.
- Guha S, Rey O, Rozengurt E (2002)Neurotensin induces protein kinase C-dependent protein kinase D activation and DNA synthesis in human pancreatic carcinoma cell line PANC-1. Cancer Res 62: 1632-1640.
- Leyton J, Garcia-Marin L, Jensen RT, Moody TW (2002) Neurotensin causes tyrosine phosphorylation of focal adhesion kinase in lung cancer cells. Eur J Phrmacol 442: 179-186.
- Kisfalvi K, Guha S, Rozengurt E (2015) Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol 202: 880-890.
- Wang Z (2016) Transactivation of epidermal growth factor receptors by G protein-coupled receptors: recent progress, challenges and future research. Int J MolSci 17: 95.
- Ouyang Q, Chen G, Zhou J, Li L, Dong Z, et al. (2016) Neurotensin signaling stimulates glioblastoma cell proliferation by upregulating c-Myc and inhibiting miR-29b-1 and miR-129-3p. NeuroOncol 18: 216-226.
- Harrison RJ, McNeil GP, Dobner PR (1995) Synergistic activation of neurotensin/neuromedin N gene expression by c-Jun and glucocorticoids: Novel effects of Fos family proteins. MolEndocrinol 9: 981-993.
- Cattaneo F, Guerra G, Parisi M, Marinis MD, Tafuri D, et al. (2014) Cell-Surface receptors transactivation mediated by G protein-coupled receptors. Int J MolSci 15: 19700-19728.
- Hassan S, Dobner PR, Carraway RE (2004) Involvement of MAP kinase, PI-3 kinase and EGF-receptor in the stimulatory effect of neurotensin on DNA synthesis in PC3 cells. RegulPept 120: 155-166.
- DiFlorio A, Sancho V, Moreno P, Fave GD, Jensen RT (2013) Gastrointestinal hormones stimulate growth of foregut neuroendocrine tumors by transactivating the EGF receptor. BiochimBiophysActa 28333: 573-582.
- Zhou Z, Xie J, Cai Y, Yang S, Chen Y, et al. (2015) The significance of NTR1 expression and its correlation with β-catenin and EGFR in gastric cancer. DiagnPathol 10: 128.
- Ye Y, Long X, Zhang L, Chen J, Liu P, et al. (2015) NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 7: 70303-70422.
- Dupouy S, Doan VK, Wu Z, Mourra N, Liu J, et al. (2014) Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget 5: 8235-8251.
- Kaminae S, Yamamoto E, Kai M, Niinuma T, Yamano HO, et al. (2015) Epigenetic silencing of NTSR1 is associated with lateral and noninvasive growth of colorectal tumors. Oncotarget 6: 29975-29990.
- Wang X, Jackson LN, Johnson SM, Wang Q, Evers BM (2010) Suppression of neurotensin receptor type I expression and function by histone deacetylase inhibitors in human colorectal cancers. Mol Cancer Ther 9: 2389-2398.
- Moody TW, Berenguer BN, Nakamura T, Jensen RT (2016) EGFR transactivation by peptide G protein-coupled receptors in cancer. Curr Drug Targ 17: 520-528.
Relevant Topics
- Agriculture & Horticulture
- Biological science or Life science
- Biomedical Technology
- Bioscience
- Clinical Science
- Environmental Science
- Food Science & Technology
- Health Science
- Imaging Science
- Medical Science
- Nuclear Science
- Ontogeny
- Pharmaceutical Science
- Physical Science
- Physiological Science
- Psychological Science
- R&D
- Social Science
- Veterinary Science & Medicine
Recommended Journals
Article Tools
Article Usage
- Total views: 3092
- [From(publication date):
March-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 2203
- PDF downloads : 889