Research Article Open Access
Efficacy of Osteolaemus tetraspis Pituitary Gland (APG) Hormone on Induced Spawning of Clarias gariepinus
Jemerigbe Richard1, Lucky E2 and Anthony O3*1College of Education, Warri, Delta State, Nigeria
2Ambrose Alli University, Department of Zoology, Ekpoma, Edo State, Nigeria
3College of Agricultural Technology, Agenebode, Edo State, Nigeria
- *Corresponding Author:
- Anthony O
College of Agricultural Technology
Agenebode, Edo State, Nigeria
Tel: +2348065284479; +2348079548388
E-mail: anthonyoyase@gmail.com
Received Date: March 01, 2016 Accepted Date: March 18, 2016 Published Date: April 07, 2016
Citation: Richard J, Lucky E, Anthony O (2016) Efficacy of Osteolaemus tetraspis Pituitary Gland (APG) Hormone on Induced Spawning of Clarias gariepinus. J Fisheries Livest Prod 4:177. doi: 10.4172/2332-2608.1000177
Copyright: © 2016 Richard J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The experiment was conducted to ascertain efficacy of Osteolaemus tetraspis Pituitary Gland (APG) hormone on induced spawning of fish. The research work was carried out between January and May 2008 at the Nigeria Institute of Oceanography and Marine Research (NIOMR) Sapele. Thirty-nine fish samples of Clarias gariepinus (36 females and 3 males) and three alligators were used. The means weight of the fish was 800 ± 20 g while that of the alligator was 5.0 ± 0.2 k g. Three replicate trials were done to observe spawning activities using three different doses, (0.5 ml, 1.0 ml and 2.0 ml) of acetone -dried APG Ovulation was recorded after 11-13 hours post-injection. Eggs were obtained by stripping and fertilized artificially with milt from the male Clarias and incubated in plastic bowls. Hatching occurred within 20-26 hours after fertilization at a water temperature of 25-26 degree centigrade. Fertilization and hatching percentages increased (p < 0.05) with increase in hormone dosage. ANOVA of the means of hatching did not show any significant different in the dosage used. The overall breeding performances of the APG hormone were found to be satisfactorily. Experiment on standardization of the APG hormone is however recommended.
Keywords
Cultivable; Spawning; Riverine; Vertebrate; Chorionic
Introduction
The availability of adequate quality of fish seed of cultivable species is the most important requirement for the development of fish culture. In time past, riverine spawning accounted for the availability of fish seeds. As time went on, fish farmers experienced difficulty in obtaining required amount of pure “seed” as the number and quality seed deteriorate due to over-fishing and various environmental problems (e.g. garbage, oil pollutants, gas flaring etc.) causing havoc with the natural habitat.
In the last few decades effort have been made by fishery scientists to overcome the pressing problem of acute shortage of quality fish seed by evolving induced breeding methods. Induced breeding as the name implies entails “forcing” a fish to spawn out of season or an unusual time. Induced breeding requires some ovulating agent, the most common and effective being fish pituitary extracts. Beside, Desoxycorticosterone Acetate popularly known as DOCA, Human Chorionic Gonadotropin (HCG), extracted from the urine of pregnant women, Luteinizing hormone etc. [1] have been used. Among other vertebrates, toad pituitary extracts have been used [1].
Recently however, new generation synthetic drugs e.g. ovopel, ovatide, ovaprim etc. have been used for induced breeding.
According to Ayinla [2] and Sukumasavin [3] fish reproduction is generally initiated by environmental factors such as temperature, rainfall, water quality, photoperiod, food quality, as well as food availability. The fish receives the signal in form of neural input through the brain and interprets them, in order to determine whether the environmental conditions are suitable for spawning [4]. Under culture conditions, the required environmental factors may not be available or may not persist for sufficient length of time for spontaneous maturation of gonads to occur.
This has led to the development of induced reproduction where pituitary hormones or synthetic agents are injected into the fish. This stimulates natural gonadotropin surge, by-passing the environmental variables of temperature, rainfall, photoperiod etc.
Though induced spawning of fish have a history of nearly 60 years old, this technology has had a very slow pace of development in Nigeria. Despite the wide gap between fish demands of 1.5 million metric tons and supply 80,000 metric (tons) [5] in Nigeria today, it has been established that hormone induced spawning could have a favorable impact on the Nigeria effort towards self sufficiency in the production of the much needed animal protein. According to Oladosun (personal communication) induced breeding in Nigeria was pioneer by Payam fish farm in Jos in early 60’s. There is a wealth of literature available on induce breeding of various fish species [2,6-8].
Use of Osteolaemus tetraspsis pituitary hormone extract does not seem to have been documented in literature. Hence no record of previous use in different seasons as this was done for only one season.
Basis of induce breeding
The reproductive cycle of almost all fishes is regulated by environmental stimuli. Appropriate sensory receptors send the environmental stimuli to the brain in the form neural inputs. This neural information, stimulate the appropriate portion of the brain to stimulate the pituitary gland to release gonadotropic hormones which influence the gonads, The gonads in turn produce sex steroidal hormones responsible for the formation of gametes, regulation of secondary sexual characters and breeding behavior, This process provide the basis for induce reproduction.
Justification of the study
This study is aimed at providing alternative source of hormone for inducing fish to spawn easily, making Osteolaemus tetraspis (i.e dwarf crocodile) most suitable for this experiment after slaughtering and flesh eaten as a protein source.
Aims of the study
Examine the efficacy of Osteolaemus tetraspis hormone for induce spawning of Clarias gariepinus; ascertaining physiological response of Clarias gariepinus to Osteolaemus tetraspis pituitary hormone and evaluating the correlation between conventional hormone (control) and the experimental hormone.
Materials and Methods
Study area
This study was carried out between January-May 2008. The brood stocks of Clarias gariepinus of known breeding records were obtained from the Nigerian Institute of Oceanography and Marine Research (NIOMR) fish farm, Sapele. Sapele is a town in Sapele Local Government Area of Delta State, Nigeria Figure 1. Sapelelies between longitude 50 46”E and latitude 50 50”N of the Equator (Wikipedia) and situated on the bank of the Ethiope River. The Ethiope River forms a boundary between Sapele and Ethiope West Local Government Area of Delta State, Nigeria.
Live dwarf crocodile (Osteolaemus tetraspis) sample was purchased from commercial hunters landing at Koko on market days. Koko is a town in Warri North Local Government Area of Delta State, Nigeria. It lies between latitude 60 N and longitude 5024”E (Wikipedia). Koko is situated on the bank of the Benin River and it is one of Nigeria’ s Seaport. The plains are always subjected to flooding annually whenever the river overflows its bank during the raining season (May to October).
Study population
This comprise of Clariasgariepinus (plate 2) and Dwarf crocodile (Osteolaemus tetraspis) an amphibious reptile (plate 1).
Sample collection and maintenance
Broodstock fish of Clarias gariepinus: A total of thirty-nine (36 females and 3 male) active live and apparently healthy Clarias spp. fish sample of 790-820 g (mean wt 800 g) having fresh skins were obtained from the Nigerian Institute of Marine and Oceanography Research fish farm in Sapele as spawners. The fishes were transported to the laboratory in the morning in two plastic buckets containing little quantity of fresh water. The small quantity of water prevented the fishes from jumping out or fighting and causing injury to each other.
The fish samples were identified using the taxonomy keys by Reed et al. [9], Babatunde and Aminu [10]and Elakhame [11]. The identified samples were then labeled in triplicates.
Osteolaemus tetraspis: This was bought from local traders at Koko who landed them from the wild. It weighed 5.0 kg and had a total length of 1.2 m. A small stick was placed across the upper and lower jaw of the mouth of the dwarf crocodile. The two jaws were then tied with twine/string to prevent the dwarf crocodile from biting /causing injury/embarrassment during handling. Its legs were also tied behind dorsally to demobilize the animal during handling.
The specimen was identified using the keys of Kofron [12] and Adam [13].
Extraction of Osteolaemus tetraspis pituitary gland: This extraction was done according to the methodology of Rottmann, et al. [14].
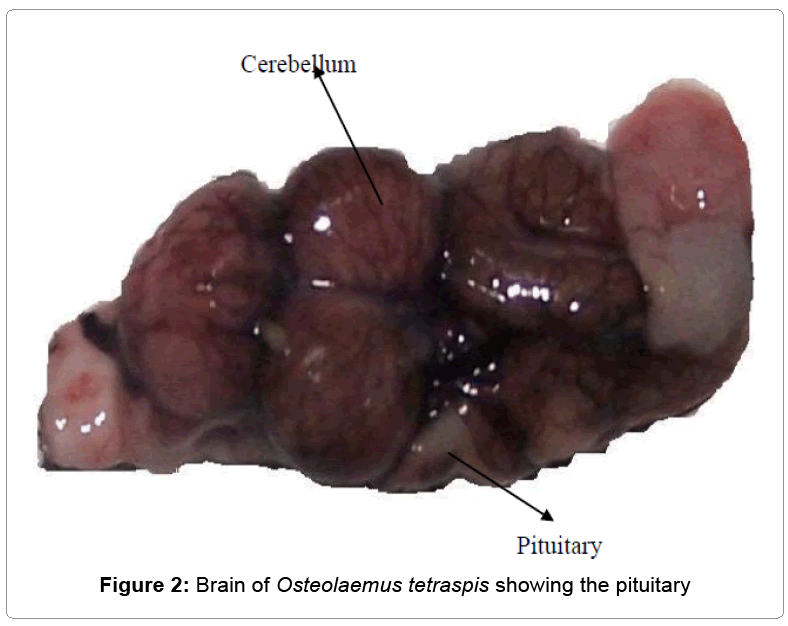
a) Preparation of hormone: At the time to be used, the acetone-dried pituitary gland (ADPG) was weighed with a metler analytical (Toledo) balance having a sensitivity of 0.0000 g. The pituitary gland extract (PGE) weighed 78.00 mg. The gland was grinded with a laboratory mortar and pestle and mixed thoroughly with 12 ml. of 0.9% normal saline to produce a uniform suspension (Figures 1 and 2).
b) Hormone injection: The hormone injection was done in the night at 9.30 pm while the selection and weighing of spawners were done during the day. Only the female fishes were given hormone injection. The grinded Osteolaemus tetraspis pituitary gland extract was sucked up with a 2.0 ml (0.1 ml calibration) hypodermal syringes fitted BDH No.22 needle as suggested by Kumar [8] and Gupta and Gupta [4].
The female fishes were injected intramuscularly at an angle of 45 degree to the body just below their dorsal fins in the region of the caudal penduncle. Three spawners designated as Clg1. Clg2 and Clg3 were injected with 0.5 ml/5.57 mg of PGE, each. Another three spawners designated as Clg4, Clg5 and Clg6 were injected with 1 ml/11.14 mg each while another three Clg7, Clg8 and Clg9 were injected with 2 ml/22.28 mg of PGE each to see if the Osteolaemus tetraspis pituitary extract can induce spawning in Clariasgariepinus. Three other spawners Clg10, Clg11 and Clg12 as control were injected with 0.5 ml each of Ovaprim (standardized inducing agent). The prevailing water temperature was 25 -26 degree centigrade. The injected female fishes were returned to their holding bowls. The male spawner was not injected since milt cannot be obtained by stripping. The injected spawners were now left till morning (Figure 3).
c) Milt collection: Prior to stripping of the female spawners, milt was collected from the male spawner. The male fish was dealt a blow to the head with a stick which stunned it. This ensured that the fish was relaxed during surgical operation. The fish was opened up on the ventral side using a surgical blade. The testes were located and gently pulled and cut out. Small incision was made on the testes. The milt was carefully squeezed out into a clean dry glass Petri-dish. The milt was then preserved in 0.9% sodium chloride solution as soon as they were collected.
d) Egg collection: This was by stripping of the female fishes. The latency period was 10-11 hours after injection. Prior to stripping of the female spawners, the stage of egg maturation was checked by pressing gently on the abdomen and observing the nature of the eggs [15]. The spawners were removed one after the other and their urinogenital regions were washed with clean water. Each spawner was held on a dry clean napkin with one hand toward the head and the other to the middorsal region. The eggs were then stripped in turn into plastic bowl and transferred with plastic spoon to glass Petri-dish for weighing. Ovulated eggs flowed out freely. Only 1 g of stripped eggs from each spawner was used. The spent female fish was returned to the holding bowl.
e) Fertilization: Few drops of milt were added to the stripped eggs to effect fertilization from a syringe and the two were mixed by gently shaking the glass Petri-dish for 1-2 minutes (Figure 4). Sperm activation was initiated by the addition of 5 ml fresh water from the incubation trough and checked for mobility by microscopic examination. The fertilization rate was assessed by counting the number of fertilized opaque and unfertilized white eggs respectively.
f) Egg Incubation: Prior to stripping of eggs and fertilization, 12 plastic tanks( 52 cm X 33 cm X 20 cm depth) were thoroughly washed clean for the incubation. Fine mesh mosquito nets to serve as hatching tray were also washed clean and placed inside the plastic tanks containing clean fresh well water. The fertilized eggs were spread evenly in a single layer on the hatching nets inside the tanks. Hatching occurred 20-26 hours after incubation at a temperature range 24-26 degree centigrade. The mosquito net ensures fry survival, as the yolk fry were able to swim through the mesh opening into the tank while their egg shell and dead eggs remain on the net. The dead eggs were siphoned out. Hatching success was later evaluated by counting the number of fry hatched out of the fertilized eggs [16-18].
g) Fry counting: This was by direct counting of the hatched fry. The eggs that did not hatch were also counted.
h) Pre-trial experiment: Three pre-trial experiments were designed and conducted.
Statistical Analysis
The raw data obtained were pooled from the treatments and compared by one way Analysis of Variance (ANOVA) and t-test to determine significant differences.
Results
The results obtained in the present study are presented herein. For the purpose of clarity, the results are presented in subsections.
Weights
The average weights of the fishes Clarias gariepinus, Osteolaemus tetraspsis and the Osteolaemus tetraspsis pituitary glands were 800 g, 5.0 k g and 78 mg respectively.
Trial observations
Three trial observations were carried out and the results are presented in the tables that follows:
In trial observation I
For each dosage, three clarias spp. were injected. 0.5 ml dosage gave an average of 48% hatchability.
2.0 ml dosage gave 81% hatchability while
1.0 ml dosage gave 76% hatchability.
In trial observation II, for each dosage, three Clarias sp. of fish were also injected. The percentage hatchability for trial I and II were also recorded as shown in Table 1.
• 0.5 ml, dosage gave an average of 50% hatchability.
• ml dosage gave an average of 89% hatchability while 2.0 ml dosage gave an average of 94% hatchability.
• 0.5 ml dosage of the control gave an average of 93% hatchability.
| Hormone dosage | Hatched Eggs | |
|---|---|---|
| T1 | T2 | |
| 0.5ml | 384 | 400 |
| 1.0ml | 608 | 712 |
| 2.oml | 648 | 752 |
| 0.5ml (control) | 656 | 744 |
Table 1: Comparison of mean values of hatchability of Clarias gariepinus in trial observation I and II.
When 0.5 ml dosage was administered in both trials, 384 eggs were hatched in trial 1 while in trial II, 400 eggs were hatched. When 1.0 ml dosage was administered, 608 eggs were hatched in trial 1 while in trial II, 712 eggs were hatched. When 2.0 ml dosage was administered, 648 eggs were hatched in trial 1 and 752 eggs were hatched in trial II.
Latency Period
This is the time interval between injections of the hormone solution and ovulation of the eggs. It was observed that eggs flow took up to 13 hours in trial I when the temperature was about 24°C. In trial II eggs flowed after 11 hrs when the temperature was 25.5°C (Table 2)
Table |
0.5ml/kg | 1.0ml/kg | 2.0ml/kg | Control (0.05ml) |
|---|---|---|---|---|
| 1 | 384 | 608 | 648 | 656 |
| II | 400 | 712 | 752 | 744 |
Table 2: Summary of mean Hatching results.
The time interval between fertilization and hatching also called incubation period depends on water temperature. The incubation period decreases with increases in temperature
Table 3 shows that the calculated t-value, of 36.952 is higher than the critical value of 2.78 at 0.05 level of significance of hatchability. Based on this result, the hypothesis was therefore rejected. This implies that there is significant difference in concentration of the hormone between the PGE and fecundity.
| Variables | N | X | SD | DF | Cal.t | Cri-t |
|---|---|---|---|---|---|---|
| 0.5ml/kg | 3 | 4 | 2 | 4 | 36.952 | 2.78 |
| 0.5ml/kg | 3 | 7.44 | 16 |
Table 3: Means, standard deviations and t-value of 0.5 ml PGE and 0.5 ml ovaprim(control).
In Table 4 it shows that the calculated t-value, of 1.337 is lower than the critical value of 2.78 at 0.05 level of significance of hatchability. Based on this result, the hypothesis was therefore accepted. This implies that there is no significant difference between the concentration of the hormone at 1.0 ml of PGE and Ovaprim (control).
| Variables | N | - | SD | DF | Cal.t | Cri-t |
|---|---|---|---|---|---|---|
| X | ||||||
| 1.0ml/kg | 3 | 6.0667 | 177.249 | 4 | 1.337 | 2.78 |
| 0.5ml/kg (Control) | 3 | 7.44 | 16 |
Table 4: Mean standard deviations and t-valve of 1.0 ml PGE and 0.5 ml ovaprim (control).
In Table 5 it shows that the calculated t-value, of 1.028 is lower than the critical value of 2.78 at 0.05 level of significance of hatchability. Based on this result, the hypothesis was therefore accepted. This implies that there is no significant difference between the concentration of the hormone at 2.0 ml of PGE and
| Experiment | N | X | SD | DF | Cal.t | Cri-t |
|---|---|---|---|---|---|---|
| 2.0ml/kg | 3 | 7.54 | 5.2915 | 4 | 1.028 | 2.78 |
| 0.5ml/kg(control) | 3 | 7.44 | 16 |
Table 5: Mean, standard deviations and t-valve of 2.0 ml PGE and 0.5 ml ovaprim (control).
Ovaprim (control)
The Null Hypothesis Ho: The hatchability of the three dosages (0.5 ml, 1.0 ml and 2.0 ml) are the same.
Alternate Hypothesis H1: The hatchability of the three dosages (0.5 ml, 1.0 ml and 2.0 ml) are not the same.
ANOVA: Single Factor
At 0.5 ml, 1.0 ml and 2.0 ml volume of hormone used, 2.0 ml generated the highest mean value of 701, followed by 1.0 ml volume of 659.5. The least number of hatched fry was 392 from 0.5 ml. However, at 95% confidence level, there was significant difference (Fo 0.05 [2,17]) among the level of hatchability of the various volume of the hormone. There was a significant difference in the mean value of x hatched between 1.0 ml and 2.0 ml of the hormone. Also, significant difference occur in the mean value of eggs hatched between 0.5 ml and 2.0 ml. The mean difference was also significant at 0.05 level for 0.5 ml and 1.0 ml of the hormone.
Consequently, optimum yield of hatchable eggs was observed at 2.0 ml of the inducing hormone. Thus indicative of the ideal volume necessary for hatching of eggs with the inducing hormone. Other factors such as temperature, quality of the water etc are also of very important consideration [19].
Discussion
Osteolaemus tetraspsis pituitary gland extract under the present study successfully induced spawning in catfish Clariasgariepinus. Only the catfish Clarias gariepinus have been subjected to this hormone. Other inducing agents like, ovopel, vatide and ovaprim are well documented in literature. Osteolaemus tetraspsis pituitary hormone for induced spawning has not been found in literature. However apart from fish, only amphibians (toad) pituitary hormone amongst the lower vertebrates have been successfully used for induced spawning of fish [4].
In the experiment undertaken, 0.5 ml, 1.0 ml and 2.0 ml of the gland extract solution were injected into the trial female fish (avg. wt 800 g) in a single dose in each replicate fish samples used. Through the study, it was found that Osteolaemus tetraspsis pituitary hormone induced spawning in the replicate fish samples with a response time varying between 11 hours to 13 hours (temperature 24°C). The trial also revealed that the 2.0 ml dose appears to be the optimum quantity at the given concentration as the eggs freely ooze out and litred the water of the fish holding bowl (Table 6).
| N6 | Mean | Std. Deviation | Std. Error | 95% confidence interval for mean | Minimum | maximum | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| 0.5ml | 6 | 392 | 8.94427 | 3.65148 | 382.6136 | 401.3864 | 382 | 402 |
| 1.0ml | 6 | 659.5 | 56,09,902 | 22.90233 | 600.6277 | 718.3723 | 606 | 714 |
| 2.0ml | 6 | 701 | 58.20996 | 23.76412 | 639.9124 | 762.0876 | 644 | 758 |
| Total | 18 | 584.1667 | 147.6475 | 34.80086 | 510.7433 | 657.5901 | 382 | 758 |
Table 6: Summar Mean Hatch.
The overall hatching percentage achieved were 50%, 89% and 94% for the 3 trial samples. The low hatching percentage may be due to several factors, such as the conditions of brood fish selected for breeding is very important as no hormone or hatchery can induce breed fish unless the condition of brood fish is good [5]. Through t-test, significant difference was recorded in number of quality eggs laid/kg body weight and in hatching percentage of the trial sample with PGE dosage of 0.5 ml against the control. There were no significant difference recorded in t-test in the number of quality eggs laid/kg body weight and in hatching percentage of the trial sample with PGE dosage of 1.0 ml and 2.0 ml against the control. However fertilization and hatching percentages increased (p < 0.05) with increases in hormone dosage. Total quality eggs spawned and fertilizations and hatching rates also did not differ significantly among the trial samples as revealed in the ANOVA. Gupta and Gupta [4] reported higher percentage hatchability with ovaprim.
The number of eggs hatched when the dosage 0.5 ml, 1.0 ml and 2.0 ml respectively were administered during each trial. It has been observed that the quantity of eggs hatched in any species of Clarias in 1 gm is 800 eggs (Adams, personal communication) Table 7.
| SS | df | MS | F | P-Value | F crit | |
|---|---|---|---|---|---|---|
| Between Groups | 337519 | 2 | 168759.5 | 76.529136 | 1.348E-07 | 3.6823167 |
| Within Groups | 33077.5 | 15 | 2205.1667 | |||
| Total | 370596.5 | 17 |
Table 7: Anova.
In a pre-trial experiment were three Clarias gariepinus fish specimens were injected. No hatching was recorded for each of the administered dosage after the time period of observation. The probable reason for this no hatching could be:
(i) Due to human error in broodstock selection.
(ii) Inadequate or inappropriate extraction of the hormone (pituitarygland
(iii) That the quantity administered may not be enough to induce hatchability.
Conclusion
 Through the present study, Osteolaemus tetraspsis pituitary hormone successfully induced spawning in the catfish Clariasgariepinus.
Through the present study, Osteolaemus tetraspsis pituitary hormone successfully induced spawning in the catfish Clariasgariepinus.
 Use of Osteolaemus tetraspsis pituitary hormone extract does not seem to have been documented in literature.
Use of Osteolaemus tetraspsis pituitary hormone extract does not seem to have been documented in literature.
 Conventional synthetic hormone like ovaprim appears to have an edge over Osteolaemus tetraspsis pituitary hormone as it is already in solution and ready for use unlike Osteolaemus tetraspsis hormone which require fresh extractions.
Conventional synthetic hormone like ovaprim appears to have an edge over Osteolaemus tetraspsis pituitary hormone as it is already in solution and ready for use unlike Osteolaemus tetraspsis hormone which require fresh extractions.
 Osteolaemus tetraspsis hormone quality needs confirmations experiment.
Osteolaemus tetraspsis hormone quality needs confirmations experiment.
 A 5 kg Alligator was able to induce 9 number of C. gariepinus of average weight of 800 g.
A 5 kg Alligator was able to induce 9 number of C. gariepinus of average weight of 800 g.
Recommendations
Further studies are required to evaluate the growth performance of induced Clariagariepinus produced with Osteolaemus tetraspsis pituitary hormone and the conventional synthetic hormone-ovaprim. Experiment on standardization of the Osteolaemus tetraspsis pituitary extract will also be required.
References
- Madu CJ (1986) Fish seed Production In: Fisheries Enterprises and Information brochure Kanji lake resource Inst. New Bussapp 27-34.
- Ayinla OA (1991) Spawning of selected culturable fish species. In: Proc. of the Fish seed propagation course, African Regional Aquaculture Center, Port Harcourt, Nigeria,pp: 25-39.
- Sukumasavin N (2002) Technical Group, Inland Fisheries research and Development Bureau. Department of Fisheries India.
- Gupta SK, Gupta PC (2006) General and Applied Ichthyology S Chand and Company, Ram Nagar, New Delhi. pp:1133.
- FGC (2005) Federal Government of Nigeria Report of Presidential Committee on Fisheries and Aquaculture Development. Consolidated Report. Federal Department of Fisheries. Federal Ministry of Agriculture and Rural Development.
- Rothbard S, Proginin Y (1975) Induced spawning and artificial incubation of Tilapia. Aquaculture 5: 315-321.
- Lam TJ (1982) Application of Endocrinology to Fish culture. Can J Fish Aquaculture Sci 39:111-137.
- Das SK (2004) Evaluation of a new spawning agent, Ovopel in induced breeding of Indian carps. Asian fisheries science 17: 313-322.
- Reed WJ, Hopson AJ, Jonnes J, Yara I (1967) Fish and Fisheries of Northern Nigeria (1stedn). Ministry of Agriculture, Northern Nigeria, Zaria.
- Olaosebikan D, Raji A (1998) Field guide to Nigeria freshwater Fishes, Federal College of Freshwater Fisheries Technology, New Bussa Niger State, Nigeria.
- Elakhame LA (2004) Freshwater fishes of the Niger-Delta, BOPECO Printer and Publisher Pp:10-39.11
- Kofron P, Steiner C (1994) Observations on the African dwarf Crocodile, Ostedaemustetraspsis, copeia: 533-535.
- Adam Britton (2009) Crocodilians nature history and conservation In. Crocodilian Species-Dward Crocodile (Osteolaemustetraspsis).
- Rottmann RW, Shireman JV, Chapman FA (1991) Techniques for taking and Fertilizing the Spawn of Fish. Southern regional Aquacultural Center.
- ElzbietaB (2003) Artificial Spawning of female Polish line 3 Carp (Cyprinuscarpio L.) after treatment with pituitaryhomogenate and / or Ovopel. Aquaculture Research Pub 34: 1321-1327.
- BromageNR, Roberts RJ (1995) Broodstock Management and egg larvaquality, Oxford, Blackwell Scientific Publicationspp: 424.
- Bruska E (1999) Artificial Spawning of herbivorous fish: Use of LHRH-a to induce ovulation in grass carp Ctenopharyngodonidella(Valenciennes) and silver carp Hypophthamichthysmolitrix (Valenciennes).Aquaculture Research Pub30: 849.
- InyangNH, HettiarachichiM (1994) Efficacy of human choronicgonatro (HCG) and crude extract of fish and frog in oocyte maturation and ovulation in African catfish Clariasgariepinus. Aquaculture fish management 25: 245-255.
- LegendreM (1986) Seasonal changes in sexual maturity and fecundity and human choronic gonadotropin (HCG) induced breeding of the catfish Heterobranchuslongifilis val.(claridae) reared in Ebrie Lagoon (Ivory Coast) Aquaculture 55: 201-213
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 14592
- [From(publication date):
September-2016 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 13522
- PDF downloads : 1070