Efficacy of Medications in Space Determined By Raman Spectroscopy: Aspirin Degradation
Received: 05-Sep-2022 / Manuscript No. jabt-22-75216 / Editor assigned: 07-Sep-2022 / PreQC No. jabt-22-75216 / Reviewed: 21-Sep-2022 / QC No. jabt-22-75216 / Revised: 23-Sep-2022 / Manuscript No. jabt-22-75216 / Accepted Date: 29-Sep-2022 / Published Date: 30-Sep-2022
Abstract
Medication is the primary defense against the deleterious effects that astronauts experience in space due to weightlessness, such bone loss and headaches. Recent studies of medications returned to earth from the International Space Station suggest that high radiation levels of solar particle events (SPE) and galactic cosmic rays (GCR) can degrade medications, and potentially render them inadequate during a long mission, such as a roundtrip journey to Mars. In an effort to predict medication shelf-lives, proton irradiation at ground-based laboratories has been used to simulate SPE and GRC conditions, as quantified by high-performance liquid chromatography. Unfortunately,such predictions do not take into account extreme SPEs and/or CGRs that can occur during such long duration missions.We believe that medications, the active pharmaceutical ingredient, as well as the potentially toxic degradants, can be tested on-board space crafts at the time of use by a compact Raman spectrometer. Here we present the forced degradation of an aspirin tablet by acid hydrolysis, representing the high energy protons that make up ~85% of the SPEs and CGRs.Raman analyses of this tablet, and 10 expired twelve-year-old aspirin tablets and their toxic degradant, salicylic acid,are presented.
Keywords
Drug degradation; Aspirin; Acid hydrolysis; Raman spectroscopy; Space radiation; Mars mission
Introduction
Long term weightlessness and radiation exposure are important considerations for future manned missions to the moon, near Earth asteroids (e.g. Eros), and Mars [1-5]. These deep space conditions can adversely affect human physiology in several ways, such as causing loss of bone and muscle mass, space motion sickness, cephalad fluid shifts increasing cranial pressure, sleep deprivation, reduced immune system response, and increased risk of cancer [6-14]. A medical kit has been developed for the International Space Station (ISS) to treat these physiological changes or their symptoms [15,16]. In effect, the kit drugs represent the first-line of defense for maintaining the health of astronauts [17]. The value of these drugs is limited to the period that they maintain 90% of their potency, as the active pharmaceutical ingredient (API) will degrade over time, usually by heat and moisture driven hydrolysis, potentially forming harmful chemicals. Unfortunately, a previous study of drugs flown on the ISS suggests that the rate of degradation for these drugs may be accelerated in space [18], most likely due to interaction with the radiation of solar particle events (SPEs) and galactic cosmic rays (GCRs). It is estimated that this combined radiation is 3.3 times greater in deep space, at ~0.7 mGy/day [19], compared to that on the ISS at an orbit of ~420 km, which is within the Earth’s magnetosphere at >1000 km. The latter deflects most of the SEPs and GRCs towards the Earth’s poles. Consequently, a corresponding increase in drug degradation must be considered for deep space missions. In an attempt to simulate SPEs and GCRs that are 85 to 90% high energy protons [20], ground-based studies have employed high energy particle colliders to bombard drugs with such protons [21]. These studies have confirmed degradation of active pharmaceutical ingredients (APIs) by as much as 17% [22]. To complicate matters, the degradation products of some drugs can be toxic. For example, aspirin, one of the most often used drugs by astronauts on the ISS, degrades into salicylic acid and acetic acid, both toxic substances [23-25]. Indeed, aspirin tablets returned from the ISS after 550 days in orbit contained salicylic acid [26]. One must also consider the existence of unusual SPEs and GRCs, such as the 1989 solar storm that produced a dose of ~35Gy/hour [27], ~50,000 times the normal flux, which could degrade all of the medication. Therefore, it is critical to know the degradation status of the medical kit drugs, as well as the degradants formed, in the deep space environment.
Pharmaceutical companies identify drug degradants during the process of establishing expiration dates. This involves tests that accelerate the degradation of the API, such as heat, hydrolysis, oxidation, and photolysis, which are used separately and in combination [28]. The degradants are often quantified by high performance liquid chromatography (HPLC) [29,30], and if they represent a significant percentage of the API, they are identified using any number of chemical analyzers, such as liquid chromatography coupled mass spectrometry and nuclear magnetic resonance spectroscopy [31,32]. While these analyzers are capable of identifying and monitoring most degradants, they require significant sample preparation, are massive, use consumables and considerable power, making them unsuitable for long space missions [33]. Raman spectroscopy overcomes these limitations, in that a laser is simply focused into the sample to generate its Raman radiation, which is collected by the spectrometer for analysis. Furthermore, the method is non-destructive, so that after analysis, a drug tablet can be used. Previously, we demonstrated that Raman spectroscopy could be used to determine the percent of a drug’s API and its primary degradant in artificial mixtures of two components [34]. This approach serves a dual purpose: determining the potency,i.e. the usefulness of the drug, while also determining if it is safe for crewmember use. However, the success of this approach requires that at the very least, the primary degradant and its Raman spectrum be known. Unfortunately, the Raman spectra of many drug degradants are unmeasured. We believe that acid hydrolysis is the simplest terrestrial method to simulate drug degradation caused by the high radiation of space, since SPE and GCR are composed largely of high energy protons. Albeit, this approach does not account for the energetics of protons or other atoms traveling at near light speeds. Here we demonstrate the ability of Raman spectroscopy to measure the extent of forcibly degraded aspirin by acid hydrolysis (Figure 1), and apply the approach to 10 twelve-year-old aspirin tablets. We chose aspirin for this study because it is one of the most common drugs used in the world and by astronauts to treat pain [16], and its degradants are well known for their role in causing stomach ulcers [20,21].
Materials and Methods
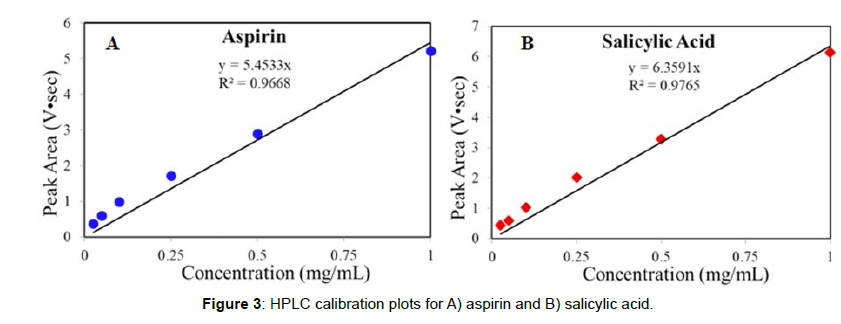
USP grade aspirin, salicylic acid, and reagent grade acetic acid, acetonitrile, de-ionized water, phosphoric acid, sulfuric acid, and trifluoroacetic acid were purchased from Sigma Aldrich (St Louis, MO). The CareOne aspirin tablets were purchased from a local pharmacy. They were 375 mg tablets, composed of 325 mg aspirin and 50 mg of the following inactive ingredients: dicalcium phosphate dihydrate, glycerol triacetate, hypromellose, starch and talc. One cm diameter, ~400 mg reference tablets of aspirin and salicylic acid were prepared using a hydraulic press that produced 1500 psi (SpecAc, UK). All Raman spectroscopic measurements were performed using a LabRaman Analyzer (Real-Time Analyzers, Inc.). The analyzer employed a 1064 nm laser, a diffraction grating and a 265 channel InGaAs array detector covering a 100 to 2300 cm-1 spectral region at ~14 cm-1 resolution. Tablets were placed in a machined plate designed to hold various size and shape tablets. The plate was mounted above the laser on an XY positioning stage (Conix Research, Springfield, OR). A cover above the plate provided eye safety and stray light rejection. Either 3x3 or 9x9 grids of ~200 μm diameter laser focal points, spaced over a 4x4 mm2 section of a tablet, were measured using the XY stage. Each spectrum employed 280 mW at each point, and consisted of 10-averaged, 4-sec integrations, taking ~6 min or ~1 hour for the two grids, respectively. The spectra were analyzed using S-Quant software (RTA, Figure 2). “Heat” maps, indicating these concentrations, were prepared using Excel software. The HPLC analysis was performed using a Shimadzu LC-10ATvp equipped with a SPD-10AVvp UV-VIS detector with D2 and W lamps and a 254 nm detector (Kyoto, JP). A Supelco C18 5 μm, 15 cm x 4.6 mm column was selected to satisfy the USP L phase requirement (Sigma-Aldrich). The column was heated to 35°C and conditioned first using a 50:50 v/v acetonitrile/DI water solution for 30 min, and second with the mobile phase consisting of 65/35 v/v water/ acetonitrile with 0.1% trifluoroacetic acid. The flow rate and duration of the conditioning solvents were 0.5 mL/min for 15 min, followed by 1.0 mL/min for an additional 15 min. A stock solution of aspirin and salicylic acid was prepared at 1.0 mg /mL in the mobile phase, which was further diluted to prepare 0.025, 0.05, 0.10, 0.25, 0.50, and 1.0 mg/mL calibration samples. Twenty μL of these calibrants were injected into a 10 μL loop that delivered each one into the column at 1 mL/min flow rate. The calibrants were measured in triplicate, and their average HPLC peak areas were used to prepare the calibration plots (Figure 3).
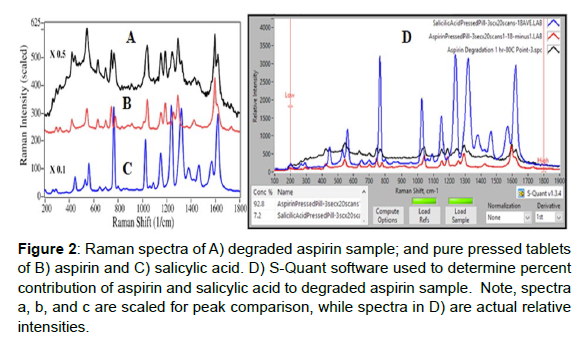
Figure 2: Raman spectra of A) degraded aspirin sample; and pure pressed tablets of B) aspirin and C) salicylic acid. D) S-Quant software used to determine percent contribution of aspirin and salicylic acid to degraded aspirin sample. Note, spectra a, b, and c are scaled for peak comparison, while spectra in D) are actual relative intensities.
Each Care One tablet, previously measured by Raman spectroscopy, was weighed, crushed by mortar and pestle, and placed in a 20 mL glass vial with 32.5 mL of the mobile phase. Each sample was then vortexes and sonicated for 20 min to completely dissolve the powder. After sonication, these samples were injected into the HPLC and measured as described for the calibration samples.
Results and Discussion
Raman Spectral Analysis
The Raman spectrum of aspirin is dominated by the low frequency phenyl C-H out-of-plane bending modes at 745 and 780 cm-1, C-H inplane bending modes at 1040 and 1190 cm-1, a C-O stretch at 1295 cm-1, and two C=C stretches at 1600 and 1616 cm-1, with the latter being a shoulder of the former (Figure 4A). The dominant salicylic acid peaks are a C-H bend at 770 cm-1, a C-H in-plane bending mode at 1030 cm-1, a C-OH stretch at 1240 cm-1, an OH bend at 1320 cm-1, and two C=C stretches at 1570 and 1620 cm-1 (Figure 4B). Acetic acid is dominated by a very intense peak at 895 cm-1, due to the C-C symmetric stretch (Figure 4C) [35,36]. As this peak does NOT appear in any of the spectra, acetic acid was not included in the spectral analysis. The absence of acetic acid is expected, as it is a liquid at room temperature (melting point is 16.7oC), and it is considered highly volatile (vapor pressure is 2.07KPa at 25oC) [37].
Acid Hydrolysis of Aspirin
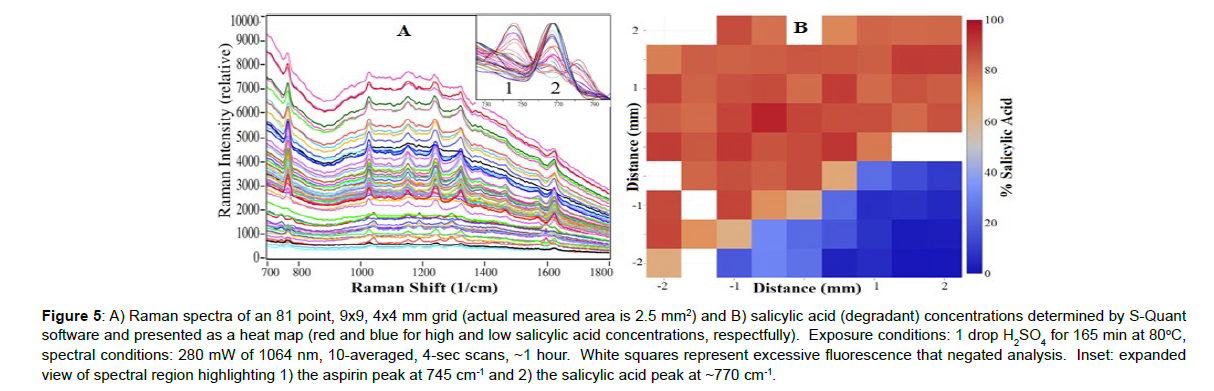
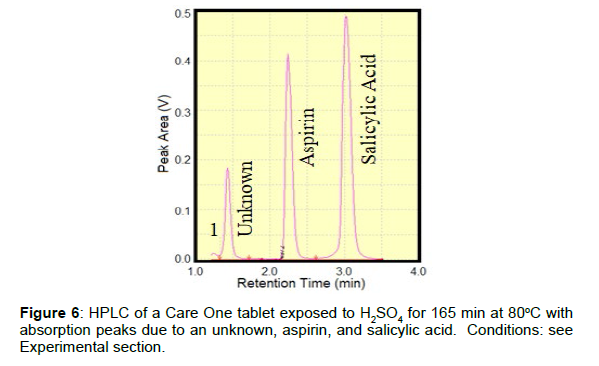
Sulfuric acid was used to accelerate the degradation of several aspirin tablets to form salicylic acid and acetic acid (Figure 1). The acid concentration, temperature and time were used to control the rate of degradation (1 to 5M H2SO4, 40 to 100oC, and 30 to 180 min, respectively). The challenge was to degrade the tablets sufficiently to measure the salicylic acid, but not so much that the tablet turned black, which produces a spectral background that obscures the Raman peaks. Of the various arrangements tried, adding 1 drop of 5M H2SO4 to the tablet surface and placing it in the oven at 80oC for 165 min degraded ~50% of the tablet. A region of a CareOne aspirin tablet, showing modest and severe degradation, prepared according to these conditions, was identified, and mapped in a 4x4 mm, 81 point, 9x9 grid. The 700 to 1800 cm-1 region contained a high spectral background, which was removed by taking the first derivative using the S-Quant software. The software was then used to determine the aspirin and salicylic acid percent concentrations by fitting each spectrum with the pure reference tablet spectra, and setting the total to100%. The S-Quant software was used to fit the first-derivative of the 700 to 1800 cm-1 region to remove the high spectral background (Figures 5A). While the average of all 81 spectra yielding 42.5% aspirin and 57.5% salicylic acid, the map showed that the extent of degradation was divided into two distinct regions (Figure 5B). This was due to selective dispensing of the acid on ~1/2 of the tablet surface. Note that fluorescence overwhelmed the Raman peaks for several of the 81 individual spectra and therefore they could not be analyzed. These spectra were not included in the S-Quant analysis, and are represented as white squares in the point-by-point heat map. The same degraded Care One tablet was then analyzed by HPLC (Figure 6). The peak areas in mg/mL, determined using a calibration plot, were again divided by the sum to produce aspirin and salicylic acid percent concentrations. The values of 42.8% (0.50 mg/mL) and 57.2% (0.67 mg/mL), respectively, were nearly identical to the Raman values, consistent with the goal of degrading 50% of the tablet. While a third peak in the chromatograph with a retention time of 1.4 min could logically be acetic acid, the other chemical component part of aspirin degradation, it does not absorb light at the HPLC detector wavelength, and must be due to one of the inactive ingredients or its degradant.
Figure 5: A) Raman spectra of an 81 point, 9x9, 4x4 mm grid (actual measured area is 2.5 mm2) and B) salicylic acid (degradant) concentrations determined by S-Quant software and presented as a heat map (red and blue for high and low salicylic acid concentrations, respectfully). Exposure conditions: 1 drop H2SO4 for 165 min at 80oC, spectral conditions: 280 mW of 1064 nm, 10-averaged, 4-sec scans, ~1 hour. White squares represent excessive fluorescence that negated analysis. Inset: expanded view of spectral region highlighting 1) the aspirin peak at 745 cm-1 and 2) the salicylic acid peak at ~770 cm-1.
Measurement of Expired Aspirin Tablets
Once the ability to detect and quantify both aspirin and salicylic acid in the same Raman spectrum was established, the approach was applied to a CareOne tablet with a Sept 2010 expiration date. The tablet was mapped as before, in a 2x2 mm, 81 point, 9x9 grid. Again, the first-derivative spectra were fit with the pure aspirin and salicylic acid spectra, but since the baseline was flat, the lower spectral region of 200-1800 cm-1 was included (Figure 7).
Figure 7: Eighty one Raman spectra for a 2x2 mm, 9x9 grid of a Care One tablet, expiration 9/2010 for A) the 200-1800 cm-1 and B) 660-860 cm-1 regions. Note that spectrum 1 contains 99.6% aspirin, while spectrum 2 contains 22.8% salicylic acid. Conditions: 280 mW at 1064 nm, ten 4-sec integrations per point, requiring ~1 hour. C) False color point-by-point aspirin concentration map of the Care One tablet.
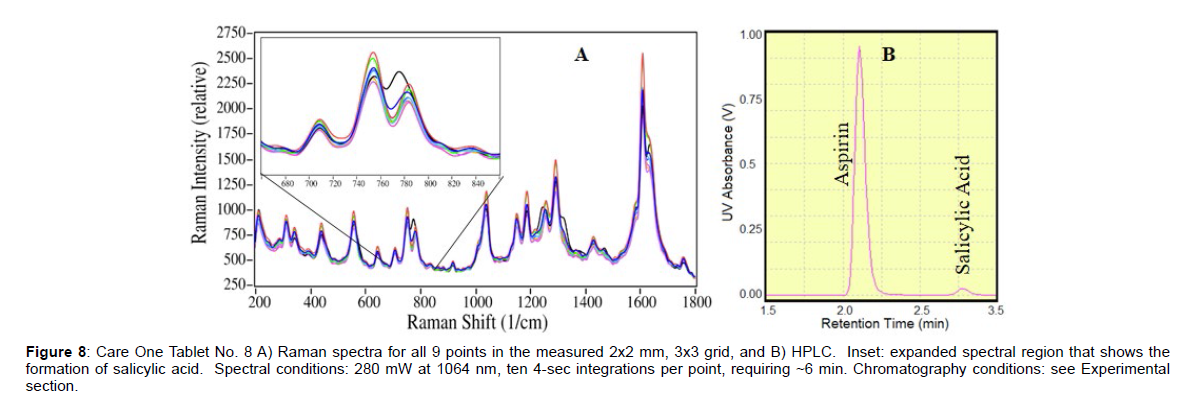
Figure 8: Care One Tablet No. 8 A) Raman spectra for all 9 points in the measured 2x2 mm, 3x3 grid, and B) HPLC. Inset: expanded spectral region that shows the formation of salicylic acid. Spectral conditions: 280 mW at 1064 nm, ten 4-sec integrations per point, requiring ~6 min. Chromatography conditions: see Experimental section.
| %Aspirin | % Salicylic Acid | |||
|---|---|---|---|---|
| Tablet No | Raman | HPLC | Raman | HPLC |
| 1 | 96.4 | 95.1 | 3.6 | 4.9 |
| 2 | 93.7 | 94.3 | 6.3 | 5.7 |
| 3 | 97.3 | 34.7 | 2.7 | 5.3 |
| 4 | 97.7 | 95.0 | 2.3 | 5.0 |
| 5 | 97.7 | 95.0 | 2.3 | 5.0 |
| 6 | 96.4 | 94.4 | 3.6 | 5.6 |
| 7 | 95.8 | 94.3 | 4.2 | 5.7 |
| 8 | 94.6 | 95.0 | 5.4 | 5.0 |
| 9 | 94.7 | 94.2 | 5.3 | 5.8 |
| 10 | 95.2 | 94.5 | 4.8 | 5.5 |
| Ave | 96.0 | 94.6 | 4.1 | 5.4 |
| StDev | 1.39 | 0.35 | 1.39 | 0.35 |
Table 1:Calculated percent aspirin and salicylic acid concentrations for 10 tablets by Raman (2x2 mm, 9 point, 3x3 grid) and HPLC.
Conclusion
This preliminary investigation demonstrates that Raman spectroscopy can be used as a straightforward method to measure drugs degraded by acid hydrolysis with the ultimate goal of 1) using the spectra to quantify the API and degradant concentrations, and 2) determining if a drug tablet is safe to use. As a test case, an aspirin tablet was degraded by adding 1 drop of 5M H2SO4 to its surface and heating it at 80°C for 165 minutes to produce nearly 50% salicylic acid, which was quantified by fitting the degraded sample spectrum with spectra of pure aspirin and salicylic acid. The Raman analysis method was then successfully used to quantify the degradation of 10 twelveyear- old aspirin tablets that on average contained 96.0 ± 1.4% aspirin, which compared favorably to the HPLC analysis of 94.6 ± 0.35%. While these concentrations suggest that the tablets are safe for use, i.e. the API was greater than 90%, the presence of ~5% salicylic acid, suggest that may not be safe. This analysis demonstrates the clear need to not only measure the API, but also its primary degradants to determine safe use. While acid hydrolysis represents the common environmental degradation of drugs by heat and humidity, we also believe it has great potential in simulating drug degradation caused by the high radiation of space, composed largely of protons. Future work will develop a compact Raman spectrometer for the analysis of drugs flown on the ISS, with the goal of using such an analyzer on future missions to evaluate drugs at the time of use.
Acknowledgement
The authors are grateful to the National Aeronautics and Space Administration (NNX14CA08C and NNX16CA16C).
References
- Williams DR (2003) The biomedical challenges of space flight. Annu Rev Med 54: 245-256.
- Williams D, Kuipers A, Mukai C, Thirsk R (2009) Acclimation during space flight: effects on human physiology. CMAJ 180: 1317-1323.
- Cucinotta FA (2014) Space radiation risks for astronauts on multiple International Space Station missions. PlOS One 9: 1-14.
- Kerr R (2013) Radiation will make astronauts’ trip to Mars even riskier. Science, 340: 1031.
- Garrett-Bakeman FE, Darshi M, Green SJ, Gur RC, Lin L, et al. (2019) The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 364: 6436.
- Blaber E, Dvorochkin N, Lee C, Finkelstein H, Dvorochkin N, Yet al. (2013) Microgravity induces pelvic bone loss through osteocloastic activity, osteocytic osteolysis, and osteoblastic cell cycle inhibition by CDKN1a/p21. PLOS One 8: 1-11.
- Muth ER (2006) Motion and space sickness: intestinal and autonomic correlates. Auton Neurosci 129: 58-66.
- Wiener TC (2012) Space obstructive syndrome: intracranial hypertension, intraocular pressure, and papilledema in space. Aviat Space Environ Med 83: 64-66.
- Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, et al. (2011) Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118: 2058-2069.
- Vein AA, Koppen H, Haan J, Terwindt GM, Ferrari MD, et al. (2009) Space headache: A new secondary headache. Cephalalgia 29: 683-686.
- Roberts DR, Albrecht MH, Collins HR, Chatterjee AR, Spampinato MV, et al. (2017) Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377: 1746-1753.
- Barger LK, Flynn-Evans EE, Kubey A, Rondaet JM, Wang W, et al. (2014) Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol 13: 904-91.
- Gueguinou N, Huin-Schohn C, Bascove M, Jean-Pol Frippiat, Legrand-Frossiet C, et al. (2009) Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth's orbit. Leukocyte Bio 86: 1027–1038.
- Barcellos-Hoffa MH, Blakely EA, Burma S, Weil MM, Shay J, et al. (2015) Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci Space Res 6: 92-103.
- Putcha L, Berens KL, Marshburn TH, Orteg HJ, Billica RD, et al. (1999) Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat Space Environ Med 70: 705–708.
- Wotring VE (2015) Medication use by US crewmembers on the International Space Station. FASEB J 29: 4417-4423.
- Wotring VE (2014) Monitoring physiology during space flight. Proc SPIE 9112-25.
- Du B, Daniels VR, Vaksman Z, Boyd JL, Cready C, et al. (2011) Evaluation of physical and chemical changes in pharmaceuticals flown on space missions. The AAPS J 13: 299-308.
- Zeitlin C, Hassler DM, Cucinotta FA, Reitz G, Rafkin S , et al. (2013) Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 340: 1080-1084.
- Ambosi TI (1998) Physics of the Space Environment Cambridge University Press.
- Blue RS, Chancellor JC, Antonsen EL, Bayuse TM, Daniels VE, et al. (2019) Limitations in Predicting Radiation-Induced Pharmaceutical Instability during Long-Duration Spaceflight. Nature Microgravity 5:15.
- Bhayani D, Mehta P, Patel M, Naik H, Nathaniel TN, et al. (2022) Ground-based selected ionizing space radiation effects on stability of APIs and their formulations. J Pharm Biomed Anal 220:115019.
- Rachel L, Chin MD, Olson KR, Dempsey D (2007) Salicylate Toxicity from Ingestion and Continued Dermal Absorption. Cal J Emerg Med 8: 23-25.
- El-Magbri M (2014) The synthesis and analysis of aspirin. J Chem Ed 2: 1-7.
- Okabe S, Amagase K (2005) An overview of the acetic acid ulcer model: the history and state of the art of peptic ulcer research. Biol Pharm Bull 28: 1321-1341.
- Wotring VA (2016) Chemical potency and degradation products of medications stored over 550 earth days at the International Space Station. AAPS J 18: 210-216.
- David B (2019) A 21st Century View of the March 1989 Magnetic Storm. Space Weather. AGU J 17:1427-1441.
- International Conference on Harmonization (2003) Stability testing of new drug substances and products Q1A(R2).
- Torriero AAJ, Luco JM, Sereno L, Raba J (2004) Voltammetric determination of salicylic acid in pharmaceutical formulations of acetylsalicylic acid. Talanta 62: 247-254.
- Sornchaithawatwong C, Vorrarat S, Nunthanavanit P (2010) Simultaneous determination of paracetamol and its main degradation product in generic paracetamol tablets using reverse-phase HPLC. J Health Res 24: 103-106.
- Wu Y (2000) The use of liquid chromatography-mass spectrometry for the identification of drug degradation products in pharmaceutical formulations. Biomed Chromatogr 14: 384-396.
- Maggio RM, Calvo NL, Vignaduzzo SE, Kaufman TS (2014) Pharmaceutical impurities and degradation products: Uses and applications of NMR techniques. J Pharmaceut Biomed Anal 101: 102-122.
- Antonsen EL, Mulcahy RA, Rubin D, Blue RS, Canga MA, et al. (2018) Prototype development of a tradespace analysis tool for spaceflight medical resources. Aerospace Med Hu. Perform 89: 108-114.
- Shende C, Smith W, Brouillette C, Farquharson S (2014) Drug stability analysis by Raman spectroscopy. Pharmaceutics 6: 651-662.
- Turi L, Dannenberg JJ (1993) Molecular orbital study of acetic acid aggregation. Monomers and dimers. J Phys Chem 97: 12197-12204.
- Socrates G (2001) Infrared and Raman Characteristic Group Frequencies 3rd Ed.
- DR Lide (1966-1967) CRC Handbook of Chemistry and Physics, 77 Ed. :6-81.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Farquharson S, Gladding Z, Brouillette C, Smith W, Farquharson A, et al. (2022) Efficacy of Medications in Space Determined By Raman Spectroscopy: Aspirin Degradation. J Anal Bioanal Tech 10: 472.
Copyright: © 2022 Farquharson S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2324
- [From(publication date): 0-2022 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 1986
- PDF downloads: 338