Efficacy of Intragastric Balloons for the Treatment of Obesity-a Systematic Review and Meta-analysis
Received: 22-Mar-2022 / Manuscript No. JOWT-22-58057 / Editor assigned: 24-Mar-2022 / PreQC No. JOWT-22-58057(PQ) / Reviewed: 07-Apr-2022 / QC No. JOWT-22-58057 / Revised: 12-Apr-2022 / Manuscript No. JOWT-22-58057(R) / Published Date: 19-Apr-2022 DOI: 10.4172/2165-7904.1000486
Abstract
Background: Intragstric balloons (IGB) are space-occupying devices that are inserted endoscopically into the stomach and removed after approximately 6 months for the treatment of obesity. IGBs are associated with short-term weight loss while having the advantage of preserving the normal anatomy of the stomach. The long-term efficacy of IGB on weight loss is still questioned. .
Objectives: To determine the short- and long-term efficacy of IGB for the treatment of obesity.
Methods: A systematic review and meta-analysis of the weight changes and BMI changes in patients who underwent an IGB procedure for obesity treatment was conducted. Articles that reported the mean and standard deviation of BMI and weight, and the number of patients before IGB insertion and at the time of IGB removal were selected. The shortterm outcomes were assessed from the IGB insertion to its removal. The Long-term outcomes were assessed from six months and beyond from removal of the IGB. The comprehensive literature search was performed using search engines, PubMed, and other sources. The methodological index for non-randomized studies (MINORS) was used to assess the methodological quality of the studies. Guidelines and protocols as per the "PRISMA guidelines" were adhered to during the systematic review and meta-analysis.
Results: A total of 27 articles were reviewed for the systematic review. The total number of patients at the time of IGB insertion was 4400. The short-term treatment effect of the IGB on obesity was assessed by meta-analysis of 15 articles. The observed standardized mean differences ranged from 0.2949 to 1.5596, with most estimates being positive (100%). The estimated average standardized mean difference based on the random-effects model was 0.7540 (95% CI: 0.5546 to 0.9535). Therefore, the average outcome differed significantly from zero (z = 7.4106, p < 0.0001).
A total of six studies were included in the analysis to assess the long-term effect of IGB treatment on obesity. The observed standardized mean differences ranged from -0.3239 to 0.0000, with most estimates being negative (83%). The estimated average standardized mean difference based on the random-effects model was -0.0961 (95% CI: -0.2113 to 0.0190). Therefore, the average outcome did not differ significantly from zero (z = -1.6364, p = 0.1018).
Conclusion: Intragastric balloons are effective in the treatment of obesity in the short-term. The meta-analysis did not show a beneficial effect on the treatment of obesity after removal.
Keywords: Obesity; Intra-gastric balloon; Bio-enteric intra-gastric balloon; Gastric balloon
Introduction
Obesity is defined as an abnormal or excessive fat accumulation in the body or a body mass index of more than 30 [1]. Over the past few decades, obesity has become a global epidemic [2]. The current data indicates that one-third of the world population is suffering from obesity and 5% of deaths are due to obesity [2]. If the current trend persists, by 2030, almost half of the world’s adult population will be affected by obesity [2]. The rates of obesity are higher in Saudi Arabia than worldwide; a study in 2016 suggested that 69.7% of the Saudi population are obese [3,4].
Obesity increases the risk of heart disease, diabetes and breathing problems such as sleep apnea; therefore, the need for effective treatments for the prevention and treatment of obesity is essential in order to stop the alarming increase in obesity [3,4].
In terms of the treatment for obesity, there are many treatment options, including surgical and medical. Laparoscopic gastric banding, sleeve gastrectomy and Roux-en-Y gastric bypass are a few of the surgical treatment options for obesity. Surgical treatment tends to come with more side effects [5,6]. Non-surgical treatments can be either pharmacologic, such as diethylpropion, sibutramine or orlistat [7], or the intragastric balloon (IGB) [8]. The IGB is a minimally invasive, non-surgical alternative [8].
Various studies and meta-analyses have reported the efficacy of the IGB in the short-term treatment of obesity [9,10]. A study from the Middle East treated 1600 obese patients with the bio-enteric-IGB, and the results of this study showed that 49.3% of patients lost > 10% of their weight, 24.7% lost > 20%, while the remaining 26.0% lost < 10% [11].
However, the literature review of various studies published on the long-term effects of the IGB have not consistently shown a decrease in weight. In some studies, weight regain was observed after the removal of the IGB. On the other hand, in a few studies, weight remained stable after the removal of the IGB [12-19].
To get a clearer understanding of the role of the IGB in the shortand long-term treatment of obesity, we conducted a systematic review and meta-analysis with the following objectives. The first objective was to conduct a systematic review of articles that studied obesity treatment with the IGB. The second objective was to conduct a meta-analysis of clinical trials that studied short- and long-term effects of the IGB for obesity management and met the inclusion and exclusion criteria for the meta-analysis.
Methods
Search strategies
The comprehensive literature search was performed using search engines including PubMed, EMBASE, Cochrane review and Web of science. For the systematic review, we included research articles that provide information on weight change or BMI changes before and after IGB insertion. We included articles published in the English language only.
Eligibility criteria
The most important criterion for a study to be qualified for the meta-analysis was the availability of data on mean weight and BMI and their standard deviations, and the number of patients studied. The above results should be available for both the time of IGB insertion and its removal for the short-term benefit analysis. For the meta-analysis of long-term effects, the data should be available from the point of IGB removal until a minimum of 6 months after removal. We included prospective, retrospective, randomized and non-randomized clinical trials for both the systematic review and meta-analysis.
We excluded articles that are letters, reviews, or guidelines, or that reported an IGB treatment duration of over 12 months.
Statistical analysis
Meta-analysis was performed using the software Jamovi [20] Guidelines and protocols from the "PRISMA guidelines" were adhered to during the systematic review and meta-analysis.
Selection and data collection process
The search engine used for the literature search was PubMed. We used the keywords “intragastric balloon” OR “balloon” for database screening. Then, we used filter options in PubMed such as “obesity,” “clinical trial,” English language, and time interval starting from January 1st, 2000 through August 31, 2019. For any additional relevant articles added from 2001 onwards, a new database search was performed on June 1st, 2021.
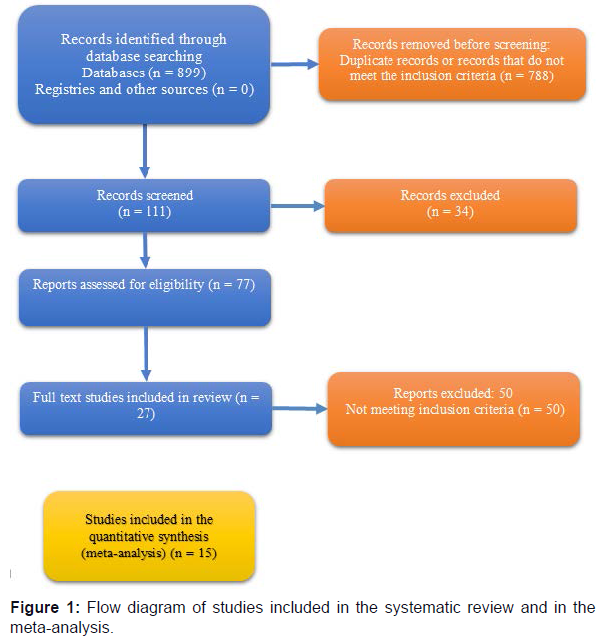
The PubMed search using the keyword “intragastric balloon” or “balloon” resulted in 8748 articles. Finally, we thoroughly studied 27 articles. The articles that provided weight or BMI values before and after removal of the IGB were used for the meta-analysis of the short-term benefits of IGB in obesity treatment. We performed a meta-analysis of the long-term effects of the IGB for the treatment of obesity using the articles that provided weight and BMI results at least 6 months after the removal of the IGB.
The selection process of the articles was carried out independently by three authors. The initial screening process involved reading the titles and abstracts to identify relevant research papers that met the inclusion criteria. Then, we retrieved the full-length articles. We resolved disagreements in the selection of studies through discussion with the primary author and reviewers.
Outcomes
For the systematic review, we studied the following weight-loss outcomes:
- Body mass index (BMI) from before IGB insertion to post IGB removal, i.e., at 6 months and 12 months. Change in BMI = initial BMI - post IGB removal BMI.
- Percent of total weight loss 6 months and 12 months after IGB removal. Percent of total weight loss = [(initial weight) - (post IGB removal weight)]/ [initial weight] × 100
- Percent excess BMI loss 6 months and 12 months after IGB removal. Percent excess BMI loss = [(initial BMI) - (post IGB removal BMI)]/ (initial BMI) - 25] × 100
- Percent excess weight loss = [(initial weight) - (post IGB removal weight)]/ [(initial weight) - (ideal weight)] × 100. Ideal weight is the weight corresponding to a BMI of 25 kg/m²
The short-term outcomes were measured from the IGB insertion to its removal. The Long-term outcomes were assessed from six months and beyond after removal of the IGB.
Outcomes of the meta-analysis of weight and the BMI change: Finally, we performed the meta-analysis of the weight and BMI change. We included an article in the meta-analysis of the short-term efficacy of IGB if the mean and standard deviation of BMI and weight, and the number of patients before IGB insertion and at the time of IGB removal were available. Similarly, we included the article in the longterm efficacy analysis of IGB if the above data were available after 6 months and 12 months of IGB removal.
Study risk of bias assessment
We used a methodological index for non-randomized studies (MINORS) to assess the methodological quality of non-randomized surgical studies, whether comparative or non-comparative [21].
The revised and validated version of MINORS contains a total of 12 items. From this, eight items are for the assessment of methodological items for non-randomized studies, and there are four additional criteria for comparative studies. The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for non-comparative studies and 24 for comparative studies.
One of the authors performed the risk of bias assessment independently and the assessment was then verified by the other authors.
Effect measures
We used the statistical software Jamovi to identify the effect measures [20]. The analysis was carried out using the standardized mean difference as the outcome measure. A random-effects model was fitted to the data.
The studies included in the meta-analysis are shown in Tables 1 and 2 and in the forest plot. Meta-analysis was performed for the changes in weight from insertion to removal, and from removal to 6 months post removal.
Statistical heterogeneity
The amount of heterogeneity (tau²) was estimated using the restricted maximum-likelihood estimator (Viechtbauer 2005). In addition to the estimate of tau², the Q-test for heterogeneity (Cochran 1954) and the I² statistic are reported. Where any amount of heterogeneity is detected (i.e., tau² > 0, regardless of the results of the Q-test), a prediction interval for the true outcomes is also provided.
Reporting bias assessment
Studentized residuals and Cook’s distances are used to examine whether studies may be outliers and/or influential in the context of the model. Studies with a studentized residual larger than the 100 × (1 - 0.05/(2 × k))th percentile of a standard normal distribution are considered potential outliers (i.e., using a Bonferroni correction with two-sided alpha = 0.05 for k studies included in the meta-analysis). Studies with a Cook’s distance larger than the median plus six times the interquartile range of the Cook’s distances are considered influential. The rank correlation test and the regression test, using the standard error of the observed outcomes as a predictor, are used to check for funnel plot asymmetry.
Results
Systematic review
A total of 27 articles were reviewed for the systematic review, published between 2004 and 2017. Twelve of the studies were prospective, five were retrospective, one was a double-blind comparative study, and the last was a case-control study. Figure 1 is a flow diagram of the selection process of the articles. Baseline characteristics of the studies with their publication details are given in Table 1.
| ARTICLE ID | PUBLICATION YEAR | JOURNAL | TITLE | FIRST AUTHOR | DESIGN | COUNTRY WHERE STUDY WAS CONDUCTED |
|---|---|---|---|---|---|---|
| 2 | 2012 | Obes Surg | Short- and long-term efficacy of intragastric air-filled balloon (Heliosphere® BAG) among obese patients. | M. Giuricin [30] | Prospective | Italy |
| 3 | 2005 | Obes Surg | What becomes of patients one year after the intragastric balloon has been removed? | J Herve [28] | Prospective | Belgium |
| 9 | 2005 | Gastrointest Endosc | Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. | Elisabeth MH Mathus-Vliegen [19] | Randomized, double-blind trial | 2 US, 1 Netherlands |
| 10 | 2017 | Surgical, laparoscopy Endoscopy & Percutaneous Techniques | Assessment of weight loss with the intragastric balloon in patients with different degrees of obesity | Gabriel C nunes [31] | Retrospective | Brazil |
| 12 | 2013 | Obesity biology and integrated physiology | An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study | Nicholas R. Fuller [32] | Randomized controlled | Australia |
| 13 | 2009 | Srugery Endoscopy | Laparoscopic sleeve gastrectomy versus intragastric balloon: a case-control study | Alfredo Genco [33] | Case-controlled | Italy |
| 15 | 2010 | Obesity surgery | Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study | Maria Luisa De Castro [34] | Double blind comparative study | Spain |
| 18 | 2004 | Obesity surgery | Brazilian multicenter study of the intragastric balloon | Jose a sallet [35] | Prospective | Brazil |
| 19 | 2012 | Obes Surg. 2012 Jun;22(6):896-903. | 500 intragastric balloons: what happens 5 years thereafter? | Katerina Kotzampassi [29] | Prospective? | Greece |
| 21 | 2013 | Turk J Gastroenterol 24(5): 387-91 | Long-term effectiveness of BioEnterics intragastric balloon in obese patients | Yüksel Gümürdülü [18] | Prospective | Turkey |
| 22 | 2010 | Obesity surgery | Bio-enteric intragastric balloon in obese patients: a retrospective analysis of King Faisal Specialist Hospital experience | Khalid Al Kahtani [36] | Retrospective | Saudi Arabia |
| 24 | 2008 | Obesity surgery | Intragastric balloon or diet alone? a retrospective evaluation | Alfredo Genco [16] | Retrospective | Italy |
| 25 | 2009 | Endoscopy | Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years | S negrin dastis [16] | Prospective | Belgium |
| 27 | 2013 | Obesity surgery | Effectiveness of intragastric balloon treatment for obese patients: one-year follow-up after balloon removal | Chi-ming tai [37] | Prospective | Taiwan |
| 28 | 2016 | Obesity surgery | Intragastric balloon device: weight loss and satisfaction degree | Silvia Palmisano [26] | Prospective | Italy |
| 29 | 2017 | International journal of obesity | Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial | A Courcoulas [24] | Randomized controlled | USA |
| 31 | 2009 | Obesity surgery | Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis | Nicola Crea [14] | Prospective | Italy |
| 33 | 2013 | Obesity surgery | Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance | Umit Bilge Dogan [17] | Prospective? | Turkey |
| 34 | 2015 | Videosurgery and Other Miniinvasive Techniques | In search of the ideal patient for the intragastric balloon – short- and long-term results in 70 obese patients | Kryspin Mitura [13] | Prospective | Poland |
| 35 | 2017 | Portuguese Journal of gastroenterology | Intragastric balloon for obesity treatment: safety, tolerance, and efficacy | Joana Ribeiro da Silva [38] | Prospective | Portugal |
| 36 | 2017 | World journal of clinical cases | Efficacy of intragastric balloon on weight reduction: Saudi perspective | Ebtissam Saleh Almeghaiseeb [39] | Retrospective | Saudi Arabia |
| 37 | 2013 | Obesity surgery | Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience | Alfredo Genco [40] | Retrospective | Italy |
| 43 | 2008 | Effectiveness, safety, and tolerability of intragastric balloon in association with low-calorie diet for the treatment of obese patients | Escudero Sanchis A [41] | |||
| 44 | 2011 | Obesity surgery | Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity |
Farina, MG [42] | ||
| 45 | 2014 | Surgery for Obesity and related diseases | Long-term multiple intragastric balloon treatment—a new strategy to treat morbid obese patients refusing surgery: Prospective 6-year follow-up study |
Alfredo G [43] |
||
| 52 | 2015 | Videosurgery | Tolerance of intragastric balloon and patient’s satisfaction in obesity treatment | Mitura K [15] |
Table 1: Baseline characteristics of the studies and their publication details.
Thirteen of the studies were from Europe, two from the USA, two from Saudi Arabia, two from Turkey, one from Australia and one from Taiwan. Nineteen of the studies used a bio enteric IGB that is filled with saline, and in three studies, heliospheres that are filled with air were used.
The total number of patients at the time of IGB insertion was 4400, with the number of patients per study ranging from 18 to 2002. The total number of cases at IGB removal was 3474, with a minimum of 18 and a maximum of 1016. Seventeen trials did not have a control arm and four trials did. All of the studies we analyzed kept the IGB in the stomach for 6 months.
Six months after IGB removal, 2312 patients were available for follow-up. The total number of patients available for data analysis 12 months after IGB removal was 767.
There were significantly more female patients than males; the IGB treatment was received by 2363 females and 665 males. The IGB treatment was received by 72.5% of female patients compared to 27.44% of males. The average age of the patients who had IGB in the trials was 39.2 years, with a range of 34 to 45 years.
Weight
The mean weight of the patients at the time of IGB insertion was 113 kg, with a standard deviation of 16.2 kg. The minimum reported weight was 95 kg and the maximum weight was 156 kg. The mean weight 6 months after removal of the IGB was 109.8 kg (SD 19.8 kg), with a minimum of 81 kg and a maximum of 148 kg.
The mean of the mean change in weight at bio-enteric intragastric balloon [BIB] removal was: 14.2 ± 3.43 kg (median: 13, min: 10 kg, max: 22 kg). The mean weight loss 6 months after IGB removal was: 14.1 ± 6.3 kg (median: 13 kg, min: 10 kg, max: 25 kg). The mean weight loss 12 months after IGB removal was: 9 ± 5.5 kg (median: 8.1 kg, min: 3 kg, max: 16 kg). The mean percentage excess weight loss at BIB removal was 36.3 ± 9.25 kg (median: 35 kg, min: 26 kg, max: 57 kg).
BMI
The mean of the mean BMI at the time of IGB insertion was 39.9 ± 5.4 kg (median: 38.2 kg, min: 32.4 kg, max: 54.1 kg). The mean of the mean BMI at IGB removal was 35.03 ± 5.3 kg (median: 34 kg, min: 29 kg, max: 47 kg). The mean of the mean BMI 6 months after removal was: 39 ± 6.7 kg (median: 41 kg, min: 31 kg, max: 48 kg). The mean of the mean change in BMI at BIB removal was 5.2 ± 1.4 kg (median: 4.8 kg, min: 3.6 kg, max: 8.8 kg). The mean of the mean change in BMI 6 months after IGB removal was 4.3 ± 1.1 kg (median: 3.9 kg, min: 3 kg, max: 6 kg).
Risk of bias in the studies
Risk of bias was assessed using the methodological index for nonrandomized studies (MINORS). The global ideal score is 16 for noncomparative studies and 24 for comparative studies. The individual score and aggregate score for each study using the MINORS scoring system is given in Table 2.
| Study ID | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow up less than 5% | Prospective calculation of the study size | An adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 15 |
| 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 15 |
| 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 9 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 10 | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 11 |
| 13 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 23 |
| 15 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 23 |
| 17 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 19 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 18 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 15 |
| 12 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 23 |
| 22 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 15 |
| 21 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 15 |
| 24 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 16 |
| 23 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 16 |
| 25 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 31 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 27 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 18 |
| 28 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 18 |
| 29 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 22 |
| 34 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 18 |
| 35 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 15 |
| 37 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 33 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
| 36 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 38 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 20 |
| 39 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 16 |
Table 2: The risk of bias assessed using the methodological index for non-randomized studies (MINORS) criteria.
Results of individual studies
Table 3 shows the details of the type of balloon and number of patients in each treatment arm at different time intervals of treatment for each study.
| Article ID | Balloon type | Number of patients in the study who had BIB | Number of patients at the time of BIB removal | Was there a control arm | What did they use in the control arm | Mean treatment duration (months of Balloon) | Number of months post removal | Number of patients studied 6 months afterremoval of IGB | Number of patients studied 12 months after removal of IGB | Number of patients studied 18 months after removal of IGB | Number of patients studied 24 months after removal of IGB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | Heliosphere bag | 45 | 32 | No | x | 6 months | 18 | x | x | 16 | x |
| 3 | BIB | 100 | 100 | No | x | 6 months | 12 | x | 100 | x | x |
| 9 | BIB | ? | 43? | Yes | Sham balloon placement for the first 3 months, followed by a balloon every 3 months for the remainder of the first year | 12 months – varied? multiple 3 month long balloons | 12? | x | |||
| 10 | Allergan and Medicone | 2002 | 1016 | No | x | 6 months | 6 | 842 | x | x | x |
| 12 | BIB | 31 | 29 | Yes | Behavioral modification program of diet and exercise | 6 months | 6 | 23 | x | x | x |
| 13 | BIB | 80 (this was the control) | 80 | Yes | BIB and the main group was laproscopic sleeve gastrectomy | 6 months | 6 | 80 | x | x | x |
| 15 | Heliosphere bag | 18 | 18 | No; however, there were two groups with two different balloons | x | 6 months | 6, 12 | 30? | 26? | x | x |
| 18 | BIB | 483 | 323 | No | x | **6 months – 15 removed at 4 months, 8 removed at 7 months | 6 (?) is this considered BIB removal bc BIB removed at different times,18 | 85 | x | 17 | x |
| 19 | BIB | 500 | 474 | No | x | 6 months | 6, 12, 24, 5 years | 395 | 352 | x | 352 |
| 21 | BIB | 32 | 32 | No | x | 6 months | 6 | 32 | x | x | x |
| 22 | BIB | 173 | 140 | No | 6 months–189 days | 6 | 137 | x | x | x | |
| 24 | BIB | 130 (BIB was control condition) | Yes | 130 (diet modification was the main condition, not control) | 6 months | 18 | 130 | x | 129 | x | |
| 25 | BIB | 100 | 86- states the mean weight loss in 100 patients rather than 86?? | No | x | 6 months | 30, 58 | x | x | x | x |
| 27 | BIB | 33 | 28 | No | x | 6 months | 6 | one year follow up, did not state change in weight, instead only BMI, which is stratified into >32 and <32 | |||
| 28 | BIB and Heliosphere (either used?) | 93 | 81 | No | x | 6 months | 12.3 | x | 72 | x | x |
| 29 | 137 (125 continued) | 136 (130 continued) | 6 months | 6 months | not long-term | ||||||
| 31 | BIB | 143 | 138 | No | x | 6 months | 6, 12 | 138 | 138 | x | x |
| 33 | BIB | 50? | 50 | No | x | 6 months | 6, 12 | 50? | 50? | x | x |
| 34 | BIB (Allergan, Santa Barbara, CA, USA) | 75 | 70 | No | x | 6 months | 24 months | 70 | x | x | 70 |
| 35 | BIB | 51 | 35 | No | x | 6 months | 6–12 months | 29 | 29 | x | x |
| 36 | BIB (MEDSIL® IGB silicon based saline filled bioenetric intragastric balloon) | 301 | 301 | No | x | 6 months | 6 months – 100 subjects removed at other times, 80 after a week or month, 20 after a day or week before 6 months | 301? | x | x | x |
| 37 | BIB | 261 | 6 months | 0 months, 36 months |
Table 3: Study characteristics.
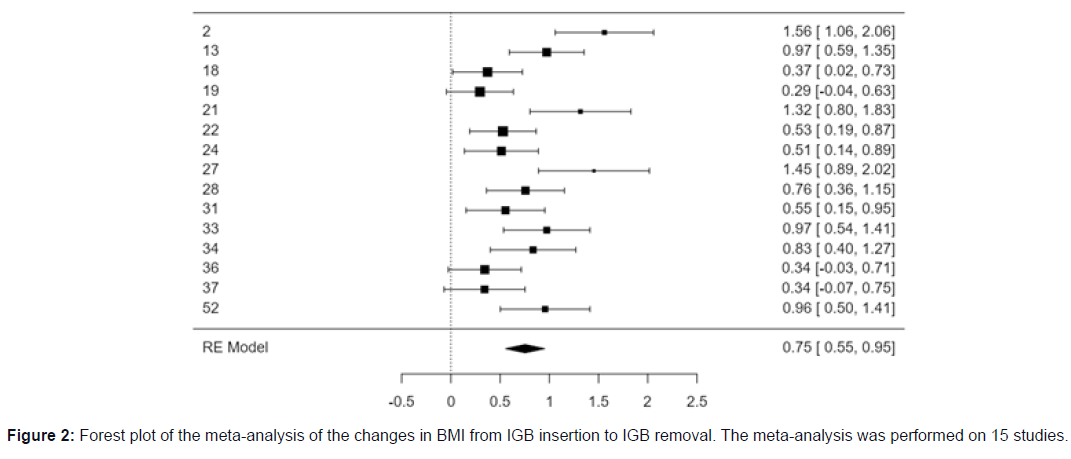
Meta-analysis of BMI changes from IGB insertion to removal
The meta-analysis was performed using 15 studies that have data on BMI before IGB insertion and at the time of IGB removal. The standardized mean difference was significant in the short term.
The observed standardized mean differences ranged from 0.2949 to 1.5596, with most estimates being positive (100%). The estimated average standardized mean difference based on the random-effects model was 0.7540 (95% CI: 0.5546 to 0.9535). Therefore, the average outcome differed significantly from zero (z = 7.4106, p < 0.0001).
Summary statistics for the meta-analysis of BMI changes from IGB insertion to removal are given in the Forest plot (Figure 2).
Heterogeneity
According to the Q-test, the true outcomes appear to be heterogeneous [Q (14) = 46.5934, p < 0.0001, tau² = 0.1101, I² = 71.9812%]. The 95% prediction interval for the true outcomes is given by 0.0738 to 1.4343. Hence, even though there may be some heterogeneity, the true outcomes of the studies are generally in the same direction as the estimated average outcome. An examination of the studentized residuals revealed that none of the studies had a value larger than ± 2.9352 and hence there was no indication of outliers in the context of this model.
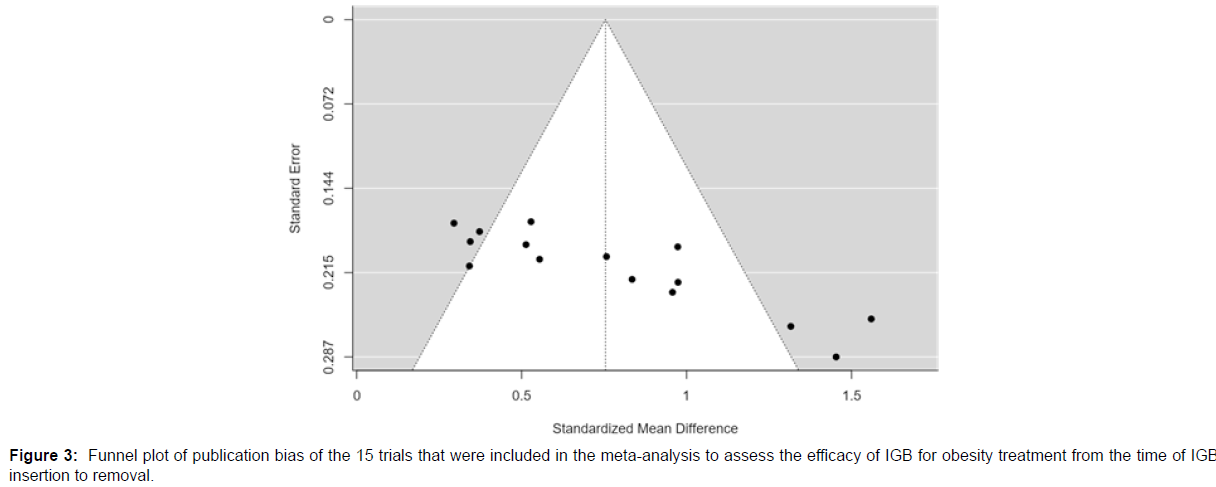
Publication bias
According to the Cook’s distances, none of the studies were overly influential. Both the rank correlation and the regression test indicated potential funnel plot asymmetry (p = 0.0003 and p < 0.0001, respectively).
Figure 3 shows the funnel plot for the publication bias of the studies used for the meta-analysis of change in BMI from IGB insertion to removal, representing standardized mean difference.
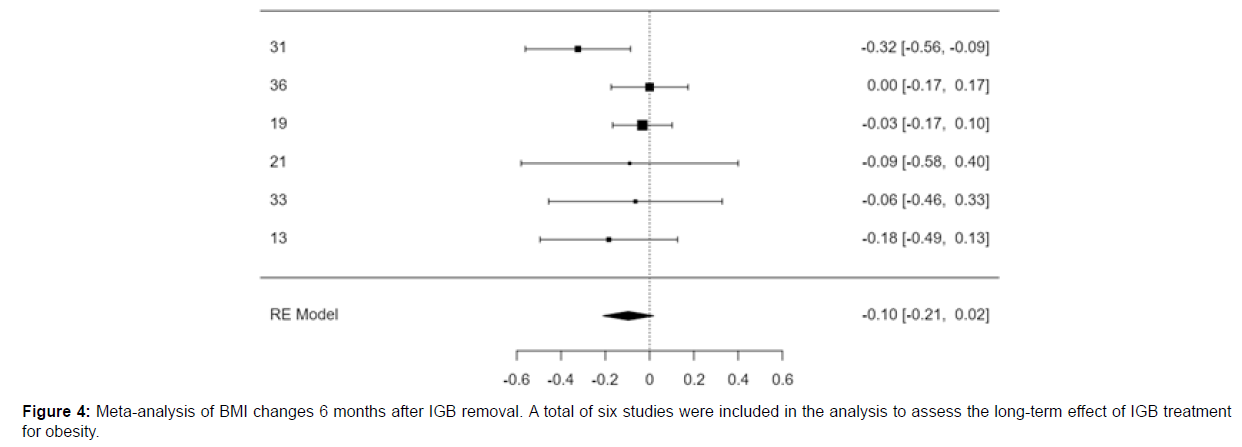
Meta-analysis of BMI changes 6 months after IGB removal
A total of six studies were included in the analysis to assess the longterm effect of IGB treatment for obesity. The observed standardized mean differences ranged from -0.3239 to 0.0000, with most estimates being negative (83%). The estimated average standardized mean difference based on the random-effects model was -0.0961 (95% CI: -0.2113 to 0.0190). Therefore, the average outcome did not differ significantly from zero (z = -1.6364, p = 0.1018).
Figure 4 shows the forest plot for the meta-analysis done for the changes in BMI. A total of six studies were included for the assessment of the long-term effect of IGB treatment for obesity.
Heterogeneity in the outcomes
According to the Q-test, there was no significant amount of heterogeneity in the true outcomes [Q (5) = 5.7878, p = 0.3274, tau² = 0.0056, I² = 28.1353%]. The 95% prediction interval for the true outcomes is given by -0.2827 to 0.0904. Hence, although the average outcome is estimated to be negative, in some studies the true outcome may in fact be positive.
Publication bias
An examination of the studentized residuals revealed that none of the studies had a value larger than ± 2.6383 and hence there was no indication of outliers in the context of this model. According to the Cook’s distances, none of the studies were overly influential. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 0.7194 and p = 0.4460, respectively).
Short-term effect of the IGB on weight change
Meta-analysis of 12 studies was included in the analysis to assess the short-term effects of IGB on obesity treatment. The observed standardized mean differences ranged from 0.2895 to 1.1130, with most estimates being positive (100%). The estimated average standardized mean difference based on the random-effects model was 0.6935 (95% CI: 0.5396 to 0.8474). Therefore, the average outcome differed significantly from zero (z = 8.8316, p < 0.0001). Even though there may be some heterogeneity, the true outcomes of the studies are generally in the same direction as the estimated average outcome. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 0.9466 and p = 0.9565, respectively).
Long-term effect on weight change in the first year after IGB removal
A total of three studies were included in the analysis. The observed standardized mean differences ranged from -0.3324 to -0.1695, with most estimates being negative (100%). The estimated average standardized mean difference based on the random-effects model was -0.3117 (95% CI: -0.4328 to -0.1906). Therefore, the average outcome differed significantly from zero (z = -5.0439, p < 0.0001).
According to the Q-test, there was no significant amount of heterogeneity in the true outcomes [Q (2) = 0.5984, p = 0.7414, tau² = 0.0000, I² = 0.0000%]. An examination of the studentized residuals revealed that none of the studies had a value larger than ± 2.3940 and hence there was no indication of outliers in the context of this model. According to the Cook’s distances, none of the studies were overly influential. Neither the rank correlation nor the regression test indicated any funnel plot asymmetry (p = 0.3333 and p = 0.4951, respectively).
Discussion
The meta-analysis showed that the IGB was beneficial for obesity treatment when the device is inside the stomach. However, our metaanalysis did not show any beneficial effect in further decreasing the weight after the removal of the IGB.
The concept of the IGB was developed in 1982 as a less invasive treatment method for obesity. The first balloons were made from latex and filled with air, but these were found not to be very effective in decreasing weight compared to dietary and behavioral therapy. Moreover, patients suffered from gastric ulcers, gastric mucosal erosion and small bowel obstruction [22].
Later balloons made of silicone and filled with saline known as Obrera [formerly known as Bio-enteric intra-gastric balloons were introduced. Other saline-filled balloons include the Spatz Adjustable balloon system, Reshape duo integrated balloon system, and the Ellipse [22-27].
The IGB is a minimally invasive, non-surgical alternative. Various studies and meta-analyses have reported that IGBs are effective and safe for the short-term treatment of obesity [9,10].
From the literature review of various studies published on the long-term effects of the IGB, the results were found to be inconsistent. For example, during a median follow-up of 3.3 ± 1.76 years after the removal of the IGB, the majority (78.7%) of patients regained weight or had further bariatric measures [12].
Similar experiences were reported by a study in which 70 patients who were treated with an IGB were interviewed 2 years after its removal, finding that 45 patients still maintained reduced weight; however, a satisfactory weight loss of >10% was achieved in only 19 patients [15]. Comparable results were observed in Italy; weight regain was frequent in the 12 months post-IGB removal [14].
In contrast to the above-mentioned studies with negative results, some other studies have shown the beneficial role of the IGB in the long-term after its removal [17-19]. A study from Germany that followed 97 patients after IGB insertion reported that a quarter of participants had successful weight loss and maintenance at 2.5 years. Successful IGB therapy was defined as weight loss at 6 months of ≥10 % of weight at baseline, that remained ≥ 10 % until 2.5 years, without bariatric surgery [17-19].
A study from Belgium followed 100 patients for a year after IGB removal, reporting encouraging results with a mean weight loss of 8.6 kg and a percent excess weight loss of 26.8% [28].
A study from Greece followed 473 patients who had IGB, 195 of whom were able to be assessed at 60 months. Overall, 23% of the patients were found to have maintained >20% excess-body-weight loss. The compliance and behavioral changes from a very early stage of the treatment were a prerequisite for successful weight loss [29].
In this study, we have systematically reviewed many studies where patients were treated with IGB for obesity treatment. In addition, we also performed a meta-analysis as per the PRISMA guidelines, looking for changes in standardized mean difference in BMI and weight. We did not find many randomized controlled studies with a control group with sham treatment to judge the long-term effect of IGB. However, from the available data, it seems that IGBs are effective for reducing weight over a short-term period in good number of patients. We recommend further good quality studies to judge the efficacy of the IGB in the short- and long-term.
Conclusions
IGBs are effective in the treatment of obesity in the short term; however, they have no role in further reducing weight after their removal.
Acknowledgment: Acknowledge Mrs. Rhea Laguerta for editing the tables.
Funding: This project was not funded by any source.
Registration: The research promotion committee of the department of medicine and King Faisal specialist hospital and research center ethics committee approved the proposal.
References
- Chen S, Binns CW, Zhang Y (2012) The importance of definition in diagnosing obesity: a review of studies of children in China. Asia Pac J Public Health 24(2): 248-62.
- Collaboration NCDRF (2017) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390(10113) : 2627-42.
- Alfadda AA, Al-Dhwayan MM, Alharbi AA, Al Khudhair BK, Al Nozha OM, et al. (2016) The Saudi clinical practice guideline for the management of overweight and obesity in adults. Saudi Med J 37(10): 1151-62.
- Balhareth A, Meertens R, Kremers S, Sleddens E (2019) Overweight and obesity among adults in the Gulf States: A systematic literature review of correlates of weight, weight-related behaviours, and interventions. Obes Rev 20(5): 763-93.
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. (2013) Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347: f5934.
- Li L, Yu H, Liang J, Guo Y, Peng S, et al. (2019) Meta-analysis of the effectiveness of laparoscopic adjustable gastric banding versus laparoscopic sleeve gastrectomy for obesity. Medicine (Baltimore) 98(9): e14735.
- Shettar V, Patel S, Kidambi S (2017) Epidemiology of obesity and pharmacologic treatment options. Nutr Clin Pract 32(4): 441-62.
- Imaz I, Martinez-Cervell C, Garcia-Alvarez EE, Sendra-Gutierrez JM, Gonzalez-Enriquez J (2008) Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 18(7): 841-6.
- Buzga M, Kupka T, Siroky M, Narwan H, Machytka E, et al. (2016) Short-term outcomes of the new intragastric balloon End-Ball((R)) for treatment of obesity. Wideochir Inne Tech Maloinwazyjne 11(4): 229-35.
- Zheng Y, Wang M, He S, Ji G (2015) Short-term effects of intragastric balloon in association with conservative therapy on weight loss: a meta-analysis. J Transl Med 13: 246.
- Abeid M, Kaddah T, Zaitoun NA, Alsamman MA (2019) Efficacy and safety of intragastric balloon placements in 1600 case, an experience from the Middle East. Obes Surg 29(7): 2087-91.
- Haddad AE, Rammal MO, Soweid A, Shararra AI, Daniel F, et al. (2019) Intragastric balloon treatment of obesity: Long-term results and patient satisfaction. Turk J Gastroenterol 30(5): 461-6.
- Mitura K, Garnysz K (2016) In search of the ideal patient for the intragastric balloon - short- and long-term results in 70 obese patients. Wideochir Inne Tech Maloinwazyjne 10(4): 541-7.
- Crea N, Pata G, Della Casa D, Minelli L, Maifredi G, et al. (2009) Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg 19(8): 1084-8.
- Mitura K, Garnysz K (2015) Tolerance of intragastric balloon and patient's satisfaction in obesity treatment. Wideochir Inne Tech Maloinwazyjne 10(3): 445-9.
- Dastis NS, Francois E, Deviere J, Hittelet A, Ilah Mehdi A, et al. (2009) Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy 41(7): 575-80.
- Dogan UB, Gumurdulu Y, Akin MS, Yalaki S (2013) Five percent weight lost in the first month of intragastric balloon treatment may be a predictor for long-term weight maintenance. Obes Surg 23(7): 892-6.
- Gumurdulu Y, Dogan UB, Akin MS, Tasdogan BE, Yalaki S (2013) Long-term effectiveness of BioEnterics intragastric balloon in obese patients. Turk J Gastroenterol 24(5): 387-91.
- Mathus-Vliegen EM, Tytgat GN (2005) Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc 61(1): 19-27.
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, et al. (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12: 77.
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, et al. (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9): 712-6.
- Choi SJ, Choi HS (2018) Various intragastric balloons under clinical investigation. Clin Endosc 51(5): 407-15.
- Caglar E, Dobrucali A, Bal K (2013) Gastric balloon to treat obesity: filled with air or fluid? Dig Endosc 25(5): 502-7.
- Courcoulas A, Abu Dayyeh BK, Eaton L, Robinson J, Woodman G, et al. (2017) Intragastric balloon as an adjunct to lifestyle intervention: a randomized controlled trial. Int J Obes (Lond) 41(3): 427-33.
- Lee PC, Dixon J (2017) Medical devices for the treatment of obesity. Nat Rev Gastroenterol Hepatol 14(9): 553-64.
- Palmisano S, Silvestri M, Melchioretto B, Giuricin M, Giudici F, et al. (2016) Intragastric balloon device: weight loss and satisfaction degree. Obes Surg 26(9): 2131-7.
- Ruban A, Doshi A, Lam E, Teare JP (2019) Medical devices in obesity treatment. Curr Diab Rep 19(10):90.
- Herve J, Wahlen CH, Schaeken A, Dallemagne B, Dewandre JM, et al. (2005) What becomes of patients one year after the intragastric balloon has been removed? Obes Surg 15(6): 864-70.
- Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E (2012) 500 intragastric balloons: what happens 5 years thereafter? Obes Surg 22(6): 896-903.
- Giuricin M, Nagliati C, Palmisano S, Simeth C, Urban F, et al. (2012) Short- and long-term efficacy of intragastric air-filled balloon (Heliosphere BAG) among obese patients. Obes Surg 22(11): 1686-9.
- Nunes GC, Pajecki D, de Melo ME, Mancini MC, de Cleva R, et al. (2017) Assessment of weight loss with the intragastric balloon in patients with different degrees of obesity. Surg Laparosc Endosc Percutan Tech 27(4): e83-e6.
- Fuller NR, Pearson S, Lau NS, Wlodarczyk J, Halstead MB, et al. (2013) An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity (Silver Spring) 21(8): 1561-70.
- Genco A, Cipriano M, Materia A, Bacci V, Maselli R, et al. (2009) Laparoscopic sleeve gastrectomy versus intragastric balloon: a case-control study. Surg Endosc 23(8): 1849-53.
- De Castro ML, Morales MJ, Del Campo V, Pineda JR, Pena E, et al. (2010) Efficacy, safety, and tolerance of two types of intragastric balloons placed in obese subjects: a double-blind comparative study. Obes Surg 20(12): 1642-6.
- Sallet JA, Marchesini JB, Paiva DS, Komoto K, Pizani CE, et al. (2004) Brazilian multicenter study of the intragastric balloon. Obes Surg 14(7): 991-8.
- Al Kahtani K, Khan MQ, Helmy A, Al Ashgar H, Rezeig M, et al. (2010) Bio-enteric intragastric balloon in obese patients: a retrospective analysis of King Faisal Specialist Hospital experience. Obes Surg 20(9): 1219-26.
- Tai CM, Lin HY, Yen YC, Huang CK, Hsu WL, et al. (2013) Effectiveness of intragastric balloon treatment for obese patients: one-year follow-up after balloon removal. Obes Surg 23(12): 2068-74.
- Ribeiro da Silva J, Proenca L, Rodrigues A, Pinho R, Ponte A, et al. (2018) Intragastric balloon for obesity treatment: safety, tolerance, and efficacy. GE Port J Gastroenterol 25(5): 236-42.
- Almeghaiseeb ES, Ashraf MF, Alamro RA, Almasoud AO, Alrobayan AA (2017) Efficacy of intragastric balloon on weight reduction: Saudi perspective. World J Clin Cases 5(4): 140-7.
- Genco A, Lopez-Nava G, Wahlen C, Maselli R, Cipriano M, et al. (2013) Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg 23(4): 515-21.
- Escudero Sanchis A, Catalan Serra I, Gonzalvo Sorribes J, Bixquert Jimenez M, Navarro Lopez L, et al. (2008) Effectiveness, safety, and tolerability of intragastric balloon in association with low-calorie diet for the treatment of obese patients. Rev Esp Enferm Dig 100(6): 349-54.
- Farina MG, Baratta R, Nigro A, Vinciguerra F, Puglisi C, et al. (2012) Intragastric balloon in association with lifestyle and/or pharmacotherapy in the long-term management of obesity. Obes Surg 22(4): 565-71.
- Alfredo G, Roberta M, Massimiliano C, Michele L, Nicola B, et al. (2014) Long-term multiple intragastric balloon treatment--a new strategy to treat morbid obese patients refusing surgery: prospective 6-year follow-up study. Surg Obes Relat Dis 10(2): 307-11.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Peedikayil MC, Edathodu Z, Khan SA, Sindi R, Sohaibani FIA, et al. (2022) Efficacy of Intragastric Balloons for the Treatment of Obesity-a Systematic Review and Meta-analysis. J Obes Weight Loss Ther 12: 481. DOI: 10.4172/2165-7904.1000486
Copyright: © 2022 Peedikayil MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2987
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2479
- PDF downloads: 508