Efficacy of Early-Season Applications of Acetochlor and Pethoxamid in Rice
Received: 25-Aug-2018 / Accepted Date: 07-Sep-2018 / Published Date: 15-Sep-2018 DOI: 10.4172/2329-8863.1000393
Keywords: Very long-chain fatty acid-inhibiting herbicides; Herbicide-resistance; Delayed preemergence
Introduction
Red rice (Oryza sativa L.), also known as weedy rice (Oryza sativa L. var. sylvatica ), is one of the most problematic weeds in Arkansas rice production [1]. Shared morphological and physiological characteristics of rice and weedy rice make it almost impossible to discern the difference between them in the field, especially early in the season [2]. However, weedy rice plants generally have a higher growth rate and are often taller and produce more tillers than cultivated rice [3-6]. Previous research demonstrated that a single weedy rice plant per m2 can reduce rice yield by 755 kg ha-1 and has the competitive ability of four cultivated rice plants [2,7].
Prior to the introduction of imidazolinone-resistant (ClearfieldTM BASF Corporation, Research Triangle Park, NC) rice in 2002, weedy rice was mainly controlled using water seeding and crop rotation with soybean [Glycine max (L.) Merr.], corn (Zea mays L.), and grain sorghum [Sorghum bicolor (L.) Moench] [1]. The ClearfieldTM technology was quickly adopted in the mid southern U.S. because it allowed growers to selectively control troublesome grasses such as weedy rice and barnyardgrass (Echinochloa crus-galli (L.) Beauv.) using acetolactate synthase (ALS)-inhibiting herbicides. In 2014, approximately 49% of Arkansas rice acreage was planted to ClearfieldTM rice [8], although that percentage has decreased slightly in recent years. In the mid-2000s, extensive use of ALS inhibitors such as imazethapyr and imazamox, in addition to poor adherence to stewardship guidelines, quickly led to resistance among several weed populations. To date, 11 species have confirmed resistance to the ALS site of action (SOA) in Arkansas, including weedy rice, barnyardgrass, junglerice [Echinochloa colona (L.) Link], yellow nutsedge (Cyperus esculentus L.), rice flatsedge (Cyperus iria L.), and palmer amaranth [Amaranthus palmeri (S.) Wats.] [9]. However, the natural hybridization and resulting outcrossing between weedy rice and cultivated rice is largely responsible for the increase in ALS-resistant weedy rice populations [10].
Aggressive growth habit, extensive root system, and prolific seed production contribute to the extreme competitiveness of barnyardgrass in rice [11,12]. Barnyardgrass infestations can cause up to 70% yield loss if not properly managed [6]. Beginning with propanil in 1990, barnyardgrass has since become resistant to seven different herbicides among four SOA including: propanil (Weed Science Society of America [WSSA] (Group 7), clomazone (WSSA Group 13), quinclorac (WSSA Group 4), and ALS-inhibitors imazethapyr, bispyribac, imazamox and penoxsulam (WSSA Group 2) [9]. In 2011 survey of crop consultants in Arkansas and Mississippi, 58% of respondents reported populations of herbicide-resistant barnyardgrass in fields they scouted, indicating widespread resistance [13].
The repetitive use of the same herbicide SOA has been shown to quickly lead to herbicide resistance. When the same SOA is repeatedly targeted, frequency of resistance alleles increases in the population as a function of selection pressure, thereby reducing herbicide efficacy and limiting control options [14]. However, the evolution of resistance among problematic weeds such as barnyardgrass and weedy rice may be delayed by rotating and mixing different herbicide SOAs [15]. Since there have been no new SOA discovered in recent years, there is a need to explore alternative herbicides that may be used to delay resistance and control resistant weeds in rice.
Very long-chain fatty acid (VLCFA)-inhibiting herbicides such as S - metolachlor, acetochlor, and pyroxasulfone are used in row crops for control of annual grasses and small-seeded broadleaves [16,17]; however, VLCFA-inhibiting herbicides are not labeled for U.S rice production. In contrast, pretilachlor and butachlor, also VLCFAinhibitors, are common in Asian rice production and have been used to control grass species such as barnyardgrass, Chinese sprangletop (Leptochloa chinensis L.), and knotgrass (Paspalum distichum L.) [18,19]. These soil-applied herbicides are primarily absorbed through seedling shoots and roots where they inhibit cell development and cell division.
Acetochlor is a widely-used VLCFA-inhibitor belonging to the chloroacetamide family. Currently labeled for use in U.S. corn, cotton, soybean, and grain sorghum, acetochlor is generally applied preemergence for control of annual grasses and small-seeded broadleaves. Warrant (Monsanto Company, St. Louis, MO) is a microencapsulated (ME) formulation of acetochlor in which herbicide molecules are protected from degradation processes by a porous, polymer shell [20]. When exposed to soil moisture, the polymer shell dissolves and allows a slow release of acetochlor, which can prolong residual activity and influence weed control and crop tolerance.
The efficacy of ME acetochlor on target weeds such as barnyardgrass and weedy rice has been reported in several row crops [16,21,22] and wet-seeded rice [23]. Studies conducted by Godwin [24] evaluated tolerance of drill-seeded rice to 630 and 1050 g ai ha-1 of ME acetochlor applied DPRE, and at the spiking, 1-2 leaf, and 1-2 leaf rice stages. Results from these experiments indicated that rice tolerance to acetochlor generally increased as application timing was delayed, and that minimal crop injury occurred when acetochlor was applied at 1-2 leaf stage or later. Additionally, increased risk may be associated with PRE or DPRE applications of acetochlor, as dry conditions at application followed by heavy rains activated the herbicide simultaneously with rice germination and resulted in rice injury.
Pethoxamid (FMC Corporation, Philadelphia, PA) is a new VLCFAinhibitor currently being developed for use in corn, cotton (Gossypium hirsutum L.), soybean, canola (Brassica napus L.), sunflower (Helianthus annuus L.), and rice in the U.S. and Canada. Similar to other chloro-acetamides such as acetochlor and metolachlor, pethoxamid is a soil-applied herbicide with activity on annual grasses and small-seeded broadleaves [25]. In preliminary studies, pethoxamid has shown initial promise, with high levels of barnyardgrass control and rice tolerance. Godwin [24] reported less than 5% rice injury and no yield reduction when pethoxamid was applied DPRE and at the spiking and 1-2 leaf growth stages. Doherty et al. [26] also evaluated rice injury and weed control following pethoxamid applications to spiking rice. There were no differences in control (97 to 99%) of barnyardgrass, Amazon sprangletop [Leptichloa panicoides (J. Presl) A.S. Hitchc.], or eclipta (Eclipta prostrata L.) 26 days after application (DAA) when pethoxamid at 420 or 560 g ai ha-1 was applied alone or in combination with imazethapyr, clomazone, quinclorac, or pendimethalin. In addition, no injury was observed following any treatment.
With only five weeds having resistance to VLCFA-inhibiting herbicides worldwide, there is relatively low risk for resistance compared to rice herbicides used today [9]. The ability of acetochlor and pethoxamid to control weedy rice, barnyardgrass, and other problematic species in row crops, in addition to the preliminary assessments of tolerance in drill-seeded rice, indicate that these herbicides may be used successfully in mid southern U.S. rice production. By targeting an alternative SOA, acetochlor and pethoxamid may help delay the onset of resistance while providing high levels of weed control and minimizing crop injury. Because limited research has been conducted on these particular VLCFAinhibitors in rice, experiments were conducted to evaluate the ability of acetochlor and pethoxamid to provide early-season weed control in drill-seeded rice. It was hypothesized that rice will be most tolerant at the 1 to 4 leaf growth stages; however, the best weed control will result from DPRE and spiking application timings.
Materials and Methods
All experiments were conducted on a Calloway silt loam (Fine-silty, mixed, active, thermic Aquic Fraglossudalfs) at the Pine Tree Research Station (PTRS) near Colt, AR. Clearfield cultivar ‘CL151’ was planted on May 11, 2016, and ‘CL172’ was planted on May 16, 2017 at 72 seeds m-1 of row, with 18 cm between rows, in 1.8 by 5.2 m plots. To mimic the beginning of a standard rice herbicide program, preemergence applications of clomazone (Command herbicide, FMC Corporation, Philadelphia, PA) were applied to both experiments at 336 g ai ha-1. Experiments were fertilized and otherwise managed according to University of Arkansas Extension recommendations [27]. Herbicide treatments were applied using a CO2-pressurized backpack sprayer calibrated to deliver 140 L ha-1 at 276 kPa. In each year, rice injury and weed control were evaluated 2 and 4 weeks after treatment (WAT) on a scale of 0 to 100, with 0 being no control or injury and 100 being complete control or crop death. In 2017, the number of weedy rice plants per m2 in each plot was counted 2 and 4 WAT. Plots were harvested on September 15, 2016, and September 19, 2017, using a small-plot combine, and weight of rice grain was adjusted to 12% moisture for determining rough rice yield.
Acetochlor experiment
Acetochlor (Warrant herbicide, Monsanto Company, St. Louis, MO) was applied at 1050 (low) and 1470 (high) g ai ha-1 at the DPRE, spiking, 1-2 leaf, and 1-2 leaf timings. Herbicide applications were made as follows: DPRE on May 16, 2016, and May 22, 2017; spiking growth stage on May 25, 2017; 1-2 leaf rice on May 25, 2016, and May 30, 2017; 1-2 leaf rice on June 2, 2016, and June 7, 2017. Spiking applications were not made in 2016.
Pethoxamid experiment
Pethoxamid (FMC Corporation, Philadelphia, PA) was applied at 420 (low) and 840 (high) g ai ha-1 at the DPRE, spiking, 1-2 leaf, and 1-2 leaf timings. Herbicide applications were made as follows: DPRE on May 16, 2016, and May 22, 2017; spiking growth stage on May 25, 2017; 1-2 leaf rice on May 25, 2016, and May 30, 2017; 1-2 leaf rice on June 2, 2016, and June 7, 2017. Spiking applications were not made in 2016.
Statistical analysis
Yield data were found to be normally distributed, via a nonsignificant Shapiro-Wilk Test; however, all other parameters were analyzed assuming beta distribution [28]. All data were analyzed as a two-factor factorial randomized complete block using the GLIMMIX procedure in SAS 9.4 (SAS Institute Inc., Cary, NC). The first factor being application timing: delayed preemergence (DPRE), spiking, 1-2 leaf, and 1-2 leaf rice; the other being herbicide rate: low and high. A weedy check plot was included in both experiments for comparison. Due to inconsistency of weed species between experimental locations, barnyardgrass, broadleaf signalgrass (Urochloa platyphylla (Nash) R.D. Webster), and large crabgrass (Digitaria sanguinalis (L.) Scop.) control was reported for 2016, while weedy rice control was reported for 2017. For these reasons, rice injury and rough rice yield were analyzed and reported separately by year. Weedy rice counts m-2 were converted to proportions of the average of the nontreated for each experiment and year, respectively, and presented as a percent reduction relative to the non-treated check. Analysis of variance indicated no significant interactions between factors in any experiment and therefore only main effects are presented. All means were separated using Fisher’s protected LSD (α=0.05).
Results and Discussion
Rice injury and weed control using acetochlor
In both years, a main effect of application timing influenced rice injury 2 WAT (p=0.0015, 0.0040). As also reported in similar studies [24], rice injury to acetochlor, averaged over rate, generally decreased as application timing was delayed although no treatment caused more than 10% injury (Table 1). The increased injury from earlier application timings was that rice was probably absorbing higher concentrations of herbicide in the soil solution during germination, resulting in more growth inhibition relative to 1 to 4 leaf applications when plants were established prior to herbicide application.
| Factor | Injury 2 WAT | Weedy Rice 2017 | Weedy Rice 2017 | BYG 2016 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2 WAT | 4 WAT | 2 WAT | 4 WAT | 2 WAT | 4 WAT | ||
| % | % control | % reduction | % control | ||||||
| Timing | DPRE | 10a | 8a | 54a | 41a | 65a | 63 | 94a | 77a |
| Spiking | - | 9a | 53a | 38ab | 73a | 65 | - | - | |
| 1-2 LF | 4b | 6a | 49a | 34b | 60a | 63 | 55b | 39b | |
| 3-4 LF | 2b | 0b | 33b | 25c | 19b | 49 | 34c | 24c | |
| Rate | 1050 g ai ha-1 | 4 | 5 | 44b | 32b | 50 | 65 | 57b | 46 |
| 1470 g ai ha-1 | 7 | 7 | 50a | 37a | 58 | 55 | 65a | 48 | |
| P-values | |||||||||
| Timing | 0.0015* | 0.0040* | <0.0001* | <0.0001* | 0.0001* | 0.3264 | <0.0001* | <0.0001* | |
| Rate | 0.1037 | 0.282 | 0.0218* | 0.0097* | >0.9999 | 0.1944 | 0.0051* | 0.5502 | |
| Timing × Rate | 0.0758 | 0.8774 | 0.3335 | 0.0526 | 0.7446 | 0.7456 | 0.0722 | 0.3356 | |
Table 1: Rice injury, weedy rice control, reduction of weedy rice density, and barnyardgrass control following early season applications of acetochlor. a,b,c,d: a) WAT, weeks after treatment; DPRE, delayed preemergence; BYG, barnyardgrass, b) At 6 weeks after planting, average weedy rice and barnyardgrass density in the nontreated plot was approximately 4 and 5 plants per m2, respectively, c) Spiking treatments were not made in 2016, therefore rice injury and BYG control were not recorded as indicated by (-), Means within a column followed by the same lowercase letter are not different according to Fisher’s protected LSD at (α=0.05). Significant P-values are indicated by (*).
Although weedy rice pressure varied within the experimental area, achievement of high levels of control from all treatments was not expected, as drill-seeded rice has shown adequate tolerance to some application timings evaluated in this experiment [24]. The challenge, of course, is finding an application timing that minimizes rice injury while maximizing suppression of weedy rice. Main effects of application timing and rate influenced weedy rice control at 2 WAT (p<0.0001 and p=0.0218) and 4 WAT (p<0.0001 and p=0.0097, respectively). DPRE, spiking, and 1-2 leaf applications provided comparable control 2 WAT; however, by 4 WAT, control was better following DPRE than 1-2 leaf applications, although spiking treatments were comparable to both (Table 1). Weedy rice control averaged over acetochlor rate decreased when applications were delayed until 1-2 leaf rice 2 WAT and 1-2 leaf rice 4 WAT. Similarly, DPRE, spiking, and 1-2 leaf application timings averaged over acetochlor rates reduced weedy rice density 2 WAT, but there were no differences among applications by 4 WAT. All treatments reduced weedy rice density relative to the non-treated (data not shown), which averaged 4 weedy rice plants per m2 six weeks after planting.
Very-long-chain fatty acid-inhibitors are primarily absorbed through emerging shoots and secondarily through roots; therefore, plants beyond the seedling stage will still absorb herbicide through roots, but translocation to shoots is limited and thus efficacy is decreased as application timing is delayed [29]. The limited translocation to shoots and resulting reduced efficacy of VLCFAinhibitors when absorbed through roots could explain why 1-2 leaf applications were comparable to DPRE and spiking applications in some instances, while 3-4 leaf applications were not. In general, the lack of control from the 3-4 leaf application timing is likely due to the presence of emerged weedy rice plants at application, which would not be controlled by acetochlor, as it has little or no effect on emerged seedlings [30]. When averaged across timings, the higher rate of acetochlor increased weedy rice control. In addition, increased rates would likely have more impact at DPRE than EPOST application timings due to aforementioned absorption characteristics.
Main effects of both application timing and rate influenced barnyardgrass, broadleaf signal-grass, and large crabgrass control 2 WAT (see Tables 1 and 2 for p-values). Nontreated plots averaged 5, 3 and 4 plants per m2 for barnyardgrass, broadleaf signal-grass, and large crabgrass, respectively, 6 weeks after planting. Overall, control ratings for all species followed trends similar to those observed in weedy rice, in that control generally decreased as application timing was delayed but increased with rate. Averaged across rates, acetochlor DPRE provided ≥ 89% control of all species 2 WAT; however, control was reduced when applications were delayed to 1-2 leaf or 3-4 leaf rice.
For all species evaluated, the best control was observed following acetochlor applied DPRE or at 1470 g ai ha-1, when averaged over acetochlor rate and application timing, respectively. In contrast, weed control was reduced when acetochlor applications were delayed to 3-4 leaf timings or applied at the lower rate. It should be noted that acetochlor applied alone at any timing is not a herbicide program and should not be relied upon to provide season-long control. No postemergence herbicides were applied in these experiments; however, in a season-long program with herbicides such as fenoxaprop, imazethapyr, and quizalofop, post herbicides could be used where appropriate to control plants that escaped acetochlor activity [31,32].
Overall, rough rice yield followed patterns similar to those observed in weed control; yield decreased as application timing was delayed (Table 2). Treatments that provided superior weed control also had higher rice yields than those that did not. Thus, rice yields were generally higher following the high rate of acetochlor and were maximized at the DPRE and spiking timings. In addition, rice in all treated plots yielded higher than in the nontreated (Table 2).
| Factor | BLSG 2016 | LCG 2016 | Yield | ||||
|---|---|---|---|---|---|---|---|
| 2 WAT | 4 WAT | 2 WAT | 4 WAT | 2016 | 2017 | ||
| % Control | kg ha-1 | ||||||
| Timing | DPRE | 93a | 82a | 89a | 83a | 7500a | 8400a |
| Spiking | - | - | - | - | - | 8200a | |
| 1-2 LF | 69b | 54b | 67b | 56b | 6500b | 7800b | |
| 3-4 LF | 39c | 34c | 54c | 44c | 5900c | 7200c | |
| Rate | 1050 g ai ha-1 | 64b | 52b | 64b | 56b | 6500b | 7700b |
| 1470 g ai ha-1 | 70a | 62a | 75a | 65a | 6800a | 8100a | |
| P-values | |||||||
| Timing | <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* | <0.0001* | |
| Rate | 0.0080* | 0.0053* | 0.0013* | 0.0007* | 0.0266* | 0.0012* | |
| Timing × Rate | 0.0615 | 0.5695 | 0.2108 | 0.1416 | 0.5386 | 0.2474 | |
Table 2: Control of broadleaf signal-grass, large crabgrass, and rough rice yield following early season applications of acetochlor. a,b,c,d: a) WAT, weeks after treatment; DPRE, delayed preemergence; BLSG, broadleaf signal-grass; LCG, large crabgrass, b) At 6 weeks after planting, broadleaf signal-grass and large crabgrass density in the nontreated plot averaged 3 and 4 plants per m2, respectively. Rough rice yield in the non-treated averaged 2700 and 4500 kg ha-1 in 2016 and 2017, respectively, c) Spiking treatments were not made in 2016; therefore BLSG, LCG, and rough rice yield were not recorded as indicated by (-), d) Means within a column followed by the same lowercase letter are not different according to Fisher’s protected LSD at (a=0.05). Significant P values are indicated by (*).
Rice injury and weed control using pethoxamid
Rice injury 2 WAT was influenced by the main effects of application timing and rate, with injury generally decreasing at the lower rate and as application timing was delayed (Table 3). Although injury did not exceed 20% for any treatment in either year, rice injury observed in these experiments was slightly higher than reported by Godwin [24] on a similar soil.
| Factor | Injury 2 WAT | Weedy rice 2017 | Weedy rice 2017 | ||||
|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2 WAT | 4 WAT | 2 WAT | 4 WAT | ||
| % | % Control | % Reduction | |||||
| Timing | DPRE | 20a | 16a | 63a | 58a | 55 | 68 |
| Spiking | - | 8b | 63a | 58a | 47 | 67 | |
| 1-2 LF | 9b | 5bc | 56b | 53a | 26 | 57 | |
| 3-4 LF | 3c | 2c | 53b | 44b | 24 | 45 | |
| Rate | 420 g ai ha-1 | 8b | 5b | 53b | 51b | 33 | 58 |
| 840 g ai ha-1 | 14a | 10a | 64a | 56a | 43 | 61 | |
| P-values | |||||||
| Timing | <0.0001* | 0.0005* | 0.0161* | 0.0002* | 0.1667 | 0.0529 | |
| Rate | 0.0064* | 0.0258* | <0.0001* | 0.0226* | 0.4172 | 0.648 | |
| Timing × Rate | 0.0817 | 0.0953 | 0.9461 | 0.8141 | 0.9931 | 0.9919 | |
Table 3: Rice injury, weedy rice control, and reduction of weedy rice density following early-season applications of pethoxamid. a,b,c,d: a) WAT, weeks after treatment; DPRE, delayed preemergence, b) 6 weeks after planting, average weedy rice density in the nontreated plot was approximately 4 plants per m2, c) Spiking treatments were not made in 2016, therefore rice injury was not recorded as indicated by (-), d) Means within a column followed by the same lowercase letter are not different according to Fisher’s protected LSD at (a=0.05). Significant P values are indicated by (*).
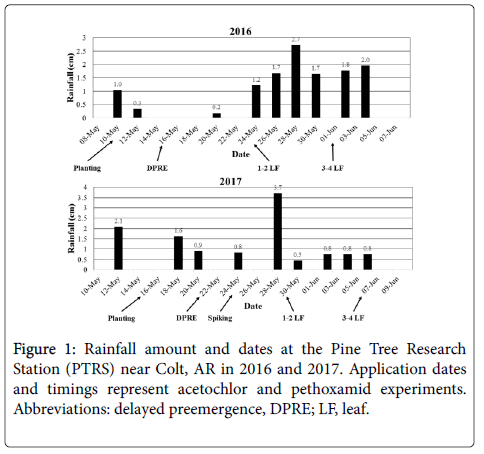
Nonetheless, 20% rice injury 2 WAT is not particularly concerning, as all plots recovered to <5% injury by 4 WAT (data not shown). Generally, 1-2 cm of rainfall is required to activate VLCFA-inhibiting herbicides [20]; however, Dhareesank et al. [33] demonstrated pethoxamid phytotoxicity to rice increases with soil moisture. Increased rice injury in this experiment can be attributed to rainfall events prior to and just after application, which increased pethoxamid availability in soil while rice was germinating (Figure 1).
Application timing and rate affected weedy rice control with pethoxamid 2 and 4 WAT (Table 3). The highest weedy rice control was achieved by DPRE and spiking treatments 2 WAT; however, 1-2 leaf timings provided comparable control 4 WAT. Generally, weedy rice control decreased as application timing was delayed past spiking (2 WAT) or 1-2 leaf timings (4 WAT), and when the 420 g ai ha-1 rate was used. The value of pethoxamid to reduce weedy rice density at 6 weeks after planting relative to nontreated rice should be noted for all treatments, even though differences among timings were not observed.
Barnyardgrass and broadleaf signal-grass populations in this experiment were similar to those in the acetochlor experiment, averaging four and two plants per m2 in the nontreated plots, respectively. At 2 WAT, barnyardgrass and broadleaf signal-grass control was influenced only by the main effect of application timing (p<0.0001); however, by 4 WAT a main effect of both application timing and rate was observed (Table 4). Similar to trends in rice injury and weedy rice control, barnyardgrass and broadleaf signal-grass control with pethoxamid decreased as application timing was delayed and at the lower rate.
| Factor | BYG 2016 | BLSG 2016 | Yield | ||||
|---|---|---|---|---|---|---|---|
| 2 WAT | 4 WAT | 2 WAT | 4 WAT | 2016 | 2017 | ||
| % Control | kg ha-1 | ||||||
| Timing | DPRE | 93a | 78a | 81a | 65a | 6900a | 7900a |
| Spiking | - | - | - | - | - | 7900a | |
| 1-2 LF | 83b | 72b | 69b | 51b | 6900a | 7300b | |
| 3-4 LF | 66c | 48c | 55c | 47b | 5900b | 6600c | |
| Rate | 420 g ai ha-1 | 78 | 63b | 66 | 48b | 6100b | 7100b |
| 840 g ai ha-1 | 83 | 69a | 70 | 61a | 7000a | 7800a | |
| P-values | |||||||
| Timing | <0.0001* | <0.0001* | <0.0001* | <0.0001* | 0.0013* | <0.0001* | |
| Rate | 0.0552 | 0.0461* | 0.094 | <0.0001* | 0.0004* | 0.0002* | |
| Timing × Rate | 0.2763 | 0.4961 | 0.1165 | 0.2915 | 0.9397 | 0.0788 | |
Table 4: Control of barnyardgrass, broadleaf signal-grass and rough rice yield following early season applications of pethoxamid.a,b,c,d: a) WAT,weeks after treatment; DPRE, delayed preemergence; BYG, barnyardgrass; BLSG, broadleaf signal-grass, b) 6 weeks after planting, average barnyardgrass and broadleaf signal-grass density in the nontreated plot was approximately 4 and 2 plants per m2, respectively. Rough rice yield in the nontreated averaged 1600 and 5600 kg ha-1 in 2016 and 2017, respectively, c) Spiking treatments were not made in 2016, therefore BYG,BLSG, and rough rice yield were not recorded, as indicated by (-), d) Means within a column followed by the same lowercase letter are not different according to Fisher’s protected LSD at (a=0.05). Significant P values are indicated by (*).
Pethoxamid applied DPRE controlled barnyardgrass 93 and 78% at 2 and 4 WAT, respectively, while broadleaf signal-grass was controlled 81% and 65%, respectively. Main effects of application timing and rate influenced rice yield in 2016 and 2017 (Table 4). Although there were no differences between DPRE and 1-2 leaf applications in 2016 and DPRE and spiking applications in 2017, yield generally decreased as application timing was delayed, likely due to higher weed interference in plots treated at later growth stages. Pethoxamid applied DPRE yielded 1000 and 1,300 kg ha-1 more than pethoxamid at the 3-4 leaf stage in 2016 and 2017, respectively, highlighting the importance applying VLCFA-inhibiting herbicides prior to weed emergence. Additionally, all pethoxamid treatments, regardless of rate or application timing, yielded higher than the nontreated, demonstrating the value of residual grass control with pethoxamid.
Practical implications
Minimal rice injury, combined with some weedy rice suppression and control of barnyardgrass, broadleaf signal-grass, and large crabgrass in these experiments indicate that acetochlor and pethoxamid could be extremely valuable in providing residual grass control prior to flooding rice. In both experiments, weedy rice and annual grass control decreased as application timing was delayed, with DPRE and spiking timings being the most efficacious. In addition, weed control and rough rice yield increased when the higher rate of either herbicide was used, with little to no increase in crop injury. The decreased control from 3-4 leaf rice application timings support the importance of applying chloro-acetamides such as acetochlor and pethoxamid prior to weed emergence. However, previous research also demonstrates the ability of VLCFA-inhibiting herbicides to cause significant rice injury when applied at the PRE or DPRE timing, warranting caution when applying prior to rice emergence [34]. The results of these experiments lead to the suggestion that acetochlor or pethoxamid be applied after rice emergence but by the 1-2 leaf rice growth stage to maximize weed control and minimize rice injury.
Acknowledgements
Funding for this research was provided by the Arkansas Rice Research and Promotion Board.
References
- Burgos NR, Norsworthy JK, Scott RC, Smith KL (2008) Red rice (Oryza sativa) status after 5 years of imidazolinone-resistant rice technology in Arkansas. Weed Technology 22: 200-208.
- Pantone DJ, Baker JB (1991) Weed-crop competition models and response-surface analysis of red rice competition in cultivated rice: a review. Crop Science 31: 1105-1110.
- Diarra A, Smith RJ, Talbert RE (1985) Growth and morphological characteristics of red rice (Oryza sativa) biotypes. Weed Science 33: 310-314.
- Diarra A, Smith RJ, Talbert RE (1985) Interference of red rice (Oryza sativa) with rice (O. sativa). Weed Science 33: 644-649.
- Kwon SL, Smith RJ, Talbert RE (1991) Interference durations of red rice (Oryza sativa) in rice (O. sativa). Weed Science 39: 363-368.
- Smith RJ (1988) Weed thresholds in southern US rice, Oryza sativa. Weed Technology 2: 232-241.
- Ottis BV, Smith KL, Scott RC, Talbert RE (2005) Rice yield and quality as affected by cultivar and red rice (Oryza sativa) density. Weed Science 53: 499-504.
- Hardke JT (2014) Trends in Arkansas rice production. AAES Research Series 626: 11-22.
- Shivrain VK, Burgos NR, Anders MM, Rajguru SN, Moore J, et al. (2007) Gene flow between Clearfield™ rice and red rice. Crop Protection 26: 349-356.
- Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977)Â The world's worst weeds. Distribution and biology. University Press of Hawaii.
- Talbert RE, Burgos NR (2007) History and management of herbicide-resistant barnyardgrass (Echinochloa crus-galli) in Arkansas rice. Weed Technology 21: 324-331.
- Norsworthy JK, Bond J, Scott RC (2013) Weed management practices and needs in Arkansas and Mississippi rice. Weed Technology 27: 623-630.
- Jasieniuk M, Brûlé-Babel AL, Morrison IN (1996) The evolution and genetics of herbicide resistance in weeds. Weed Science, pp: 176-193.
- Norsworthy JK, Ward SM, Shaw DR, Llewellyn RS, Nichols RL, et al. (2012) Reducing the risks of herbicide resistance: best management practices and recommendations. Weed Science 60: 31-62.
- Krausz RF, Young BG, Kapusta G, Matthews JL (2000) Application timing determines giant foxtail (Setaria faberi) and barnyardgrass (Echinochloa crus-galli) control in no-till corn (Zea mays). Weed Technology 14: 161-166.
- Zemolin CR, de Avila LA, Agostinetto D, Cassol GV, Bastiani M, et al. (2014) Red rice control and soybean tolerance to S-metolachlor in association with glyphosate. American Journal of Plant Sciences 5: 2040.
- Chauhan BS, Abeysekara AS, Kulatunga SD, Wickrama UB (2013) Performance of different herbicides in a dry-seeded rice system in Sri Lanka. Weed Technology 27: 459-462.
- Mutanal SM, Kumar P, Joshi VR, Honnannavar SY (1998) Effect of butachlor for weed control in sprouted direct seeded paddy field under rainfed conditions. Karnataka J Agric Sci 11: 487-489.
- Cahoon CW, York AC, Jordan DL, Everman WJ, Seagroves RW, et al. (2015) Weed control in cotton by combinations of microencapsulated acetochlor and various residual herbicides applied preemergence. Weed Technology 29: 740-750.
- Janak TW, Grichar WJ (2016) Weed control in corn (Zea mays L.) as influenced by preemergence herbicides. International Journal of Agronomy.
- Eleftherohorinos IG, Dhima KV (2002) Red rice (Oryza sativa) control in rice (O. sativa) with preemergence and postemergence herbicides. Weed Technology 16: 537-540.
- Godwin JA (2017) Evaluation of Very-Long-Chain Fatty Acid-Inhibiting Herbicides in Arkansas Rice Production.
- Dhareesank A, Kobayashi K, Usui K (2005) Phytotoxic activity of pethoxamid in soil under different moisture conditions. Weed Biology and Management 5: 197-202.
- Doherty RC, Barber LT, Norsworthy JK, Hill ZT (2016) Residual weed control and crop response to pethoxamid systems in rice. Arkansas Agricultural Experiment Station Research Series 634: 159-162.
- Hardke JT (2013) Arkansas Rice Production Handbook. Arkansas Cooperative Extension Service Miscellaneous Publications, p: 192.
- Gbur E (2012)Â Analysis of generalized linear mixed models in the agricultural and natural resources sciences. Crop Science Society of America.
- Senseman SA (2007)Â Herbicide handbook. Lawrence, US: Weed Science Society of America.
- Babczinski P, Watanabe Y, Nakatani M, Yoshimura T, Hanai R, et al. (2011) Herbicides disturbing the synthesis of very long-chain fatty acids. Modern Crop Protection Compounds, pp: 305-337.
- Buehring NW, Talbert RE, Baldwin FL (2006) Rice (Oryza sativa) response and annual grass control with graminicides. Weed Technology 20: 738-744.
- Scott RC, Barber LT, Boyd JW, Seldon G, Norsworthy JK, et al. (2018) Rice. Recommended chemicals for weed and brush control. Little Rock, AR: The Arkansas Cooperative Extension Service Publication MP 44: 91.
- Dhareesank A, Kobayashi K, Usui K (2006) Residual phytotoxic activity of pethoxamid in soil and its concentration in soil water under different soil moisture conditions. Weed Biology and Management 6: 50-54.
- Fogleman ME, Norsworthy JK, Barber T, Gbur EE (2018) Influence of formulation and rate on rice tolerance to early-season applications of acetochlor. Weed Technol (in review).
Citation: Norsworthy JK, Fogleman M, Barber T, Gbur EE (2018) Efficacy of Early-Season Applications of Acetochlor and Pethoxamid in Rice. Adv Crop Sci Tech 6: 393. DOI: 10.4172/2329-8863.1000393
Copyright: © 2018 Norsworthy JK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4105
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 3267
- PDF downloads: 838