Research Article Open Access
Efficacy of a Silicon Based Continuous Scalp Cooling System with Thermostat on Chemotherapy Induced Alopecia
Tony Ibrahim*, Joseph Kattan, Tarek Assi, Georges Chahine, Fadi El Karake, Fadi Nasr and Marwan Ghosn
Hematology - Oncology Department, Faculty of Medicine, Saint Joseph University, Beirut - Lebanon
- *Corresponding Author:
- Tony Ibrahim
Hematology -Oncology Department
Faculty of Medicine, Saint Joseph University
Beirut - Lebanon
Tel: 0096170971758
E-mail: ibrahim_toni@hotmail.com
Received date: December 25, 2014; Accepted date: February 17, 2015; Published date: March 02, 2015
Citation: Ibrahim T, Kattan J, Assi T, Chahine G, Karake FE, et al. (2015) Efficacy of a Silicon Based Continuous Scalp Cooling System with Thermostat on Chemotherapy Induced Alopecia. J Palliat Care Med 5:209. doi: 10.4172/2165-7386.1000209
Copyright: © 2015 Ibrahim T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Introduction: Alopecia although reversible, is one of the most distressing side effects of chemotherapy. Preventive measures mainly focus on scalp cooling with variable and unpredictable efficacy reported in the literature. The objective of this work is to test the tolerance and effectiveness of a new silicon based cooling technique in preventing chemotherapy induced alopecia (CIA) after each of six cycles of chemotherapy. Method: This is a one center prospective descriptive study. Included patients were females receiving Taxanes and/or Adriamycin based regimens at the oncology department of Hotel-Dieu de France University Hospital. Cooling was done using the Orbis-Paxman scalp cooling system. Hair loss severity was evaluated using the World Health Organization (WHO) grading system. Failure or success of cooling was defined on the basis of wig or head cover use. Independent factors like age, type of hair, type and dose of chemotherapy, total and post infusion cooling time hemoglobin and creatinine at each course. Results: 81 patients were included. Success ranged between 84.8% and 96% for the repeated cycles. No association was found with the independent factors, except for the dose of chemotherapy in the first course with higher doses of Adriamycin (99 mg v/s 77 mg, p value 0.037) as well as taxanes (140 mg v/s 122 mg, p value 0.035) in patients experiencing failure. Conclusion: Scalp cooling has proved to be a successful and well tolerated prevention method for CIA in breast cancer patients treated by Taxanes and Adriamycin.
Keywords
Scalp cooling; Chemotherapy induced alopecia; Hair loss; Orbid-paxman scalp cooling
Introduction
Alopecia is one of the most distressing side effects of chemotherapy. Although reversible, it may affect body image in cancer patients and have great influence on treatment acceptance [1-3]. In fact chemotherapy, by acting on rapidly growing cells, affects mainly hair follicles on the scalp which are in majority in the growth phase [4,5]. Patients may also lose their eyebrows and eyelashes [6]. Hair shredding usually occurs one to two weeks after infusion and its’severity depends both on the type and dose of chemotherapy like Taxanes and doxorubicin, two crucial drugs in the treatment of woman with breast cancer [6-12].
Currently, preventive measures mainly focus on scalp cooling [13]. This later was first introduced in the 1970s, and has been used since to reduce and prevent chemotherapy induced alopecia (CIA) [14]. Its’ efficacy in the literature, however, is highly variable and unpredictable which could be due to variations in cooling techniques, hair loss evaluation methods and chemotherapeutic regimens [6,8]. Several other factors have been proposed to influence success of this procedure like cooling type and time, scalp temperature and hepatic function [6,15].
The objective of this work is to test the tolerance and effectiveness of a new silicon based cooling technique in preventing CIA after each of six cycles of Taxanes and/or doxorubicin based chemotherapy.
Materials and Methods
This is a one center prospective descriptive study. All included patients were females receiving Taxanes and/or Adriamycin based regimens from June- 2013 till June-2014 at the oncology department of Hotel-Dieu de France University Hospital. We excluded patients having cold induced urticaria, cold agglutinin disease, cryoglobulinemia, and post-traumatic cold dystrophy. Cooling was done using the Orbis- Paxman scalp cooling system as written on machine protocol. Hair loss severity was evaluated by trained medical staff three weeks after each course and reported in grades using the World Health Organization (WHO) grading system for alopecia: grade 0 for no hair loss, grade 1 for mild hair loss, grade 2 for pronounced hair loss, and grade 3 for total hair loss [1,16]. Failure or success of cooling was defined on the basis of whether the patient used a wig or head cover. Tolerance at each course was described, and side effects of cooling were graded as following: grade 1-2 for mild or moderate without a need for intervention (stopping or reducing cooling duration); grade 3-4 for severe or very severe with a need for intervention. Independent variables studied were age, type of hair as defined by Van den Hurk et al. (thin Caucasian type v/s thick African type) [3], type of chemotherapy (Taxanes based v/s Adriamycin based v/s Taxanes and Adriamycin based), dose of chemotherapy (in mg), post infusion cooling time (minutes), total duration of cooling (minutes), hemoglobin (g/dl) and creatinine (mmol/l) at each cure.
Results
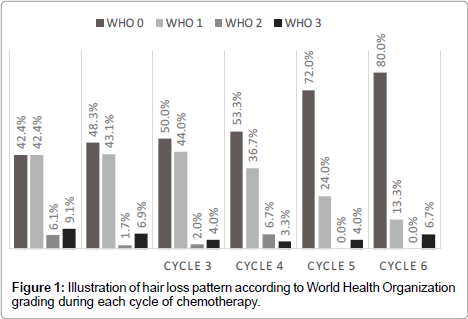
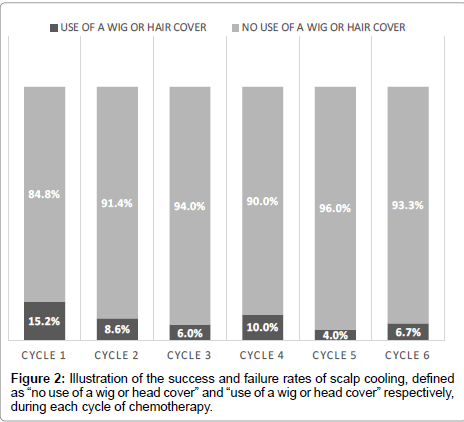
In total, 81 patients were included in this study. The mean and median age were respectively 50 and 49 years old (standard deviation of 12), with a maximum of 78 years old and a minimum of 19 years old. Breast cancer patients constituted the largest group (75 patients-92.6%) followed by ovarian cancer (4 patients-4.9%) and the last 2 (2.5%) patients had Hodgkin’s lymphoma. As for hair type, subjects were nearly equally divided with 28 (46.7%) having thick hair and 32 (53.3%) had thin hair. Cooling was well tolerated in the majority of patients all along the 6 courses (Table 1) with a small number of patients experiencing adverse events severely enough to affect the cooling procedure (Table 2). Most of patients had no or minimal hair loss (Table 3 and Figure 1) during the repeated cycles, and success rate (defined as no need for a wig or head cover) ranged between 84.8% for the first cycle to more than 90% for the subsequent cycles (Figure 2). We analyzed the association between success rate and independent variables (defined above) for each course. We did not found any association except for the dose of chemotherapy in the first cycle (data only shown for the first cycle in Table 3). In fact patients experiencing failure of cooling had significantly higher doses of Adriamycin (99 mg v/s 77 mg, p value 0.037) as well as Taxanes (140 mg v/s 122 mg, p value 0.035).
| Cycle n°1 | Cycle n°2 | Cycle n°3 | Cycle n°4 | Cycle n°5 | Cycle n°6 | ||
| Tolerance | No | 3 (3.7%) |
0 (0.0%) |
4 (7.3%) |
1 (2.0%) |
1 (3.4%) |
0 (0.0%) |
| Yes | 78 (96.3%) |
64 (100.0%) |
51 (92.7%) |
48 (98.0%) |
28 (96.6%) |
25 (100.0%) |
|
| Adverseevents | No | 70 (86.4%) |
58 (90.6%) |
46 (83.6%) |
44 (89.8%) |
25 (86.2%) |
19 (76.0%) |
| Yes | 11 (13.6%) |
6 (9.4%) |
9 (16.4%) |
5 (10.2%) |
4 (13.8%) |
6 (24.0%) |
|
| Hair loss WHO |
0 | 28 (42.4%) |
28 (48.3%) |
25 (50.0%) |
16 (53.3%) |
18 (72.0%) |
12 (80.0%) |
| 1 | 28 (42.4%) |
25 (43.1%) |
22 (44.0%) |
11 (36.7%) |
6 (24.0%) |
2 (13.3%) |
|
| 2 | 4 (6.1%) |
1 (1.7%) |
1 (2.0%) |
2 (6.7%) |
0 (0.0%) |
0 (0.0%) |
|
| 3 | 6 (9.1%) |
4 (6.9%) |
2 (4.0%) |
1 (3.3%) |
1 (4.0%) |
1 (6.7%) |
|
| Stopping hair cooling because of hair loss | No | 64 (97.0%) |
55 (94.8%) |
50 (100.0%) |
30 (100.0%) |
23 (100.0%) |
15 (100.0%) |
| Yes | 2 (3.0%) |
3 (5.2%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
|
| Need for a wig or hair cover | No | 56 (84.8%) |
53 (91.4%) |
47 (94.0%) |
27 (90.0%) |
24 (96.0%) |
14 (93.3%) |
| Yes | 10 (15.2%) |
5 (8.6%) |
3 (6.0%) |
3 (10.0%) |
1 (4.0%) |
1 (6.7%) |
|
Table 1: Summary of tolerance, adverse event, hair loss pattern, cooling arrest due to hair loss and success rate during each cycle.
| GRADE 1-2 | GRADE 3-4 | |||
|---|---|---|---|---|
| Cycle1 | ||||
| Headache | 5 | 6.20% | 2 | 2.50% |
| Cold | 2 | 2.50% | 1 | 1.20% |
| Vertigo | 1 | 1.20% | - | - |
| Cycle2 | ||||
| Headache | 5 | 6.20% | - | - |
| Cold | 1 | 1.20% | - | - |
| Cycle3 | ||||
| Headache | 1 | 1.20% | - | - |
| Cold | 2 | 2.50% | 1 | 1.20% |
| Nausea/vomiting | 2 | 2.50% | 3 | 3.70% |
| Cycle4 | ||||
| Headache | 3 | 3.70% | 1 | 1.20% |
| Cold | 1 | 1.20% | - | - |
| Cycle5 | ||||
| Headache | 1 | 1.20% | 1 | 1.20% |
| Cold | 2 | 2.50% | - | - |
| Cycle6 | ||||
| Headache | 1 | 1.20% | - | |
| Cold | 5 | 6.20% | - | - |
Table 2: Type and grade of adverse events due to cooling during each cycle.
| First cycle mean (std.dev.) | No Wig or Hair cover use | Use of a Wig or Hair cover | p value | |
|---|---|---|---|---|
| Age mean (std.dev.) |
49 (11) |
48 (10) |
.862† | |
| Hemoglobin (g/dl) mean (std.dev.) |
12.4) (1.6) |
11.7 (2.5) |
.362† | |
| Creatinine (mmol/l) mean (std.dev.) |
50 (11) |
52 (5) |
.729† | |
| Type of hair | Thick | 20 (83.4%) |
4 (16.6%) |
.409‡ |
| Thin | 24 (92.3%) |
2 (7.7%) |
||
| Chemotherapy Protocol |
Adriamycin based | 28 (90.3%) |
3 (9.7%) |
.223° |
| Taxanes based | 27 (79.4%) |
7 (20.6%) |
||
| Adriamycin dose (mg) mean (std.dev.) |
77 (17) |
99 (18) |
.037†* | |
| Taxanes dose (mg) mean (std.dev.) |
122 (10) |
140 (40) |
0.035†* | |
| Post infusion cooling time (minutes) mean (std.dev.) |
227 (20) |
234 (13) |
0.294 † | |
| Total cooling time (minutes) mean (std.dev.) |
466 (24) |
465 (21) |
0.948† | |
‡Fisher exact test.
°Chi square test.
†Independent samples t test (equal variances was demonstrated by the Levene’s test and normality of distribution by Kolmogorov-Smirnov test).
Table 3: Summarizes the association between success rate and independent variables: age, hemoglobin/creatinine concentration, type of hair, chemotherapeutic protocol and doses and time of cooling during the first cycle.
Discussion
Scalp cooling is based on two principles; first it reduces blood flow to the hair follicles during peak plasma concentrations of the chemotherapeutic agents and second minimizes biochemical activity of cells which makes them less sensitive to chemotherapy [15]. Practically, however, this technique is not frequently offered for cancer patients to prevent of CIA. For example, in February 2008, less than 40% of all Dutch hospitals offered scalp cooling [1]. One of the reasons is the underestimation of the impact of hair loss for patients and their relatives by medical professionals [6]. In fact, CIA could be a reason to refuse chemotherapy or to choose less effective regimens in up to 8% [7,17]. Another reason could be the overestimation of scalp cooling burden on a patient and the lack of knowledge on its’ current effectiveness [6].
In the majority of the published studies tolerance rates were high and side effects were minimal [8,18-22], as in our study where we found that more than 90% of patients tolerated scalp cooling very well (Table 1). Adverse effects were the same signaled in the literature, like headache, cold sensation, nausea and vomiting [15] with less than 5% being grade 3 or 4 as defined in the methodology section above.
Concerning the success of cooling, a recent meta-analysis of 1,098 participants including eight randomized controlled trials and nine controlled clinical trials, has shown that scalp cooling is the most successful method that significantly reduce the risk of CIA (RR = 0.38, 95% CI = 0.32-0.45) [13]. In our study we found a success rate of more than 80% remaining constant all along the subsequent cycles. It is one of the highest in the literature where success rate ranged from 50 and 80 percent [15,23]. This relatively large difference in success rates is mainly due to different type of cooling used in the different studies as well as chemotherapy regimens and variation in defining failure and hair loss severity [1,8].
A review made by Komen et al. [1] defined several factors that could affect success, beginning by the types of chemotherapy, doses of chemotherapy, time of perfusion, time of cooling, type of hair, hemoglobin concentration and the alteration in liver function. This later was not included in our study, but for the other factors we only found an association between success and chemotherapeutic doses. We also found a trend towards less success rate when Taxanes have been used compared to Adriamycin based chemotherapy which was not found to be statistically significant regarding the small sample size. In addition some authors consider scalp temperature to be the most important factor, with the optimal scalp temperature still debated [15].
The high success rate in this study, however, confirms the findings of Komen et al. [6] which focus on the importance of the cooler type, with devices equipped with a thermostat providing constant cooling for longer duration especially when silicon based medium is used.
Long-term adverse consequences
One of the debated late side effect of cooling is scalp metastasis. Theoretically, tumor cells that have seeded in the scalp might not receive adequate treatment during hypothermia. But this condition have rarely been reported in the literature. A first study published in 1983, 2 subjects out of 7800 breast cancer patient had recurrence in the scalp following cooling [24] whereas a review done by Grevelman et al. on 56 studies found 9 recurrences out of 2500 which were especially hematological neoplasia [8]. On another hand findings of Lemenager et al. (15 years’ experience) [9] and Ridderheim et al. seem to be reassuring (median follow up 9 & 15 months respectively) [21]. Actually it is advised not to use that scalp hypothermia in neoplastic diseases known to metastasize to the scalp like leukemias [15].
Limits of the study
This study is mainly limited by the small sample size. Also a more objective evaluation method of hair loss should be used, like pre and post cooling scalp picture evaluation by a third party.
References
- Mols F, van den Hurk CJ, Vingerhoets AJ, Breed WP (2009) Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations. Support Care Cancer 17: 181-189.
- Rosman S (2004) Cancer and stigma: experience of patients with chemotherapy-induced alopecia. Patient Educ Couns 52: 333-339.
- van den Hurk CJ, Mols F, Vingerhoets AJ, Breed WP (2010) Impact of alopecia and scalp cooling on the well-being of breast cancer patients. Psychooncology 19: 701-709.
- Botchkarev VA, Komarova EA, Siebenhaar F, Botchkareva NV, Komarov PG, et al. (2000) p53 is essential for chemotherapy-induced hair loss. Cancer Res 60: 5002-5006.
- Trüeb RM (2010) Chemotherapy-induced hair loss. Skin Therapy Lett 15: 5-7.
- Komen MM, Smorenburg CH, van den Hurk CJ, Nortier JW (2013) Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist 18: 885-891.
- Batchelor D (2001) Hair and cancer chemotherapy: consequences and nursing care--a literature study. Eur J Cancer Care (Engl) 10: 147-163.
- Grevelman EG, Breed WP (2005) Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol 16: 352-358.
- Lemenager M, Lecomte S, Bonneterre ME, Bessa E, Dauba J, et al. (1997) Effectiveness of cold cap in the prevention of docetaxel-induced alopecia. Eur J Cancer 33: 297-300.
- Münstedt K, Manthey N, Sachsse S, Vahrson H (1997) Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support Care Cancer 5: 139-143.
- National Comprehensive Cancer Network. Breast Cancer (2014).
- Trüeb RM (2009) Chemotherapy-induced alopecia. Semin Cutan Med Surg 28: 11-14.
- Shin H, Jo SJ, Kim do H, Kwon O, Myung SK (2015) Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. Int J Cancer 136: E442-454.
- Breed W, van den Hurk CJ, Peerbooms M (2011) Presentation, impact and prevention of
- chemotherapy induced hair loss: Scalp cooling potentials and limitations. Dermatology 6: 109-25.
- Payne, AS (2014) Chemotherapy induced alopecia. In: UpToDate, Post TW (Ed), UpToDate, Waltham,
- World Health Organisation (1979) Handbook for reporting results of cancer treatment. WHO Offset Publ, Geneva.
- Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, et al. (2004) Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer 12: 543-549.
- Katsimbri P, Bamias A, Pavlidis N (2000) Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur J Cancer 36: 766-771.
- Massey CS (2004) A multicentre study to determine the efficacy and patient acceptability of the Paxman Scalp Cooler to prevent hair loss in patients receiving chemotherapy. Eur J Oncol Nurs 8: 121-130.
- Protière C, Evans K, Camerlo J, d'Ingrado MP, Macquart-Moulin G, et al. (2002) Efficacy and tolerance of a scalp-cooling system for prevention of hair loss and the experience of breast cancer patients treated by adjuvant chemotherapy. Support Care Cancer 10: 529-537.
- Ridderheim M, Bjurberg M, Gustavsson A (2003) Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer 11: 371-377.
- Ron IG, Kalmus Y, Kalmus Z, Inbar M, Chaitchik S (1997) Scalp cooling in the prevention of alopecia in patients receiving depilating chemotherapy. Support Care Cancer 5: 136-138.
- Friedrichs K, Carstensen MH (2014) Successful reduction of alopecia induced by anthracycline and taxane containing adjuvant chemotherapy in breast cancer - clinical evaluation of sensor-controlled scalp cooling. Springerplus 3: 500.
- Dean JC, Griffith KS, Cetas TC, Mackel CL, Jones SE, et al. (1983) Scalp hypothermia: a comparison of ice packs and the Kold Kap in the prevention of doxorubicin-induced alopecia. J Clin Oncol 1: 33-37.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 15090
- [From(publication date):
March-2015 - Jul 18, 2025] - Breakdown by view type
- HTML page views : 10413
- PDF downloads : 4677