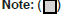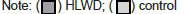Efficacy and Safety of Huanglian Wendan Decoction Combined with Donepezil in Patients with Mile Alzheimerâs disease: A Randomized Controlled Trial
Received: 12-Oct-2022 / Manuscript No. JADP-22-77036 / Editor assigned: 14-Oct-2022 / PreQC No. JADP-22-77036 (PQ) / Reviewed: 28-Oct-2022 / QC No. JADP-22-77036 / Revised: 04-Nov-2022 / Manuscript No. JADP-22-77036 (R) / Published Date: 11-Nov-2022 DOI: 10.4172/2161-0460.1000554 QI No. / JADP-22-77036
Abstract
Background and Objective: Currently, drugs for Alzheimer's disease (AD) are unable to slow or prevent neuronal damage. Clinical studies have shown that a Traditional Chinese Medicine (TCM) decoction combined with conventional Western medicine has a synergistic effect in the treatment of AD. This study aimed to investigate the clinical efficacy and safety of the Huanglian Wendan Decoction (HLWD) combined with donepezil in patients with AD.
Methods: Fifty-two eligible patients were randomly and equally assigned into two groups: one receiving HLWD and donepezil and one control group receiving donepezil alone for 12 weeks. The primary endpoints were measured by the Mini-Mental State Examination (MMSE) and serum levels of Interleukin-1β (IL-1β) and Interleukin-6 (IL-6). Secondary outcomes were evaluated using the Wechsler Memory Scale (WMS), the activity of daily living (Barthel index), and the TCM Symptom Grading Quantitative Scale Score (TCMS).
Results: After 12 weeks of treatment, the MMSE, WMS, and Barthel index scores in the patients of both the groups were higher than those before treatment (P<0.05). TCMS was lower than before treatment in both the groups (P<0.05), whereas the MMSE, WMS, and Barthel index scores of the HLWD group were significantly higher than those of the control group (P<0.05). After treatment, the TCMS score of the HLWD group was significantly lower than that of the control group (P<0.05). Additionally, IL-1β and IL-6 levels in both the groups decreased (P<0.05), while they were significantly lower in the HLWD group than those in the control group (P<0. 05).
Conclusion: HLWD combined with donepezil has significant clinical efficacy in the treatment of patients with mild AD, improving their cognition, memory function, self-care ability, TCM symptoms, and reducing the levels of IL-1β and IL-6.
Keywords: Alzheimer’s disease; Phlegm turbidity and obstruction; Huanglian Wendan Decoction; Cognitive memory
Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disease characterized by memory, cognition, language, and behavior disorders, along with personality changes. According to Traditional Chinese Medicine (TCM), deficiency syndromes in the spleen and stomach can easily cause phlegm production, leading to mental dysfunction. The spleen is located in the middle energizer. One of the main physiological functions of the spleen is to transform ingested water and grain into foodstuff essence (grain essence). If the spleen is obstructed by phlegm and there is no way to clear it up, it will be unable to transport this water upwards, and the brain will lose nourishment, thus leading to dementia [1]. This is the core pathogenesis of AD from the perspective of TCM [2]. More than 55 million people worldwide suffer from dementia [3].
In China, a nationwide cross-sectional study in 2020 showed that there were 15.07 million patients with dementia among the population aged ≥ 60 years, including 9.83 million patients with AD [4].
To date, clinical trials have failed to identify drugs that can slow dementia caused by AD. There are currently two classes of drugs available for the symptomatic treatment of AD. Cholinesterase inhibitors (donepezil, rivastigmine, and galantamine) prevent the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine, which is implicated in the encoding and maintenance of memory. N-Methyl-D-Aspartate-receptor antagonists block the effects of glutamate, which is released in excess in the brains of patients with AD and causes damage to brain cells. Of this class of drugs, donepezil is indicated in the treatment of AD [5].
The general consensus is that two proteins in the brain are closely involved in the pathophysiology of AD. One is β-amyloid, commonly known as amyloid, which reaches abnormal levels in the brains of people with AD and forms plaques that accumulate between neurons and disrupt cell function. The other protein, Tau, can also reach abnormal levels and form tangles within neurons that block their transport systems. Further details of these mechanisms are still under investigation.
As mentioned above, the core pathogenesis of AD involves a deficiency syndrome in the spleen and stomach, which increases phlegm production, leading to mental dysfunction [2]. As it is understood in TCM, phlegm is a hidden expression of physiological imbalance, and it is widely pathogenic and can change form so that it can often invade internal organs, such as the viscera, limbs, and central nervous system parts. The clinical manifestations of phlegm are very consistent with those of clinical dementia, various syndromes, and complex conditions [6]. Clinical studies have preliminarily proved that a TCM decoction combined with conventional Western medicine has a synergistic effect in the treatment of AD: the deterioration rate (Mini-Mental State Examination (MMSE) score ≥ 4 points) is reduced by 48.71%, and the benefit is greater when treatment is administered early in the course of the disease rather than in the middle or late phases5. Furthermore, TCM treatment of AD can be individualized based on clinical stages and syndrome differentiation [7].
The Huanglian Wendan Decoction (HLWD) is a preparation made from Chinese herbal medicines, such as Coptis chinensis, bamboo shavings, Fructus aurantii, Pinellia ternata, tangerine peel, licorice, ginger, and Poria cocus (Wolfiporia extensa). Liu Yin et al., mentioned its effect in regulating qi and reducing phlegm [8]. Studies have shown that berberine, a compound present in HLWD, can enhance cholinergic neurotransmission, improve cerebral blood flow, protect neurons from inflammation, limit Tau hyperphosphorylation, promote the clearance of β-amyloid peptide [9], and significantly improve the learning and memory ability of APP/PS1 transgenic mice. The decrease in the expression of Aβ42 reduces the formation of senile plaques [10]. According to TCM, the components of HLWD have the following medicinal properties: Curcumin has a significant protective effect on the nerves of patients with AD through anti-inflammatory and antioxidant effects, inhibition of hyperphosphorylation of Tau protein, and promotion of neuroplasticity. Tangerine peel can improve the symptoms of nerve function defects, relieve cerebral edema and reduce the volume of cerebral infarction [11], protect the vascular endothelial injury induced by high glucose [12], and has the potential of being an anti-AD treatment option. Poria cocos acid and Poria cocos polysaccharide are the main active substances in Poria [13]. Glycyrrhizin (LG) has a variety of biological activities, including antioxidant, anti-inflammatory, anti-tumor, and anti-viral effects [14,15] which can improve the inflammatory response and memory decline induced by Aβ in the AD model mice [16,17]. For AD with phlegm turbidity, Pinellia can reduce stomach reflux, dampness, and phlegm; Aurantii fructus immaturus enhances qi to eliminate phlegm; bamboo shavings clear away heat and reduce phlegm, stop vomiting, and eliminate irritation; tangerine peel regulates qi, dryness dampness and reduces phlegm; Tuckahoe invigorates the spleen, helps permeate moisture and eliminates phlegm; Coptis chinensis can clear heat and dryness, purge fire and detoxify; licorice, ginger, and jujube nourish the spleen and stomach and eliminate the source of phlegm. According to clinical studies, adding or reducing HLWD can significantly inhibit the inflammatory response of rats with vascular dementia and play a role in the treatment of dementia. Additionally, HLWD has a definite effect on mild cognitive impairment after stroke [18,19]. However, the clinical efficacy and safety of HLWD combined with donepezil in AD are still unclear. The purpose of this study was to compare the clinical efficacy and safety of HLWD and donepezil with donepezil alone in patients with AD.
Materials and Methods
Study design
This study was designed as a randomized, single-blind, controlled study. Fifty-four patients with AD were enrolled in the Eighth People’s Hospital of Chengdu, according to inclusion and exclusion criteria. The study was conducted in accordance with the Declaration of Helsinki and good clinical practice and national regulations. The protocol was approved by the local ethics committee before starting the study, and informed consent was obtained from all participants. The protocol of this study was registered at the Chinese Clinical Trial Registry (ChiCTR2200055918). Eligible patients were randomly assigned to HLWD group or control group in a ratio of 1:1. The random number table method was used to randomize the random list generated by the computer. All patients turn a blind eye to the assignment. A total of 54 hospitalized patients diagnosed with phlegm turbidity obstructive mild type mild AD were enrolled. Fifty-four patients, who met the inclusion and exclusion criteria were in accordance with the Chinese Guidelines for the diagnosis and treatment of AD dementia (2020), were randomly divided into the treatment group (n=27) and control group (n=27) according to the random number table method. The control group received conventional Western medicine (donepezil 10 mg/d), while the treatment group received conventional Western medicine and HLWD (twice a day) for 12 weeks. Based on these guidelines, the experimental group took HLWD Compound Granule, one dose (taken twice a day) divided into two doses, every day. Patients were evaluated at baseline. The intervention effect was evaluated after 12 weeks of treatment. Apart from HLWD and donepezil, any other drugs that may affect the therapeutic effects of HLWD and donepezil were prohibited. In the case of comorbidities, only drugs that meet the appropriate guidelines were permitted.
Study materials
An HLWD Compound Granule is composed of 3 g Coptis chinensis, 20 g Pinellia fragrans, 18 g dried tangerine peel, 20 g Poria, 6 g Licorice, 12 g Citrus aurantium, 20 g Bamboo Ru, and 12 g ginger. The granules are produced by Sichuan New Green Pharmaceutical Science and Technology Development Co., LTD.; the department of Pharmacy of Chengdu Eighth People’s Hospital was responsible for quality control.
Donepezil hydrochloride tablets are manufactured by Eisai China Pharmaceutical Co., LTD.; the preparation is 5 mg/tablet. The Il-1 β kit and IL-6 kits were purchased from Nanjing Camilo Bioengineering Co., LTD.
Study participants
According to the diagnostic criteria of AD by the National Institute of Neurology, Speech and Stroke and the AD and Related Disorders Association (NINCDS), the diagnostic criteria of phlegm and turbidity obstruction were based on the Clinical Research Guidelines for Traditional Chinese Medicine New Drugs (Trial) [20]. The study included the following criteria: men and women with mild AD; TCM dialectical phlegm turbidity obstruction of the body; the diagnostic criteria for mild AD based on MMSE scores ≥ 21 and ≤ 24. Clinical Dementia Rating (CDR) [21] was 1 (1 was mild dementia), and informed consent was submitted.
The exclusion criteria were as follows: (1) patients with severe primary diseases, complicated and unpredictable conditions; (2) patients with a consciousness disorder or mental illness who cannot cooperate with the examination; or with hearing impairment, visual impairment, aphasia, agnosia, and other serious neurological defects that affect the functional evaluation; (3) patients with severe indigestion, or gastrointestinal obstruction, or stomach and duodenal ulcers, and other gastrointestinal diseases that affect drug absorption; (4) patients with external treatment (such as acupuncture or physiotherapy); (5) complications of acute myocardial infarction, cardiac shock or severe arrhythmias with hemodynamic changes; (6) severe liver dysfunction (index value of liver function more than double the normal value); (7) renal insufficiency creatinine clearance rate >20%, serum creatinine>3 mg/dl or >265 mmol/L; (8) stroke; (9) participation in other clinical trials within the last month; and (10) refusal to use or allergy to the study drugs.
Clinical endpoints
The main efficacy indexes of this study were MMSE scores and IL-1β and IL-6 levels. Secondary efficacy indicators were the TCM symptom grading scale of phlegm and WMS and Barthel index scores. The scale categories were evaluated by two participants not involved in the study.
Laboratory tests
The levels of IL-1β and IL-6 in the serum were detected according to the instructions of the related assay kits; 5 mL of venous blood was taken on an empty stomach in the morning, kept at room temperature for 30 min, and centrifuged at 3000 r/min for 10 min, and Il-1 β and IL-6 were detected by double-antibody sandwich enzyme-linked immunosorbent assay.
Sample size
According to the formula 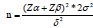 and using α=0.05, Zα=1.96, Zβ=1.28, σ (standard deviation)=1.3, δ (difference)=1.3, which is according to the pre- test, N1=N2=21. The sample sizes of the experimental group and the control group was 21 each. Considering the loss to follow-up rate of approximately 20%, the sample size of the experimental group and the control group was 26 patients, and the total sample size was 52 patients.
and using α=0.05, Zα=1.96, Zβ=1.28, σ (standard deviation)=1.3, δ (difference)=1.3, which is according to the pre- test, N1=N2=21. The sample sizes of the experimental group and the control group was 21 each. Considering the loss to follow-up rate of approximately 20%, the sample size of the experimental group and the control group was 26 patients, and the total sample size was 52 patients.
Statistical analysis
Adopt SPSS 26 0 statistical software (IBM, USA) for statistical analysis, count data expressed in n (%), grade data (clinical efficacy) using Ridit test, disordered classification data using χ 2 inspection. The measurement data (the scores of each scale and the number of inflammatory factors) are expressed in mean ± standard deviation (x ± s). One way ANOVA is used in the group. LSD test is used for the comparison of those who obey the normal distribution, and Mann Whitney U test is used for the comparison of those who do not obey the normal distribution; Independent sample t-test was used for those who obey normal distribution among groups, and rank sum test was used for those who do not obey normal distribution, p<0 05 means the difference is statistically significant.
Results
Patient characteristics
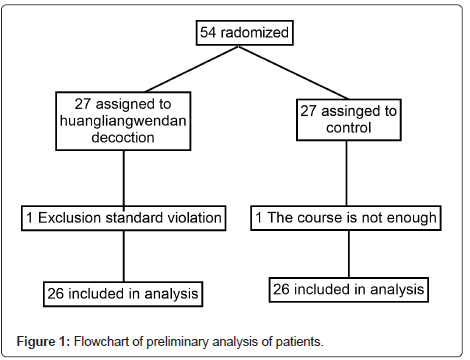
The patients were enrolled between March 2021 and June 2021 (Figure 1). Of the 54 patients, 52 (HLWD, n=26; control, n=26) fit the preliminary analysis. One case (HLWD, n=1; control, n=0) was excluded from the main analysis due to violation of exclusion criteria. The most common reason for exclusion was the cessation of HLWD or donepezil due to changes in the development or behavior of the disease. In one case (HLWD, n=0; control, n=1), treatment was excluded due to discharge or transfer. All participants did not stop using HLWD and donepezil tablets due to side effects, and the dose was not modified during the study. The demographic and baseline characteristics of the HLWD and control groups were well balanced.
Changes in MMSE
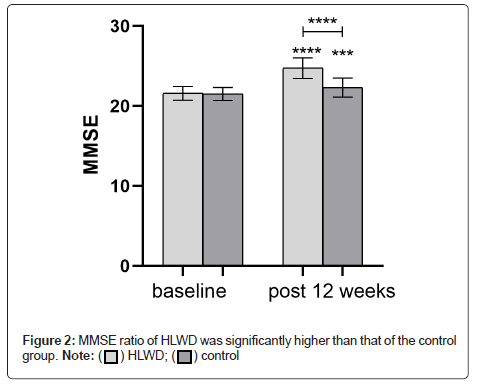
The enrolled patients had mild AD with phlegm turbidity and obstruction of the orifice. MMSE score at baseline was similar in both the groups (P>0.05). After 12 weeks of treatment, the MMSE score of each group increased (HLWD, P<0.001; control group, P<0.001). The MMSE ratio of HLWD was significantly higher than that of the control group (P<0.001), (Figure 2 and Table 1).
| HLWD | Control | p-value | |
|---|---|---|---|
| Baseline | 20.54 ± 1.92 | 20.27 ± 1.71 | 0.521 |
| 12 Weeks | 24.96 ± 1.56 | 22.23 ± 1.48 | <0.001 |
Table 1: Comparison of MMSE scores between the two groups.
Changes in IL-1β and IL-6 levels
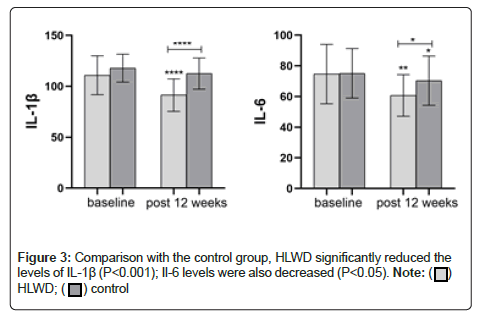
As shown in Figure 3, baseline IL-1β and IL-6 levels were similar in both the groups (P>0.05). After 12 weeks of treatment, IL-1β in the HLWD group was significantly lower (P<0.001). Il-6 was decreased in both the groups (P<0.01; control group, P<0.05). Compared with the control group, HLWD significantly reduced the levels of IL-1β (P<0.001); Il-6 levels were also decreased (P<0.05) (Figure 3 and Table 2).
| HLWD | Control | p-value | ||
|---|---|---|---|---|
| IL-1β (pg/ml) | Baseline | 110.92 ± 19.08 | 117.90 ± 13.71 | 0.142 |
| 12 Weeks | 91.42 ± 15.94 | 112.50 ± 15.35 | <0.001 | |
| IL-6 (pg/ml) | Baseline | 74.67 ± 19.37 | 75.16 ± 16.12 | 0.934 |
| 12 Weeks | 60.71 ± 13.55 | 70.32 ± 16.01 | 0.043 |
Table 2: Comparison of IL-1β and IL-6 scores between the two groups.
Changes in TCMS, WMS, and Barthel Index Scores
Before treatment, there was no statistically significant difference between the two groups in the TCMS scale (P>0.05) (Table 3). After treatment, the TCMS score of the HLWD group decreased compared with the score before treatment (P<0.001), and this score of the HLWD group was lower than that of the control group (P<0.001), as shown in Figure 4. Before treatment, there was no significant difference in WMS and Barthel index scores between the two groups (P>0.05) (Table 4). After 12 weeks of treatment, the above scores in both the groups were significantly higher than those before treatment (P<0.05), and the WMS score and Barthel index in the HLWD group were higher than those before treatment (P>0.001). After treatment, the WMS score and Barthel index in the HLWD group were higher than those in the conventional treatment group (P<0.001), as shown in Figure 5.
| HLWD | Control | p-value | |
|---|---|---|---|
| Baseline | 19.54 ± 5.78 | 19.58 ± 7.68 | 0.915 |
| 12 Weeks | 13.04 ± 5.88 | 18.73 ± 6.09 | <0.001 |
Table 3: Comparison of TCMS scores between the two groups.
| HLWD | Control | p-value | ||
|---|---|---|---|---|
| LMS | Baseline | 20.92 ± 5.53 | 20.77 ± 5.20 | 0.372 |
| 12 Weeks | 24.92 ± 4.99 | 22.00 ± 5.64 | <0.001 | |
| Barthel index | Baseline | 70.00 ± 11.49 | 69.42 ± 12.19 | 0.376 |
| 12 Weeks | 80.19 ± 12.53 | 72.31 ± 10.70 | <0.001 |
Table 4: Comparison of LMS and Barthel index scores between the two groups.
Discussion
Neuroinflammation is a hallmark of neurodegenerative diseases, such as AD, and is defined as the stimulation of astrocytes and microglia and the activation of pro-inflammatory factors. Interleukin and other inflammatory factors promote aging [22]. From a biological perspective, IL-1 β and IL-6 are the most critical inflammatory cytokines involved in the activation of microglia and astrocytes in the nervous system to produce AD [23]. IL-1β is locally released and stimulates IL-6 production [24]. Functionally, IL-1β -activated IL-6 stimulates microglia and astrocytes in AD patients, thereby, stimulating the production of pro-inflammatory cytokines and C-reactive protein. Prevention and treatment of inflammation are thought to help prevent dementia. The study results suggest that HLWD combined with donepezil can improve the clinical efficacy of phlegm turbidity and obstruction of orifice type mild AD, and the two can play a synergistic role. HLWD combined with donepezil can effectively inhibit serum IL-1β and IL-6 levels and improve patients' cognitive function, memory ability, and self-care ability. Additionally, the MMSE, WMS, Barthel index, and TCM Symptom Grading Quantitative Scale of Phlegm and obstruction scores suggested that HLWD combined with conventional Western medicine treatment was more effective than conventional Western medicine treatment. It can also significantly improve patients' cognition, memory, self-care ability, and TCM clinical symptoms. In terms of safety, there were no liver and kidney function damage observed in the two groups before and after treatment, indicating good safety.
Conclusion
The combined application of HLWD and conventional Western medicine in the treatment of AD has definite clinical efficacy and comprehensive efficacy of improving patients' cognition, memory, self-care ability, TCM clinical symptoms, and reduces the occurrence of adverse events, which is worthy of further clinical promotion. In this study, the sample size was small, and the observation time was short. In the future, the authors will continue to increase the sample size and extend the observation and follow-up time to observe the overall endpoint of HLWD for AD and to provide a better clinical plan for the comprehensive prevention and treatment of AD.
Ethics Statement
The protocol of this study was registered at the Chinese Clinical Trial Registry (ChiCTR2200055918). The protocol was approved by the Ethics Committee of Chengdu Eighth People’s Hospital. The studies involving human participants were reviewed and approved by the Ethics Committee of Chengdu Eighth People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LL, YQH: designed the experiments and wrote the first draft of the manuscript; DR, LTT: assisted in completing the experiment and analyzed the data; HJX, WQ: assisted in completing the experiment. All authors read and approved the final manuscript. All authors declare no competing interests.
Role of the funding source
This study was supported by the Natural Science Foundation of Sichuan Province (YLZLZ1701).
Data Availability
All datasets generated for this study are included in the article/supplementary material.
References
- Zhang Juan, Zhang Jianying, Dong Shengshan, Yang Ping (2005) Current status of application of therapeutic drugs for Alzheimer's disease. Chin J Integr Med 3(4):343-344.
- Guo Jing,WANG Zhichao,WU Yuxiao (2020) Discussion on Treatment of Alzheimer ’s disease from Gallbladder According to Theory of Earth Zang-organs Depending on Gallbladder. J Tradit Chin Med 38(01):93-95.
- WHO. Global status report on the public health response to dementia.
- Jia L, Du Y, Chu L, Zhang Z, Li F, et al. (2020) Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Glob Health 5(12):e661-671.
[Crossref] [Google Scholar] [PubMed]
- Schneider LS, Dagerman KS, Higgins JP, McShane R (2011) Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol 68(8):991-998.
[Crossref] [Google Scholar] [PubMed]
- Cui Yuanwu, Zhang Yulian (2015) The understanding of traditional Chinese medicine on senile dementia and the analysis of syndrome differentiation thinking. China J Gerontol 35(05):1419-1422.
- Tian Jinzhou, Xie Hengge, Wang Luning (2021) Guidelines for the diagnosis and treatment of dementia in Alzheimer's disease in China (2020 edition). China J Gerontol 40(3):269-83.
- Zhang Haichao, Meng Ting, Lv Zhikun (2012) Examples of new clinical application of Huanglian Wendan Decoction. J New Chin Med 44(02): 157-158.
- Shinjyo N, Parkinson J, Bell J, Katsuno T, Bligh A (2020) Berberine for prevention of dementia associated with diabetes and its comorbidities: A systematic review. J Integr Med 18(2):125-51.
[Crossref] [Google Scholar] [PubMed]
- Lu Taotao, Wang Yangyang, Huang Zhenting (2019) The Influence of Berberine on learning and memory ability and amyloid expression in APP/PS1 Transgenic Mice Chin J Integr. Med 17: 1155-1161.
- YU Jing-Jing, SU Jie, LV Gui-Yuan (2016) Research progress in anti-cardiovascular and cerebrovascular disease activity of Citri Reticulatae Pericarpium. Chin Tradit Herb Drugs 173:127-3132.
- Qin Liyue, Wu Xiaojun (2016) Review and prospect of pharmacological studies on the prevention and treatment of Parkinson's disease by medicinal plant flavonoids. Chin J Pharmacol Toxicol 30(11):1125-1135.
- Yu M, Xu X, Jiang N, Wei W, Li F, et al. (2017) Dehydropachymic acid decreases bafilomycin A1 induced β-Amyloid accumulation in PC12 cells. J Ethnopharmacol. 23;198:167-173.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Shi J, Li H (2018) Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways. Biomed Pharmacother 106:976-982.
[Crossref] [Google Scholar] [PubMed]
- Ramalingam M, Kim H, Lee Y, Lee YI (2018) Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front Aging Neurosci 10:348.
[Crossref] [Google Scholar] [PubMed]
- Jo DS, Shin DW, Park SJ, Bae JE, Kim JB, et al. (2016) Attenuation of Aβ toxicity by promotion of mitochondrial fusion in neuroblastoma cells by liquiritigenin. Arch Pharm Res 39(8):1137-1143.
[Crossref] [Google Scholar] [PubMed]
- Hongyan L, Suling W, Weina Z, Yajie Z, Jie R (2016) Antihyperuricemic effect of liquiritigenin in potassium oxonate-induced hyperuricemic rats. Biomed Pharmacother 84:1930-1936.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Liu J, Xia H, Zhai Y, Wu X (2020) Clinical study of Huanglian-Wendan Decoction combined with Estazolam tablets in the treatment of post-stroke sleep disorders with syndrome of internal disturbance of phlegm-heat. J Tradit Chin Med. 2020:319-23.
- Zheng XY (2012) Guiding principle of clinical research on new drugs of Chinese medicine (trial).
- Liu F, Wu S, Ren H, Gu J (2011) Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13(3):254-62.
[Crossref] [Google Scholar] [PubMed]
- Dursun E, Gezen-Ak D, Hanagasi H, Bilgic B, Lohmann E, et al. (2015) The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer's disease, mild cognitive impairment or Parkinson's disease. J Neuroimmunol 283:50-7.
[Crossref] [Google Scholar] [PubMed]
- de Gonzalo-Calvo D, Neitzert K, Fernandez M, Vega-Naredo I, Caballero B (2010) Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic Biol Med. 49(5):733-7.
- Querfurth HW, LaFerla FM (2010) Mechanisms of disease. N Engl J Med. 362(4):329-344.
[Google Scholar] [PubMed]
- Park JK, Lee KJ, Kim JY, Kim H (2021) The Association of Blood-Based Inflammatory Factors IL-1β, TGF-β and CRP with Cognitive Function in Alzheimer’s Disease and Mild Cognitive Impairment. Psychiatry Investig 18(1):11.
[Crossref] [Google Scholar] [PubMed]
Citation: Lin L, QiHan Y, TingTing L, JiXin H, Ran D, et al. (2022) Efficacy and Safety of Huanglian Wendan Decoction Combined with Donepezil in Patients with Mile Alzheimer’s disease: A Randomized Controlled Trial. J Alzheimers Dis Parkinsonism 12: 554. DOI: 10.4172/2161-0460.1000554
Copyright: © 2022 Lin L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 898
- [From(publication date): 0-2022 - Dec 23, 2024]
- Breakdown by view type
- HTML page views: 720
- PDF downloads: 178