Research Article Open Access
Effects of using Blood Meal on the Growth and Mortality of Catfish
Njieassam ES*University of Buea, molyko to Buea town Rd, Buea, Cameroon.
- *Corresponding Author:
- Njieassam ES
University of Buea
molyko to Buea town Rd
Buea, Cameroon
Tel: +237677160363
E-mail: stanley_emann@yahoo.co.uk
Received Date: May 25, 2015; Accepted Date: July 29, 2016; Published Date: August 08, 2016
Citation: Njieassam ES (2016) Effects of using Blood Meal on the Growth and Mortality of Catfish. J Ecosys Ecograph 6:204. doi:10.4172/2157-7625.1000204
Copyright: © 2016 Njieassam ES. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The nutritional value of blood meal was investigated for its effects on the growth and mortality in African Catfish Clarias gariepinus Juveniles during a 56 days experiment. Blood was gotten from a local abattoir in the Muea neighbourhood and used to produce blood meal which was included in the fish feed at 0%, 5%, 10%, 15% and 20%. A total of 255 Clarias gariepinus Juveniles of mean weight 7.8833 ± 1.481 g and standard length 63.000 ± 2.361 mm were stocked equally in fifteen rectangular plastic tanks of size (0.32 m × 0.45 m × 0.24 m) arranged in three replicates per treatment with water level maintained at 75% volume. The fish were fed blood meal containing Crude Protein ranging from 18.74 20.98% and Gross Energy ranging from 3361.89 3607.34 kcal/kg at 5% of their body weight daily in two rations. Weekly weights recorded and feed supplied was used to compute the growth nutrient utilization parameters. The non-parametric tests were used to compare the significant differences for the four treatment groups while a paired sample correlation was used to compare weight gain within treatments. At the end of the 56 days study period, the growth performance parameters were best at treatment fed with 10% blood meal inclusion level, no mortality recorded and with the best feasible cost. The poorest was found at treatment fed with 15% blood meal Inclusion level which also recorded the highest mortality rate. The non-parametric Spearman’s Rho test also gave a negative correlation between weights gained and dissolved oxygen values for all treatments and within weeks, hence the need for a proportionate increase in dissolve oxygen supply during aquaculture practices so as to reduce the oxygen deficiency in fish tanks.
Keywords
Growth; Nutrition; Blood meal; Mortality; Feed conversion ratio
Introduction
Research objectives
Main Objective: The general objective is to assess the inclusion levels and lowest cost protein meal and water quality parameters that influence the growth and survival of Clarias gariepinus in a tank reared system.
Specific Objectives:
(i) Determine the food conversion and protein efficiency ratio of the formulated feed in Clarias gariepinus.
(ii) Determine if there is significant difference in the various feed components with respect to growth and survival of Clarias gariepinus.
(iii) Establish a growth survival relationship in relation to water quality conditions in plastic tanks.
(iv) Evaluate the feasibility of large scale production of Clarias gariepinus using locally affordable protein source under favorable water quality conditions.
Background of the Study
World aquaculture has been growing at a rapid pace and is indeed the fastest growing food industry with a growth rate of around 10% per annum. Total capture fisheries is declining and it is forecast that increase in the production of farmed fish in the world would surpass that of captured fisheries in the near future. Aquaculture has been the world’s fastest growing food production system over the past decade and the average growth rate for aquaculture has been 8.9% per year since 1970, compared to only 1.2% for capture fisheries and 2.8% for terrestrially farmed meat-production over the same period [1].
In 2002 the total contribution of aquaculture towards total world fish requirements was 29.9% [2]. North American and European markets have shown a continuous growth of 10 to 15% per year, particularly in respect to shrimp, salmon, trout, catfish and tilapia. This implies that a production of 16 000 tons of aquaculture products per year is needed to meet the increase in demand [1].
The African catfish belongs to the family Clariidae. It is a native fish species in African countries and it has been introduced and commercially cultured in several countries in Europe (Netherlands, Germany, Belgium) and Asian countries (Indonesia, Thailand, Malaysia) and South America (Brazil). Clariid catfishes occur in most freshwater bodies of South East Asia and Africa where they constitute a significant component of the catches. The highest generic diversity is found on the African continent where some 14 genera have been reported. The African catfish is also an indigenous fish in Cameroon and inhabits a wide range of water bodies like swamps, lakes and rivers. They are hardy and are able to thrive in harsh environmental conditions in muddy, turbid and oxygen depleted water bodies with the help of their accessory air-breathing organ (labyrinth organ) that allows them to breathe atmospheric oxygen. The growth performance of African catfish fingerlings (Clarias gariepinus) can be significantly influenced by feeding regimes that strongly affect the feed ingestion and assimilation hence optimum feeding frequency for maximum growth of fish generally depends upon fish size, age and culture conditions including water temperature, food quality and amount of food provided [3].
The African catfish is suitable for aquaculture because it grows fast, feeds on a large variety of agriculture by products, tolerates high concentrations of ammonia (NH3), nitrite (NO2), and resist also low oxygen concentrations in water because the fish can be able to utilize atmospheric as well as dissolved oxygen due to the fact that it has well developed air breathing organs [4].
In Cameroon, the collection of wild catfish Juveniles for aquaculture is specific to the Nkam River basin while it remains a marginal activity in other rivers where Clarias spp. are fished but nevertheless, the holding and feeding of fingerlings is problematic in Cameroon [5].
Protein is one of the major dietary nutrients affecting growth, survival and yield of fish, by providing essential and non essential amino acids to synthesize body protein and energy for maintenance Jena et al. [6]. The development of fish feed is essentially based on the information of nutrients digestibility and its conversation rate.
These two processes provide the basis for growth, which is suitable and compatible for fish Abid and Ahmed [7]. In fish farming, nutrition is critical because feed represent 40-50% of production cost Craig and Helfrich [8]. The general problem of high feed cost associated with fish culture has been addressed by studies on the use on cheaper ingredients as protein sources Abid and Ahmed [7].
Fish is known to need a high proportion of protein in their diets because they metabolize protein as an energy source and in intensive culture, the cost of feed input is unbearable, primarily because of relatively large percentage of animal protein that has to be incorporated into diets Abebi et al. [7].
Furthermore, the need to maximize the economic and the environmentally sound disposal of slaughterhouse by-products stimulated a renewed interest in the investigation of these by products for possible use as protein feedstuff for animals and several studies have reported successful replacement of fish meal with blood meal without any adverse effect on growth and nutritional indices Otubusin et al. [9].
Material and Methods
Experimental site
This experiment was carried out in the Life Science Laboratory of the University of Buea, South West Region of Cameroon between July and September 2012. This area is situated in the tropical region and characterized by mean monthly rainfall ranging from 2416 to 2465 mm. The mean monthly temperature ranges from 21-24°C. High relative humidity value ranges from 60-99.8%. (Meteorological Reports- Ekona Banana, Molyko Buea).
Experimental design
The experiment was carried out using rectangular plastic tanks of 0.32 m × 0.45 m × 0.24 m installed in the University of Buea Lab. The tanks were aerated throughout the experiment using 2 mm pressure tubes connected from an aquarium air-pumps (Tetratec APS 150, Germany) in order to help replenish the amount of dissolved oxygen in the water found in the tanks and also to produce some current for the movement of food particles in the water. Also the tanks were covered with a net of 2 mm mesh size in order to prevent the fishes in the tanks from skipping out and also to protect the tanks from foreign materials or predators. Five treatments were used with three replicates, where each of the replicates in the various treatments had labels T0D1, T0D2, T0D3 for treatment zero and T1D1, T1D2, T1D3 for treatment one, T2D1, T2D2, T2D3 for treatment two, T3D1, T3D2, T3D3 for treatment three and T4D1, T4D2, T4D3 for treatment four. Uneaten feed and faeces were siphoned every morning prior to feeding using an 8 mm pressure tube. The fish in each of the tanks was weighed weekly using an Ohaus sensitive electronic balance (model CS200, China).
Experimental fish
Two hundred and fifty five African catfish Juveniles (Clarias gariepinus) of similar weight range and standard length was procured from Institute of Agronomic Research and Development (IRAD) Foumban. The Juveniles were placed in a plastic bag containing 70% water and 30% oxygen and were transported overnight to the University of Buea Life Science Laboratory the next morning at about 9:43 am. The plastic bag containing the Juveniles were immediately placed in a 70 litres aluminium pot containing half-filled dechlorinated water and allowed for 10-12 minutes for exchange in temperature and thereafter a horizontal cut was made across the plastic bag using a Tiger Razor Blade to allow the Juveniles move out of the bag into the pot containing fresh dechlorinated water freely. Two to three minutes later, part of the water in the pot was reduced and replaced with fresh dechlorinated water. Five hours later, the fish were then distributed equally in six plastic tanks labeled A1, A2, A3, A4, A5, and A6 for acclimatization. During the acclimatization period, each tank was monitored for daily fish mortality and dead fishes were removed from the tanks. The weight and standard length of the biggest and smallest fish in each of the six tanks were measured using an Ohaus sensitive electronic balance and a Mitutoyo shock proof clipper respectively. Also the total biomass in each of the six tanks were measured in order to provide them 5% of their body weight feed from the control diet containing 35.5% crude protein and 3.05 kcal/g of energy in the evening. The juveniles were fed every 7-8 am and 5-6 pm for five days and were then starved for 24 hours prior to the start of the experiment before evenly stocked into the 15 plastic tanks at 17 Juveniles per tank arranged at five trials with three replicates.
Experimental diets
Locally procured feed ingredients including protein ration and energy sources were used to formulate the meal for the Juveniles throughout the experiment. The feed components include red maize, fish meal, blood meal, soya beans, wheat offal, iodized salt and vitamin premix.
The method used for the feed formulation was the trial and error method at 18.74-20.98% crude protein and 3-4 kcal/g respectively. Fresh blood was collected from a local abattoir in Muea early in the morning, warm slowly for 20 minutes, was later chipped to smaller sizes then sundried for 3 days and made to fine powder while the other ingredients were obtained from Muea market. Each component of the feed was grinned to dust using a Victorian hand grinding machine. All the various components were mixed in their various ratios in order to produce the various diets needed for the experiment. A total of five diets were formulated with the varying proportions at 0% (To), 5% (T1), 10% (T2), 15% (T3), and 20% (T4) inclusion with the dried blood meal. Where to be the control diet with 0% blood meal inclusion. The experimental feed samples were analyzed for dry matter (%), ash (%DM), crude protein (%DM), crude fibre (%DM), Lipids (%DM), NFE (%DM), and Gross energy (kcal/kgDM). These analyses were carried out at the Nutritional Laboratory of the University of Dschang, using the Association of Official Analytical Chemists official methods of analysis 15th edition, Washington DC to analyse for dry matter (%), ash (%DM), crude protein (%DM), crude fibre (%DM), Lipids (%DM) and NFE (%DM) while the Wiseman and Lessire analytical methods was used to analyze for Gross Energy.
Feed stuff for each trial was weighed according to their various proportions and then placed in a clean plastic bucket, thoroughly stirred to obtain homogenous mixture. Later one liter of warm water was poured into the mixture and stirred again for 2 minutes in order to form a molten mixture. The molten mixture was then placed on the opposite side of a hand grater and then hand pressed through the 2 mm pore space in the grater to produce rice-like pellets. The pellets were spread on a fertilizer bag and then sun dried for 3-4 days. Treatment 0 (T0) served as a control with 100% fish meal protein to be of standard commercial diet. The other four diets (T1, T2, T3 and T4) contained 5%, 10%, 15% and 20% blood meal protein respectively in order to replace fish meal protein.
Fish feeding and tank management
The fish were fed 5% of their body weight in two rations, during the morning at 7.00 - 8.00 am and the evening at 5.00 - 6.00 pm throughout the experiment. The ration was adjusted every week when the new weights of the juveniles for the various experimental tanks were determined. Left over feed and faeces in each tank were siphoned every morning prior to feeding. Ammonia and Nitrite were removed during the siphoning of left over feed and faeces with about 4-5 litres of water and replaced with fresh dechlorinated water.
Monitoring of water quality
Physico-chemical parameters in the various fish tanks were taken twice per week for eight weeks specifically every Wednesdays and Saturdays during the early morning periods prior to siphoning and feeding. The temperature was measured using EXTECH Instruments (EXTECH Digital Thermometer 39240), dissolved oxygen was measured using EXTECH Instruments (EXSTIK II, Dissolve Oxygen Module DO600, made in Taiwan), pH was measured using HANNA Instruments (Woonsocket RI USA, HI98107 made in Europe), electrical conductivity was measured using HANNA Instruments (MS Dist4, HI98304, made in Mauritius) while salinity and total dissolve solids (TDS) were calculated from the electrical conductivity readings as described by Paul Dohrman. Ammonia and nitrite was extruded from the tanks by reducing two litres of water from each tank and replacing it with fresh dechlorinated water every morning prior to feeding.
Determination of growth and nutrient utilization
The weekly weights recorded and feed supplied was used to compute the growth nutrient utilization parameters. Weight of the fish was taken weekly using a hand net to capture all the fish in each tank, swirled for 4-5 seconds to allow water from the fish to drip out before weighing on an Ohaus sensitive electronic balance. The growth performance parameters calculated include weight gain, specific growth rate, and feed conversion. The growth performance and feed conversion ratio was measured in terms of final fish weight (g), survival rate (%), specific growth rate (SGR, %/day ), feed conversion ratio (FCR), protein efficiency ratio (PER) and food intake (% body weight/ g fish). Growth and nutrient utilization parameters were calculated as follows:
SGR (% /day) = ({In Wt - In Wi}/T) × 100
Where Wt is the weight of fish at time t,
Wi is the initial weight of fish at time 0 and
T is the rearing period in days.
Food conversion ratio (FCR) = total dry feed fed (g/fish) / total wet weight gain (g/fish).
Protein efficiency ratio (PER) = weight gain (g/fish) / amount of protein fed (g/fish).
Feed intake = total dry feed fed (g/fish).
Survival Rate (%) = Number of fish that survived / Number of fish stocked × 100.
Protein index (PI) = Survival × [Final mean body weight (g) – initial mean body weight (g)] / T.
Nitrogen metabolism (NM) = [0.54) (b-a) h]/2. (Where a = Initial weight (g), b = Final weight (g), h = Experimental period (days) and 0.54 experimental constant).
Statistical Analysis
Exploratory statistics was carried out to screen the data for invalid entries. The case summaries procedures was used to determine the means, median, standard deviations and standard error of means, minimum and maximum values of feed intake, weight gain and other performance records. Data was also screened for normality and homogeneity of variance using Kolmogorov-Smirnov and Shapiro- Wilk tests.
Results
Data processing and analysis
All data collected were analyzed using the Statistical Package for Social Sciences (SPSS) Standard version, Release 17.00 [10]. The case summaries procedures was used to determine the means, median, standard deviations and standard error of means, minimum and maximum values of feed intake, weight gain and other performance records. After conducting the aforementioned determinations on the data, they were then screened for normality and homogeneity of variance using Kolmogorov-Smirnov and Shapiro-Wilk tests. The normality tests failed and non-parametric tests were used to compare the significant differences for the four treatment groups (T0-T4).
Sample description
The species of fish used during the experiment was pure breed Clarias gariepinus juvenile of weight Mean ± SEM 7.8833 ± 1.481 and standard length 63.000 ± 2.361 (Table 1).
| Fish Weight (g) | Standard Length (mm) | |
|---|---|---|
| N | 12 | 12 |
| Mean ± Â SEM | 7.8833 ± 1.481 | 63.000 ± 2.361 |
| Median | 7.5500 | 63.500 |
| Minimum | 1.70 | 50.00 |
| Maximum | 15.40 | 73.00 |
| Range | 13.70 | 23.00 |
| Std. Deviation | 5.13134 | 8.180 |
Table 1: Characterization of fish used.
The various composition for the various nutrient composition for the treatments shows that T2 has the highest Dry Matter % (91.97) while T0 has the least. The highest Crude Protein % was found at T3 (20.98) and the least was T0 (18.74) with T3 has the highest Gross Energy (3481.82) and T1 has the least Gross Energy (3361.89) and there is no significant difference in Dry Matter% between T0 and T1, between T1,T2, T3 and T4 and between T2 and T3 and T3 and T4. Also there is no significant difference in the ash content between T0 and T2 and between T3 and T4. The lipid content in T0, T1 and T4 had no significant difference while there is no significant difference in the Crude Fibre (CF) content between all the treatments. More so there is a significant difference in T3 from all other treatments with respect to percentage Crude Protein (%CP) while T2 and T4 and T3 and T4 had no significant difference in their % Nitrogen Free Extract (%NFE) and there is a significant difference in the Gross Energy (GE) values content for all treatments (Table 2).
| Ingredient | Level of Inclusion (%) | ||||
|---|---|---|---|---|---|
| Treatment | |||||
| T0 | T1 | T2 | T3 | T4 | |
| Yellow Maize (YM) | 45 | 45 | 45 | 45 | 45 |
| Fish Meal (FM) | 40 | 35 | 30 | 25 | 20 |
| Blood Meal (BLM) | 0.0 | 5 | 10 | 15 | 20 |
| Soya Beans Cake (SBC) | 10 | 10 | 10 | 10 | 10 |
| Wheat Offal (WO) | 5 | 5 | 5 | 5 | 5 |
| Vitamin Premix (VP) | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Common Salt (CS) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100.75 | 100.75 | 100.75 | 100.75 | 100.75 |
| Where F is the fisher’s test and x2 is the chi square testa, b,c: MW-U test: Pair with the same letter are not significantly differentat the 0.05 Level | |||||
Table 2: Nutrient composition for the various treatments for Clarias gariepinus fed BLM for 56 days.
Table 3 shows the various feed components and the percentages that were used to incorporate into the various treatments. YM, SBC, WO, VP and CS were all included in the same proportion in the various treatments while the level of FM and BLM were varied with 40% FM and 0% BLM inclusion for T0 and 35% FM and 5% BLM for T1, 30% FM and 10 BLM for T2, 25% FM and 15% BLM for T3 and 20% FM and 20 BLM for T4.
| Parameters | T 0 | T 1 | T 2 | T 3 | T 4 | Test |
|---|---|---|---|---|---|---|
| DM (%) | 90.02a | 91.1ab | 91.97bc | 91.92bcd | 91.53bd | F=4.49 P=0.015 |
| Ash(DM %) | 26.38a | 28.53 | 25.74a | 23.75b | 22.85b | F=35.21 P=0.000 |
| Lipids (DM %) | 3.1abc | 3.72ad | 2.7b | 1.51 | 3.37cd | F=5.08 P=0.010 |
| CF(DM %) | 2.98abcd | 2.51aefg | 2.26behi | 2.05cfhj | 2.93dgij | F=1.16 P=0.369 |
| CP(DM %) | 18.74abc | 19.15ade | 19.31bdf | 20.98 | 19.62cef | F=5.11 P=0.009 |
| NFE(DM %) | 38.79 | 37.18 | 41.95a | 43.63b | 42.76ab | F=52.97 P<0.001 |
| GE (Kca/kg) | 3423.58 | 3361.89 | 3432.75 | 3481.82 | 3607.34 | F=59211.27 P<0.001 |
Table 3: Feed Ingredients used with the various level of inclusion.
Total feed given
T1 had the highest feed given (49.163 ± 0.261 g) and T0 (Control) the lowest (43.847 ± 0.121). The comparison amongst the various treatments and per weeks shows that T0 is not significantly different from T2, T1 is not significantly different from T3 and T3 is not significantly different from T4 for week 0 (Table 4).
| Weeks | Control(N=51) Mean ± SEM |
T1 (N=51) Mean ± SEM |
T2 (N=51) Mean ± SEM |
T3 (N=51) Mean ± SEM |
T4 (N=51) Mean ± SEM |
KW (P-Value) |
|---|---|---|---|---|---|---|
| Week 0 | 4.677 ± 0.018a | 5.060 ± 0.043b | 4.737 ± 0.048a | 5.133 ± 0.079bc | 5.253 ± 0.026c | χ2=79.224 P<0.001 |
| Week 1 | 4.940 ± 0.025 | 5.463 ± 0.041 | 5.160 ± 0.043a | 5.373 ± 0.088a | 5.633 ± 0.026 | χ2=90.714 P<0.001 |
| Week 2 | 5.273 ± 0.028 | 5.987 ± 0.052 | 5.477 ± 0.037 | 5.737 ± 0.060a | 5.787 ± 0.004a | χ2=101.933 P<0.001 |
| Week 3 | 5.537 ± 0.021a | 6.260 ± 0.056 | 5.470 ± 0.005a | 5.743 ± 0.077 | 5.923 ± 0.010 | χ2=129.196 P<0.001 |
| Week 4 | 5.530 ± 0.020a | 6.167 ± 0.035 | 5.507 ± 0.019a | 5.763 ± 0.080b | 5.843 ± 0.013b | χ2=129.802 P<0.001 |
| Week 5 | 5.723 ± 0.013a | 6.473 ± 0.036 | 5.827 ± 0.026a | 5.503 ± 0.123 | 6.097 ± 0.018 | χ2=140.908 P<0.001 |
| Week 6 | 5.930 ± 0.010a | 6.767 ± 0.036 | 6.263 ± 0.050 | 5.487 ± 0.095 | 5.977 ± 0.077a | χ2=125.258 P<0.001 |
| Week 7 | 6.237 ± 0.009 | 6.987 ± 0.047 | 6.627 ± 0.081 | 5.523 ± 0.125 | 5.980 ± 0.123 | χ2=130.024 P<0.001 |
| Total Feed Given |
43.847 ± 0.121a | 49.163 ± 0.261 | 45.067 ± 0.124b | 44.263 ± 0.723abc | 46.493 ± 0.301c | χ2=113.090 P<0.001 |
| a, b,c: MW-U test: Pair with the same letter on the same row are not significantlydifferent at the 0.05 Level | ||||||
Table 4: Comparison of feed given between weeks and within treatments.
Weight gained
More so, the comparison done amongst the various treatments and per week on weight gained shows that T2 has the highest total weight gained (43.133 ± 0.244) while T3 has the least total weight gained (8.200 ± 1.669). There is also no significant difference in weight gained for all the treatments in week 0 for week 1, there was no significant difference between feed given in T2 and T3. There is no significant difference in weight gained for T0, T2 and T3 and between T1 and T4 for week 4 and also no significant difference exists in feed given in week 5 for T1 and T4, and T1 and T3 (Table 5).
| Weeks | Control (N=51) Mean ± SEM |
T1 (N=51) Mean ± SEM |
T2 (N=51) Mean ± SEM |
T3 (N=51) Mean ± SEM |
T4 (N=51) Mean ± SEM |
KW (P-Value) |
|---|---|---|---|---|---|---|
| Week 0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - |
| Week 1 | 5.200 ± 1.64 | 8.433 ± 0.077a | 7.967 ± 0.098 | 4.767 ± 0.245 | 7.633 ± 0.013a | χ2=208.329 P<0.001 |
| Week 2 | 4.200 ± 0.411 | 6.367 ± 0.320 | 10.467 ± 0.289a | 7.267 ± 0.583a | 3.000 ± 0.462 | χ2=107.648 P<0.001 |
| Week 3 | 7.667 ± 0.523 | 0.200 ± 0.801 | 5.500 ± 0.469a | 0.133 ± 0.228a | 2.700 ± 0.240 | χ2=96.935 P<0.001 |
| Week 4 | -0.067 ± 0.219b | 0.433 ± 0.300a | -1.867 ± 0.528b | 0.333 ± 0.228b | -1.567 ± 0.363a | χ2=20.562 P<0.001 |
| Week 5 | 3.833 ± 0.157 | 6.333 ± 0.258ab | 6.133 ± 0.284 | -4.533 ± 0.799b | 5.067 ± 0.120a | χ2=160.836 P<0.001 |
| Week 6 | 4.133 ± 0.107b | 8.700 ± 0.624b | 5.867 ± 0.219 | -1.167 ± 0.551a | -2.400 ± 1.780a | χ2=131.233 P<0.001 |
| Week 7 | 6.167 ± 0.173bc | 7.300 ± 0.803b | 4.500 ± 0.232c | 0.933 ± 0.633a | 0.067 ± 1.074a | χ2=67.521 P<0.001 |
| Week 8 | 4.300 ± 0.312b | 5.367 ± 0.133a | -.133 ± 0.427b | 0.467 ± 0.644a | 0.333 ± 1.585a | χ2=64.825 P<0.001 |
| Total weight gained (N=51) |
35.433 ± 0.765a | 38.433 ± 1.073ab | 43.133 ± 0.244b | 8.200 ± 1.669 | 14.833 ± 3.498 | χ2=120.348 P<0.001 |
| a, b,c: MW-U test: Pair with similar letter on the same row are not significantly differentat the 0.05 Level | ||||||
Table 5: Comparison of weight gained (g) between weeks and within treatments.
Growth
Comparison of growth between weeks and within treatments shows that there is no significant difference in growth for T0 and T2, T1 and T3 and T3 and T4 in week 0. For week 1, there was no significant difference between growth in T1 and T3. For week 2, growth in T3 and T4 had no significant difference and for week 3, there is no significant difference between growth in T1 and T2 (Table 6). There is also no significant difference in growth for T0 and T2 and between T3 and T4 for week 4 and also no significant difference exist in growth in week 5 for T1 and T2.
| Weeks | Control (N=51) Mean  ± SEM |
T1 (N=51) Mean  ± SEM |
T2 (N=51) Mean  ± SEM |
T3 (N=51) Mean  ± SEM |
T4 (N=51) Mean  ± SEM |
KW (P-Value) |
|---|---|---|---|---|---|---|
| Week 0 | 93.567 Â ± 0.364a | 101.233 ± 0.864b | 94.733 ± 0.959a | 102.700 ± 1.575bc | 105.067 ± 0.537c | χ2=79.224 P<0.001 |
| Weeek1 | 98.767 ± 0.518 | 109.200 ± 0.814a | 103.167 ± 0.884 | 107.467 ± 1.779a | 112.700 ± 0.530 | χ2=90.714 P<0.001 |
| Week 2 | 102.967 ± 0.351 | 119.667 ± 1.030 | 109.533 ± 0.745 | 114.733 ± 1.206a | 115.700 ± 0.076a | χ2=123.976 P<0.001 |
| Week 3 | 110.633 ± 0.419a | 125.167 ± 1.104 | 109.733 ± 0.081a | 114.867 ± 1.534 | 118.400 ± 0.186 | χ2=128.210 P<0.001 |
| Week 4 | 110.567 ± 0.398a | 123.300 ± 0.710 | 110.167 ± 0.381a | 115.200 ± 1.602b | 116.833 ± 0.271b | χ2=129.802 P<0.001 |
| Week 5 | 114.400 ± 0.250a | 129.433 ± 0.707 | 116.500 ± 0.515a | 110.667 ± 2.333 | 121.900 ± 0.367 | χ2=140.656 P<0.001 |
| Week 6 | 118.533 ± 0.203a | 135.300 ± 0.725 | 125.200 ± 1.007 | 109.500 ± 1.871 | 119.500 ± 1.532a | χ2=123.976 P<0.001 |
| Week 7 | 124.700 ± 0.177 | 139.800 ± 0.951 | 132.500 ± 1.611 | 110.433 ± 2.504 | 119.567 ± 2.453 | χ2=130.024 P<0.001 |
| Week 8 | 129.000 ± 0.401 | 139.667 ± 0.718a | 137.867 ± 1.616a | 110.900 ± 3.132 | 119.900 ± 4.035 | χ2=87.086 P<0.001 |
| a, b,c: MW-U test: Pair with the similar letter on the same row are notsignificantly different at the 0.05 Level | ||||||
Table 6: Comparison of growth (g) between weeks and within treatments.
Feed conversion ratio
The highest Feed Conversion Ratio is found in T2 (0.955 ± 0.054) while the least is found in T3 (0.156 ± 0.038) Comparison of growth between weeks and within treatments shows that there is no significant difference in feed conversion ratio for the various treatments in week 1. For week 8, FCR is not significantly different in T0 and T2 and between T1, T3 and T4 while the Total Feed Conversion Ratio (TFCR) had no significant difference between T0 and T1 (Table 7).
| Weeks | Control (N=51) Mean ± SEM |
T1 (N=51) Mean ± SEM |
T2 (N=51) Mean ± SEM |
T3 (N=51) Mean ± SEM |
T4 (N=51) Mean ± SEM |
KW (P-Value) |
|---|---|---|---|---|---|---|
| Week 1 | 1.107 ± 0.032 | 1.585 ± 0.030 | 1.798 ± 0.036 | 0.907 ± 0.039 | 1.4553 ± Â Â 0.009 | χ2=211.062 P<0.001 |
| Week 2 | 0.868 ± 0.086 | 1.909 ± 0.043 | 1.255 ± 0.071a | 1.470 ± 0.144a | 0.5527 ± 0.087 | χ2=101.600 P<0.001 |
| Week 3 | 1.443 ± 0.093 | 0.925 ± 0.082 | 0.086 ± 0.145a | -0.008 ± 0.061a | 0.4678 ± 0.042 | χ2=113.090 P<0.001 |
| Week 4 | -0.009 ± 0.040b | -0.269 ± 0.080a | 0.077 ± 0.055b | 0.051 ± 0.038b | -0.2611 ± 0.061a | χ2=20.562 P<0.001 |
| Week 5 | 0.699 ± 0.030a | 0.999 ± 0.047bc | 1.148 ± 0.046c | -0.887 ± 0.158 | 0.8656 ± 0.019ab | χ2=140.910 P<0.001 |
| Week 6 | 0.724 ± 0.020b | 0.908 ± 0.034b | 1.481 ± 0.104 | -0.108 ± 0.102a | -0.3621 ± 0.288a | χ2=102.205 P<0.001 |
| Week 7 | 1.041 ± 0.030b | 0.657 ± 0.031 | 1.137 ± 0.127b | 0.062 ± 0.125a | -0.0853 ± 0.189a | χ2=67.733 P<0.001 |
| Week 8 | 0.689 ± 0.049b | -0.005 ± 0.060a | 0.816 ± 0.022b | -0.062 ± 0.132a | -0.2516 ± 0.189a | χ2=64.710 P<0.001 |
| Total Feed Given |
43.847 ± 0.121a | 49.163 ± 0.261 | 45.067 ± 0.124b | 44.263 ± 0.723abc | 46.493 ± 0.301c | χ2=113.090 P<0.001 |
| Total Feed conversion ratio |
0.811 ± 0.020a | 0.782 ± 0.022a | 0.955 ± 0.054 | 0.156 ± 0.038 | 0.301 ± 0.227 | χ2=135.467 P<0.001 |
| a, b,c: MW-U test: Pair with the similar letter on the same row arenot significantly different at the 0.05 Level | ||||||
Table 7: Comparison of feed conversion ratio between weeks and within treatments.
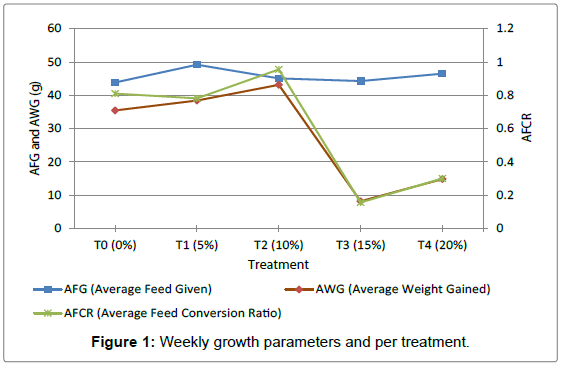
Figure 1 shows the relationship between feed given, Weight Gained, and Average Feed Conversion Ratio for the various treatments. It shows that T1 had the highest Feed Given while those for T0, T2, T3 and T4 are similar. Also the best Feed Conversion Ratio and Average Weight Gained can be observed with T2 followed by T0 which has a least Feed Given and a highest Average Feed Conversion Ratio but a lesser Weight Gained compared to T1. T3 has the lowest Feed Given, Feed Conversion Ratio and Weight Gained values followed by T4 as indicated in Figure 1.
Water quality parameters for the various treatments
All water quality parameters remains fairly insignificant within weeks and between treatments The correlation between water parameters and growth performance was assessed using the nonparametric Spearman Rho’s Correlation at the 0.05 significance level (Alpha=0.05) (Table 8). This shows that there is a negative correlation between weight gained and Dissolve Oxygen concentration in water between all the treatments during the experiment which is indirectly proportional. It shows that as the fish are increasing in weight, there is a significant drop in Dissolve Oxygen concentration from the initial week to the last week while the other water quality parameters did not vary significantly with weight gained.
| Dependent (Weight gain) | pHW | TempW | DOW | ECW | TDSW | SalW |
|---|---|---|---|---|---|---|
| R | 1.000 | 0.100 | 0.600** | -0.400* | -0.400* | -0.400* |
| P-Value | 0.000 | 0.599 | 0.000 | 0.029 | 0.029 | 0.029 |
| R | -0.872** | -0.205 | -0.400 | 0.354 | 0.354 | 0.354 |
| P-Value | 0.001 | 0.570 | 0.252 | 0.316 | 0.316 | 0.316 |
| R | 0.300 | 0.564** | -0.800** | 0.000 | 0.000 | 0.000 |
| P-Value | 0.107 | 0.001 | 0.000 | 1.000 | 1.000 | 1.000 |
| R | 0.100 | -0.975** | 0.100 | -0.447* | -0.447* | 0.100 |
| P-Value | 0.599 | 0.000 | 0.599 | 0.013 | 0.013 | 0.599 |
| R | -0.667** | 0.224 | -0.600** | 0.000 | 0.000 | 0.000 |
| P-Value | 0.000 | 0.235 | 0.000 | 1.000 | 1.000 | 1.000 |
| R | -0.700** | -0.821** | -0.700** | 0.866** | 0.866** | 0.866** |
| P-Value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| R | -0.700** | -0.400* | -0.300 | 0.707** | 0.707** | 0.707** |
| P-Value | 0.000 | 0.029 | 0.107 | 0.000 | 0.000 | 0.000 |
| R | -0.600** | -0.564** | -0.051 | 0.000 | 0.000 | 0.000 |
| P-Value | 0.000 | 0.001 | 0.788 | 0.000 | 0.000 | 0.000 |
| R is the Rho’s correlation; P is the 95% confidence level; pHW is the water acidity of the various weeks; TempW is the temperature at the various week; DOW is the Dissolve Oxygen value within the various weeks; ECW is the Electrical Conductivity Value within the weeks; TDSW is the Total Dissolved Solids within the weeks; SalW is the Salinity values at the various weeks |
||||||
Table 8: Correlation test.
Figure 2 shows the relationship between feed given, weight gained, and average feed conversion ratio and protein efficiency ratio for the various treatments. It shows that T1 has the highest feed given while those for T0, T2, T3 and T4 are similar and lower. Also the highest Protein Efficiency Ratio, Feed Conversion Ratio and Average Weight Gained can be observed with T2. Comparatively T0 has the highest Protein Efficiency Ratio and Feed Conversion Ration compared to T1 which has the highest average weight gained compared to T0 which is the control diet. T3 has the lowest Protein Efficiency Ratio, Feed conversion Ratio, Weight Gained values and also the least Feed Given followed by T4.
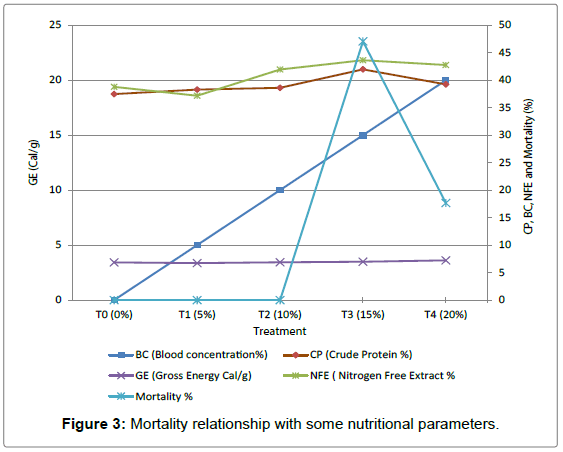
Figure 3 shows the relationship between mortality relationships with some nutritional Parameters in the various treatments. The Cow Blood meal inclusion has a linear increase from T0 to T4, also the energy relationship in the various treatments are similar while T3 has the highest Crude Protein percentage and Nitrogen Free Extract percentage. There was no Mortality in T0, T1 and T2 where as the Mortality in T3 was higher than that in T4. The high mortality recorded in T3 could be due to the high amount of Crude Protein value which has made it difficult for the feed to be utilized by the juveniles especially as the mortality rate increased as the juveniles gets older.
Cost analysis
The cost analysis of the total feed consumed per fish was calculated at the end of the trial period using the formula below;
Cost Ti=Σ cost of ingredients *Feed Factor:
I=treatment labelled i varied from 0 to 4.
Feed Factor=Total feed intake per fish/Total feed compounded. The cost for each feed ingredient in the various treatment is the same except for Fish Meal (FM) which has the highest cost in T0 and the cost reduces as we move from T0 to T4 where we have the least cost of FM due to an increase in the level of BLM inclusion since BLM is free of charge thus also reducing the total cost of feed as we move from T0 to T4. T0 which is the control diet had the highest total cost of feed while T4 has the least. T1 has the highest feed factor while T0 has the least feed factor and also the cost of feed per animal is highest in T1 while T3 has the least cost per animal. Since T2 had the best growth and nutrient utilization compared to the other treatments, it means if juveniles are fed with T2, it is going to reduce the cost from 393frs CFA (T0) to 373frs CFA (T2) per 100 grams of feed thus given a cost reduction of 20frs CFA/100 g of feed.
Ingredient
Costs per component in each treatment (CFA Frs)
Feed Conversion Ratio, Total Weight Gained and Cost per Fish in CFA Francs above it can be seen that T3 has the least cost per fish (159.357) which is slightly similar to T2 and T4 while T1 has the highest cost per fish (186.904). Also T2 has the highest Feed Conversion Ratio (0.955) and the highest Total Weight Gained (43.133 g) while the least Feed Conversion Ratio (0.156) and Weight Gained (8.2) were observed in T3.
Discussion
Throughout the world the efficiency of various alternative protein sources as partial or complete dietary replacement for fish meal has been evaluated in fish diets [11]. It is however important that the selected protein source and inclusion level does not conflict with human food security interest but should greatly reduce expenditure on fish meal without affecting the growth performance of the African catfish.
There was no significant difference in initial mean weight but there was a significant difference in the final mean weight of the Juveniles with T2 recording the highest mean weight which was statistically significant. The Feed Conversion Ratio was highest in T2 indicating the best utilization of the diet by juveniles. The Specific Growth Rate was highest in T2 which was significantly different from that of the control diet (T0) and from the rest of the treatments. Treatment two also had the best Protein Efficiency Ratio indicating the best feed utilization. Treatment two with 10% BLM inclusion, gave the best percentage weight gained, specific growth rate, feed conversion ratio and protein efficiency ratio which was significantly different in comparison to the control T0. Mortality throughout the experiment was relatively low except for T3. The result is different from that of De Graaf et al. [4] who reported a survival rate of 41.5% for Clarias gariepinus reared under medium stocking density for short duration in protected tanks. The mortality and poor growth in T3 and T4 can be due to the excessive inclusion of blood meal above 10% hence reducing the feed Conversion Ratio and Protein Efficiency ratio for both T3 and T4.
The feed conversion ratio was significantly lower and different from that stated by Olukunle et al. [12] who reported the best feed conversion ratio and specific growth rate to be found in fish fed the control diet. There is a significant difference (p<0.05) for Feed Conversion Ratio within weeks. T2 had the best Feed Conversion Ratio while the least was recorded by T3. There was also no significant difference in the Feed Conversion Ratio between T0 and T1 which are significantly different from those of the other treatments. The total feed conversion ratio increased from T1 to T3 before it dropped.
The water quality parameters were similar to those reported by Sogbesan et al. [13]; Amisah et al. [14] and Eyo et al. [15]. There was a significant difference (p<0.05) within weeks for pH except for week 1, while the temperature had no significant difference (p>0.05) within weeks. Also there was a significant difference (p<0.05) for dissolve Oxygen levels within weeks except for weeks 1, 2, 7 and 8 while the electrical conductivity, total dissolved solids and salinity had no significant difference (p>0.05) for weeks 2, 3, 4, 6 and 7 within weeks while all the water quality parameters remained fairly non significant between treatments throughout the experimental period. The reason for the significance of pH at week 1 can be due to the fact that at the beginning of the experiment, there was little or no dissolved feed materials and faeces in the plastic tanks which can cause a rise in pH. Also the Spearman rho’s test gave a negative correlation between dissolve oxygen and weight gained and this was merely due to the fact that as the fish increases in size and in age, their activity and metabolism increases thereby increasing the demand for dissolve oxygen in the plastic tanks; but in the context of this study, water replacement and supply of oxygen in the tanks was constant in all the treatments as the weights in the various treatments was increasing thus causing a negative correlation between the dissolved oxygen concentration and weight gained.
The growth performance was significantly higher (p<0.05) in T2 fed 10% BLM than those of other diets. The specific growth rate and feed conversion ratio was significantly higher in T2; this result is different from that indicated by Adejoke [16] on the use of bovine blood and rumen digest in catfish diet to replace fish meal at 0%, 25%, 50%, 75 and 100% where he reported that the best growth performance in fish fed the control diet and treatment with inclusion of bovine blood and ruminant digest meal at 25%.
The average values of final weight gained per fish and the average daily weight gain were all significantly different (p<0.05). The highest mean weight gained (43.133 g) was recorded in T2 with 10% blood meal inclusion. This was followed closely by T1 (38.433 g) with 5% blood meal inclusion while the least 8.200 g was recorded under T3 with 15% blood meal inclusion. The specific growth rate of fish under all the treatments were not significantly different (p<0.05). The highest average value of 0.67 was recorded under T2 and the lowest (0.13) was recorded under T3. The performance of fish fed 15% and 20% blood meal was significantly different from the other diets. The performance of fish fed 5% was not significantly different from that of T0 which is the control diet but there was a significant difference in the performance of fish fed 10% BLM from that of all other treatments.
As the level of blood meal inclusion increased after 10%, the response of the fish to the diet became poor. This finding indicate that fishmeal in the diet of Clarias gariepinus can only be efficiently replaced with 10% Blood Meal and this observation agree with the result of Otubusin [17] who reported that the replacement of fish meal with 10% blood meal in pelleted feeds was adequate for tilapia, Oreochromis niloticus production in floating bamboo net-cages but disagree with Agbebi et al. [9] who reported that a 25% blood meal substitution of fishmeal in diet gave the best growth performance.
Blood meal has long been recognized as a valuable feed and a rich source of essential amino acids [17]. The highest mean final weight, SGR, PER and FCR values were recorded for fish fed at T2 with 10% BLM diets which is significantly different from that reported by Abdel- Warith et al. [18] who stated the highest mean final weight, SGR, PER and FCR values were recorded for fish fed the control diet and 20% Inclusion of a commercial poultry by-product meal as a protein replacement of fish meal in practical diets for African catfish Clarias gariepinus. These results showed that fish meal can be replaced partially with blood meal at 10% inclusion level with no adverse effects on the growth, mortality and feed conversion ratio of Clarias gariepinus juvenile which is different from that of Agbebi [9] who stated that fish meal can be replaced completely blood meal at 100% with no adverse effects on the growth, survival and feed conversion ratio of Clarias gariepinus juveniles. Furthermore, the result also revealed that not all feed treatments especially T3 and T4 were accepted by fish which is similar to the report given by Olukunle et al. [12] who stated that blood meal can be harmful to fish after 5% inclusion. Cost evaluation indicated that the incorporation of blood meal as a substitute of fish meal up to 10% decreased feed costs and increased weight gained and survival thus giving a high feasibility for aquaculture production in Cameroon. This is similar to the report given by Natacha et al. [19] indicating that the incorporation of blood and feather meal as a substitute of fish meal decreased feed costs leading to a better economic conversion ratio.
Given the urgent need to provide an alternative and sustainable protein source, replacement of fish meal with alternative plant and animal by-products offers the scope to produce flexible solutions whilst minimizing the final cost of the diet. Though prices of raw materials and feed ingredients vary, depending on each country importation tariffs, energy costs, seasonal factors and the economic status of the country, prices are based on fluctuating global markets and are considered to be major commodities for trading [20].
Conclusion
(i) Determining food conversion and protein efficiency ratio of the formulated feed in Clarias gariepinus
There is no significant difference in Feed Conversion Ratio (FCR) between T0 and T1 while there was a significant difference in FCR between T2, T3 and T4. Also, there is no significant difference in the PER between T3 and T4 which is significantly different from T0, T1 and T2. Protein Efficiency Ratio (PER) is not also significantly different between T0 and T2, T1 and T2 and between T0 and T1.
(ii) Determining significant difference in the various feed components with respect to growth and survival of Clarias gariepinus
There is a great significance difference with respect to growth and mortality in the various feed treatments. The Crude Protien (CP) level in T3 is significantly different from that of all the other treatments. T2 had the highest growth performance which is significantly different from the others while T3 had the least growth performance followed by that of T4. There was no significant difference in the growth performance between T0 and T1. There was also no significant difference in the mortality between T0, T1 and T2 which recorded no mortality while T3 had the highest mortality followed by T4. The mortality rate between T3 and T4 are significantly different.
(iii) Establishing growth survival relationship in relation to water quality conditions in plastic tanks
All water quality parameters remained fairly non significantly different within weeks and between treatments except for the fact that the Spearman rho’s test gave a negative correlation between dissolve oxygen and weight gained thus making dissolve oxygen to have a significant effect with the weight gained. This was merely due to the fact that as the fish increases in size and in age, their activity and metabolism increases thereby increasing the demand for dissolve oxygen in the plastic tanks while oxygen supply remained constant thus it will be always important to increase the dissolve oxygen supply as weight gained increases.
(iv) Evaluating the feasibility of large scale production of Clarias gariepinus using locally affordable protein source (Blood Meal) under favorable water quality conditions:
T2 had the best growth, nutrient utilization and one of the most affordable cost compared to that of the other treatments, it means if juveniles are fed with T2 deviating from the standard commercial diet which is the control T0, it is going to reduce the cost if feed from 393frs CFA (T0) to 373frs CFA (T2) per 100 grams of feed thus given a cost reduction of 20 frs CFA/100 g of feed. This implies that working on a large scale fish production say 10,000 kgs (10,000,000 g) of feed, it is going to reduce cost of affording feed as follows;
Using fish meal/g = 393 frs/100 g = 3.93 frs/g
Hence for 10000 kg = 3.93 × 10.000.000 = 39,300,000 frs CFA
Using T2 (10% Blood Meal inclusion) /g = 373 frs/100g = 3.73 frs/g
Hence for 10000 kg = 3.73 × 10.000.000 = 37,300,000 frs CFA
Thus using T2 = 39,300,000 frs CFA – 37, 300, 000 frs CFA
= 2,000,000 frs CFA/10000 kg
Therefore, Blood meal can be included in fish feed up to 10% after which it is deleterious to the animal. Abattoir blood is currently a waste and a major source of pollution in Cameroon since most slaughter houses are located just besides the stream where the blood from the slaughter houses is being disposed directly into stream thereby causing serious threat to water quality. The use of blood meal at 10% inclusion (T2) will greatly reduce the cost of fish feed for Cameroon fish farmers thereby reducing the cost of fish farming with a feasible opportunity for large scale aquaculture serving as an approach to “blue revolution”, sustainable development and poverty alleviation in Cameroon, Africa and the world at large while also serving as a sound practice and an emerging solution to water pollution problems.
Recommendations
Since the study proves that the Dissolve Oxygen has a negative correlation with weight gained, it is recommended that there should be also a proportionate increase in dissolve oxygen supply during aquaculture practices so as to reduce the oxygen deficiency in fish tanks.
There is need for further research to be carried out in order to determine the effects of the various experimental diets on the reproductive performance on Clarias gariepinus brood stock.
Although blood meal has proven to be effective substitutes and secondary protein source to fish meal in aquaculture practice, its role should be addressed in the light of new information and public confidence in commercial animal based feeds.
References
- Brink D (2001) Aquaculture production in South Africa. Proceedings of the Animal Feed Manufacturers Association. Pretoria, South Africa.
- FAO (2004). The state of world fisheries and aquaculture (SOFIA) 2004. FAO Corporate Document Repository. FAO Fisheries Department. Rome: Food and Agriculture Organization of the United Nations.pp: 153.
- Marimuthu KA, Muralikrishnan S (2010) Effect of different-feeding frequency on the growth and survival of african catfish (clariasgariepinus) fingerlings. Adv Environ Biol 4: 187-193.
- De Graaf G, Janssen H (1996) Artificial reproduction and pond rearing of African catfish Claris gariepinusin sub-saharan Africa - A handbook. FAO Fisheries Technical Paper No 362, Rome. pp: 73.
- Brummett RE, Pouomogne V, Gockowski J (2006) Development of integrated aquaculture-agriculture systems for smallscale farmers in the forest margins of Cameroon. DFID Final technical report. World Fish Center, Malaysia. pp: 32.
- Jena JK, Mitra G, Biswal S (2012) Effects of dietary protein levels on the growth and nutrient utilization of fringe-lipped carp, Labeofimbriatus(Bloch) fingerlings. Journal of Aquaculture Nutrition18: 628-639.
- Abid M, Ahmed MS (2009) Growth response of LabeoRohitafingerlings fed with different feeding regimes under intensive rearing. J Anim Plant Sci19: 45-49.
- Craig S, Helfrich IA (2002) Understanding fish nutrition, feeds and feeding. Department of fisheries and wildlife science, Virginia tech.
- Agbebi OT, Otubusin SO, Ogunleye FO (2009) Effects of different levels of substitution of fishmeal with blood meal in pelleted feeds on catfish (Clariasgariepinus) culture in net cages. Eur J Sci Res 31: 6-10.
- Nana C (2008) Research Methods and Statistical Analysis, A practical guide for applied statistics using SPSS. Buea: GOOAHEAD & FASTDAM. pp: 335.
- El-Sayed A-FM (2004) Protein nutrition of farmed tilapia: searching for unconventional sources. In: Bolivar RB, Mair GC, Fitzsimmons K (eds.) New dimensions on farmed tilapia Proceedings of the Sixth International Symposium on Tilapia in Aquaculture. pp: 12-16.
- Olukunle AO, Ogunsanmi AO, Taiwo VO, Samuel AA (2002) The nutritional value of Cow blood on the growth performance, haemathology and plasma enzymes of hybrid cat fish. Nigerian Journal of Animal Science 5: 75-85.
- Sogbesan OA, Ugwumba AAA, Madu CT (2006) Nutritive potentials and utilization of garden snail (Limicolaria aurora) meat meal in the diet of Clariasgariepinusfingerlings, Afr J Biotechnol 5: 1999-2003.
- Amisah S, Oteng MA, Ofori JK (2009) Growth and performance of the African cat fish, Clariasgariepinus fed varying inclusion level of Leucaenaleucocephataleaf meal. J ApplSci Environ Manag 13: 21-26.
- Eyo AA, Falayi BA, Adetunji OM (2004) Response of genetically improved HeterobranchusLongifillesjuveniles to different diets containing beans meal and extrude soya beans meal. J ApplSci Environ Manag 8: 29-33.
- Adejoke AA (2012) Bovine blood and ruminant digesta in Catfish Clariasgariepinus(Burchell 1822) diet. Eur J Sci Res 83: 167-172.
- Otubusin SO (2001) Economics of small scale table size Tilapia production in net- cages. ASSET Series A 1: 83-90.
- Abdel-Warith AA, Russell PM, Davies SJ (2001) Inclusion of a commercial poultry by-product meal as a protein replacement of fish meal in practical diets for African catfish Clariasgariepinus(Burchell 1822). Aquaculture Research 32: 296- 305.
- Natacha N, Nereida C, Carlos A, Tiago A (2012) Inclusion of low levels of blood and feather meal in Practical diets for Gilthead Seabream (Sparusaurata). Turkish Journal of Fisheries and Aquatic Sciences 12: 641-650.
- Bekibele DO, Ansa EJ, Agokei OE, Opera JY, Alozie-Chidi VC, et al. (2012) The comparative effect of fish meal and blood meal based diets on growth and survival of juvenile Tilapia (Oreochromisniloticus) in concrete tanks. J Fish AquatSci 8: 184-189 .
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 37876
- [From(publication date):
September-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 35756
- PDF downloads : 2120