Research Article Open Access
Effects of Unprocessed Versus Vinegar-Processed Schisandra chinensis on the Activity of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 Enzymes in Rats
Lianlin Su1,2, Lijun Wang2, Ping Li2, Chunqin Mao2*, Min Hao2 and Tulin Lu1,2*
1The Key Laboratory of Chinese Herbal Medicine Processing of Jiangsu Province, Nanjing, 210046, PR China
2College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, 210046, PR China
- *Corresponding Author:
- Chunqin Mao
College of Pharmacy, Nanjing University of Chinese Medicine
Nanjing, 210046, PR China
Tel: 862585811001
E-mail: mcq63@163.com
Tulin Lu
The Key Laboratory of Chinese Herbal Medicine Processing of Jiangsu Province
Nanjing, 210046, PR China
Tel: 02585811835
E-mail: ltl209@163.com
Received Date: August 21, 2017; Accepted Date: August 26, 2017; Published Date: August 29, 2017
Citation: Su L, Wang L, Li P, Mao C, Hao M, et al. (2017) Effects of Unprocessed Versus Vinegar-Processed Schisandra chinensis on the Activity of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 Enzymes in Rats. J Anal Bioanal Tech 8:375. doi: 10.4172/2155-9872.1000375
Copyright: © 2017 Su L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Schisandra chinensis (SC) is a well-known traditional Chinese herbal medicine that has been used in clinical practices for thousands of years. However, the differences between the effects of unprocessed and vinegarprocessed Schisandra chinensis (VSC) on cytochrome P450 (CYP450) activities are poorly understood. To evaluate the differences between processed and unprocessed SC on the metabolism of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 substrates in rats using a cocktail method based on a developed and validated high performance liquid chromatography-mass spectrometry (HPLC-MS) method. Six probe substrates (coumarin (CYP2A6), bupropion (CYP2B6), paclitaxel (CYP2C8), tolbutamide (CY2C9), omeprazole (CYP2C19) and metoprolol (CYP2D6)) were delivered simultaneously into rats treated with single or multiple doses of processed or crude SC extract. The plasma concentrations of the six probes were profiled by HPLC-MS, and their corresponding pharmacokinetic parameters were calculated. Treatment with single or multiple doses of either extract of SC or VSC induced CYP2A6, CYP2B6 and CYP2C9 enzyme activity and inhibited CYP2D6, CYP2C19 and CYP2C8 enzyme activity in rats. Furthermore, the inhibitory or induced effect of multiple doses of SC was more potent after vinegar processing than without vinegar processing. CYP2A6, 2B6 and 2C9 enzyme activity were induced significantly after treatment with multiple doses but not after a single dose. CYP2C19 enzyme activity were inhibited significantly after treatment with multiple doses but not after a single dose. These results provide useful scientific data for the safe clinical application of either extract of SC in combination with other drugs, which should lack the side effects induced by other herb-drug interactions.
Keywords
Schisandra chinensis; Vinegar-processed; CYP450; Pharmacokinetic; Cocktail
Introduction
Schisandra chinensis(Wuweizi in China), the dry ripe fruits of Schisandra chinensis (Turcz.) Baill., officially listed as a sedative and tonic in the China Pharmacopoeia, has been used as an important component in various prescriptions in traditional Chinese medicine (TCM).
In recent decades, SC has been shown to have some pharmacologically useful properties for the management of certain intractable diseases, such as hepatitis [1]. SC has the ability torestore injured hepatocytes and remove free radicals from the body [2]. The results of many studies showed that the chemical constituents of Schisandra chinensiswere mainly lignans, polysaccharides, volatile oils, three terpenoids, sesquiterpene and organic acids [3-5]. Lignans with dibenzocyclooctadiene skeletons are regarded as the main bioactive components, these include schizandrin A, B and C, schizandrol A and B, schizandrer A and B, gomisin J, γ-schisandrin, gomisin N and angeloylgomisin H [6-9]. These compounds are known to possess a wide variety of biological activities, including anti-hepatotoxicity, anti-oxidation, detoxification and inhibition of hepatocarcinogenesis [10-12]. Recently, several studies have shown that some different types of lignan compounds present in SC are able to induce or inhibit CYP450 activity in human liver microsomes [13-16]. Thus far, most pharmacological and pharmacokinetic studies have examined single components of SC. However, no single component can be completely responsible for the effects of the complete SC extract. In addition, the effects of unprocessed versus vinegar-processed SC on CYP450 activities are poorly understood. The purpose of the present study was to evaluate the difference between unprocessed SC and SC processed with vinegar (VSC) on CYP450 activities.
It is well known that the liver is the main organ responsible for drug metabolism, nearly 60% of the most commonly prescribed drugs are metabolized by the CYP450 system [17]. CYP450 enzymes constitute the major drug metabolism enzyme system in humans [18,19], which participate in the formation of steroid hormones, bile acids and bile pigments, as well as in transformation of many drugs [20,21]. In liver cells, in addition to a small part of drug metabolizing enzymes in nucleus, cytoplasm, cell membrane and mitochondria, the vast majority of drug metabolism is involved in microsomal enzymes located in the endoplasmic reticulum, and metabolic enzyme CYPP450 enzyme is one of the most important, there are more than 90% involved in the metabolism of drugs, as an important phase I metabolic enzyme. The CYP450 enzyme system plays a major role in drug metabolism in the body,it has 9 major isoforms enzymes of the three families included CYP1, CYP2 and CYP3, namely CYP1A2, CYP2A6, CYP2B6, CYP2C8, CY2C9, CYPZC19, CYP2D6, CYP2E1, CYP3A4[22-24]. All of them accounted for about 8.9%, 5%, 7.2%, 4.7%, 12.8%, 30.2%, 6.8%, 20%, 7% in liver cells, respectively [24,25].
CYP1A2, CYP2E1 and CYP3A4 are the three major enzyme subtypes in the CYP450 system. We have researched on the effects of SC and VSC on the activity of CYP1A2, CYP2E1 and CYP3A4 enzymes in rats in previous studies [26]. The 10 most significant drug metabolizing CYPs in the human liver include CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, CYP2D6 and CYP3A4/5. So, in the present study, we will continue to focus on the activity of the other 6 CYP450 enzymes, such as CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6. Herbs that contain potent inhibitors of one or more CYP450 enzyme subtypes may cause herb–drug interactions with adverse effects. Therefore, the evaluation of potential herb-drug interactions is important for the selection of candidate drugs during preclinical evaluation. Here, we evaluated the effect of SC on the CYP450 system using the ‘cocktail’ approach, which involves multiple probe drugs. This approach has been widely used to monitor the influence of drugs on the P450 activities and potential drug-drug or herb–drug interactions [27-30]. The activity of drug metabolism enzymes can be assessed in vivo by the use ofspecific probe drugs [31]. For example, tolbutamide and ibuprofen are often used as the probe substrates of CYP2C9, dextromethorphan, codeine and propranololare used for CYP2D6, and omeprazole and amitriptyline are often used for CYP2C19[32].
SC and VSC are commonly used in Chinese patent drugs and TCM prescriptions. In the Chinese pharmacopoeia, there are more than ten Chinese patent drugs containing SC or VSC, including Sheng-mai-yin and Hu-gan-pian [33-35]. With the aim of avoiding possible side effects induced by herb-drug interactions, we evaluated the effects of SC and VSC on the activities of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 enzymes in rats. We used a cocktail strategy of probes containing coumarin, bupropion, paclitaxel, tolbutamide, omeprazole and metoprolol and an approach based on a developed and validated HPLC-MS method to assess CYP450 activities in vivo. We predict that the results may be useful for the clinical safety evaluation of herb-drug interactions involving SC or VSC.
Experimental
Chemicals and reagents
HPLC-grade methanol and acetonitrile were purchased from Shandong Yuwang Industrial Co. Ltd. (Shandong, China) and Merck (Merck, Darmstadt, Germany), respectively. Ultra-pure water was purified using an Milli-Q Gradient A10 superpurification system (Millipore, Bedford, MA, USA). The distilled water was used for sample extraction and preparation. Omeprazole, tolbutamide, coumarin, paclitaxel and diazepam (internal standard, IS) were purchased from the Shanghai yuanye Biotechnology Co., Ltd. (Shanghai, China). Bupropion hydrochloride andchrysophanol (internal standard, IS) were obtained from China National Institute for drug and biological products (Beijing, China).
Materials
The crude SC herb was purchased from Anhui Fengyuan Tongling Herbal Pieces Co. Ltd.(China), Batch number: LOT#140524. All the raw materials were identified by the corresponding author. The voucher specimens (NO. 101215) were deposited in the Jiangsu Key Laboratory of Chinese Herbal Medicine ProcessingResearch of the Nanjing University of Chinese Medicine.
Instrumentation
HPLC-MS was performed on an Agilent 1200-6120B. The instrument (Agilent Technologies, USA) consisted of a G1322A vacuum degassing machine, G1312B double pump, G1367DA automatic injector, G1316 column temperature box and ESI.
Extract preparation
VSC: SC (100 g) and 20 mL of a vinegar-water mixture (20:80, v/v) were mixed well in a suitable airtight container. After being moisturized for 1.5 h, the mixture was steamed for 5 h, removed from the container and dried.
SC and VSC extract: Pieces of SC or VSC (100 g) were boiled in 85% ethanol with a reflux condenser 2 times for 2 h each time. The extracts were then merged and evaporated off under vacuum at 40ΓΆΒ?Β?. The fraction was dissolved in 0.5% sodium carboxymethyl cellulose at a concentration of 1 g/mL and administered to rats.
Chromatographic conditions
HPLC separation was achieved on a Purospher® STAR LP RP-18 end capped (Hibar® RT, 250 mm × 4.6 mm, 5 µm) with the column temperature set at 25ΓΆΒ?Β?. The mobile phase consisted of (A) 0.01 mol·L-1ammonium acetate and (B) methanol, and a gradient elution of 20%-40% B at 0-15 min, 40%-55% B at 15-30 min,55%-58% B at 30-40 min, 58%-70% B at 40-50 min, 70%-80% B at 50-58 min, and 20% B at 58-60 min was employed. The flow rate was 1 mL·min-1. The injection volume was 10 µL.
Mass spectrometry condition
In this study, for the mass detection, the instrument was operated in positive and negative ion electro spray mode. Selective reaction ion monitoring(SIM) was used in the detection mode. The conditions of the MS detector were as follows: Fragmentor voltage: 150 V; Drying gas flow rate: 8 L · min-1; dry gas temperature: 350ΓΆΒ?Β?; capillary voltage: 3000 V; atomizer pressure: 35 psi.
Plasma collection
Male Sprague-Dawley (SD) rats weighing 180~200 g was purchased from Shanghai Jiesijie Lab Animal Co. Ltd, License number: SCXK (Shanghai) 2014-0006. The experiments were conducted following an approved protocol from the Nanjing University of Chinese Medicine Animal Care and Use Committee. The animals were acclimatized to laboratory conditions for more than a week before the experiments.
Plasma collection (single dose): Eighteen rats were randomly divided into an SC group, a VSC group and a control group, which were given a single 3.5 g/kg dose of SC, VSC and vehicle (5%CMC-Na), respectively. After five minutes, a probe solution mixed with metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide (25 mg/kg) was administered orally to the rats. Blood samples (0.25 mL) were collected in heparinized tubes pre-dose (0 hour) and at 0.05, 0.15, 0.25, 0.5, l, 1.5, 2, 2.5, 4, 6, 8, 10, 12 and 24 hours post-dose. Plasma samples were collected after centrifugation at 12, 000 rpm for 10 min and stored at -80ΓΆΒ?Β?.
Plasma collection (multiple doses): Eighteen rats were randomly divided into an SC group, a VSC group and a control group, which were given a 3.5 g/kg dose of SC, VSC or vehicle, respectively. After oral administration of SC or VSC extracts for 7 consecutive days, each group was given a probe solution mixed with metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide (25 mg/kg) on the eighth day. Blood samples (0.25 mL) were then collected into heparinized tubes pre-dose (0 hour) and at 0.05, 0.15, 0.25, 0.5, l, 1.5, 2, 2.5, 4, 6, 8, 10, 12 and 24 hours post-dose. The plasma samples were collected after centrifugation at 12, 000 rpm for 10 min and then stored at -80ΓΆΒ?Β?.
Preparation of plasma
TenµL of a reference substance and 10µL of each internal standard solution (diazepam: 41.20µg/mL; chrysophanol: 40.40 µg/mL) were added to a 100 µL plasma sample. The plasma sample was then spiked with 450µL acetonitrile and vortexed for 3 min. The precipitated protein was removed by centrifugation at 12, 000 rpm for 10 min. The organic layer (400 µL) was evaporated to dryness under nitrogen and stored at -20ΓΆΒ?Β? until analysis. For HPLC-MS analysis, the residue was dissolved in 100 µL acetonitrile-water (50:50, v/v), and a 10 µL aliquot was injected into the column. The standards were prepared in the same way.
Preparation of the standard solutions and quality control samples
The “Guidance for Industry-Bioanalytical Method Validation” document from the FDA was used as a guide for the assay validation, which is described below.
Calibration curve: A series of stock standard solutions were added to blank plasma to yield eight different concentrations: for metoprolol, they were 0.0424, 0.0848, 0.2120, 0.4241, 1.0602, 1.6963, 2.5445 and 3.3926 μg/mL; for coumarin, they were 0.2058, 0.4115, 1.0288, 2.0576, 5.1440, 8.2304, 12.3456 and 16.4608 μg/mL; for omeprazole, they were 0.1384, 0.2768, 0.6920, 1.3840, 3.4600, 5.5360, 8.3040 and 11.0720 μg/mL; for bupropion, they were 0.1014, 0.2029, 0.5072, 1.0144, 2.5360, 4.0576, 6.0864 and 8.1152 μg/mL; for paclitaxel, they were 0.1974, 0.3948, 0.9870, 1.9740, 4.9350, 7.8960, 11.8440 and 15.7920 μg/mL;and for tolbutamide, they were 0.1201, 0.2402, 0.6006, 1.2012, 3.0030, 4.8048, 7.2072 and 9.6096 μg/mL. TenµL of two internal standards were then added, respectively. The samples were extracted and analyzed according to the sample preparation procedures described above. Each calibration curve was constructed by plotting the peak area ratio of diazepam and chrysophanol/IS+/- versus the concentration of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide using linear regression.
Recovery: The extraction recoveries (ER) of metoprolol, coumarin,omeprazole, bupropion, paclitaxel and tolbutamide from plasma were determined at three different concentrations of quality control (QC) samples (for metoprolol: they were 0.0848, 1.0602 and 2.5445 μg/mL; for coumarin: they were 0.4115, 5.1440 and 12.3456 μg/mL; for omeprazole: they were 0.2768, 3.4600 and 8.3040 μg/mL; for bupropion: they were 0.2029, 2.5360 and 6.0864 μg/mL; for paclitaxel: they were 0.3948, 4.9350 and 11.8440 μg/mL; and for tolbutamide: they were 0.2402, 3.0030 and 7.2072µg/mL). Five µL of an internal standard was added to 100 µL blank plasma; we then extracted and analyzed the samples according to the aforementioned procedure. For the reference material, the same concentrations of standard solutions were injected directly into the HPLC-MS system. The ER of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide were calculated by comparing the mean peak area (n=6 at each concentration) of the extracted QC samples with the unextracted standard solution containing the equivalent amount of analyses.
Accuracy and precision
To assess the intra-day precision,inter-day precision and accuracy of the assay, QC samples at threedifferent concentrations were prepared under the same samplepreparation procedures. The intra-day precision of the assay wasassessed by calculating the relative standard deviation (RSD) for theanalysis of samples for quality control in six replicates, and theinter-day precision was determined by analysis of samples forquality control in three consecutive days. The accuracy wasexpressed by the relative error (RE).
Stability: The stability of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide were evaluated under conditions mimicking the situations during sample storage and the analytical process by analyzing three replicates of QC samples for each analysis. Thefreeze-thaw stability was determined after one freeze and thaw cycle. The QC samples were stored at -80ΓΆΒ?Β? for 30 days and thawed unassisted at room temperature for 24 hours.
Data and statistical analysis: The plasma concentrations of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide were expressed as the mean ± SD, and the mean concentration-time curves were plotted. Pharmacokinetic parameters were computed using the software programs DAS 1.0 (China) and SPSS 16.0 (USA). Differences among group mean values were assessed using a two-tailed, two sample t-test that assumed equal variance. A difference of p<0.05 was considered statistically significant.
Results and Discussion
Specificity
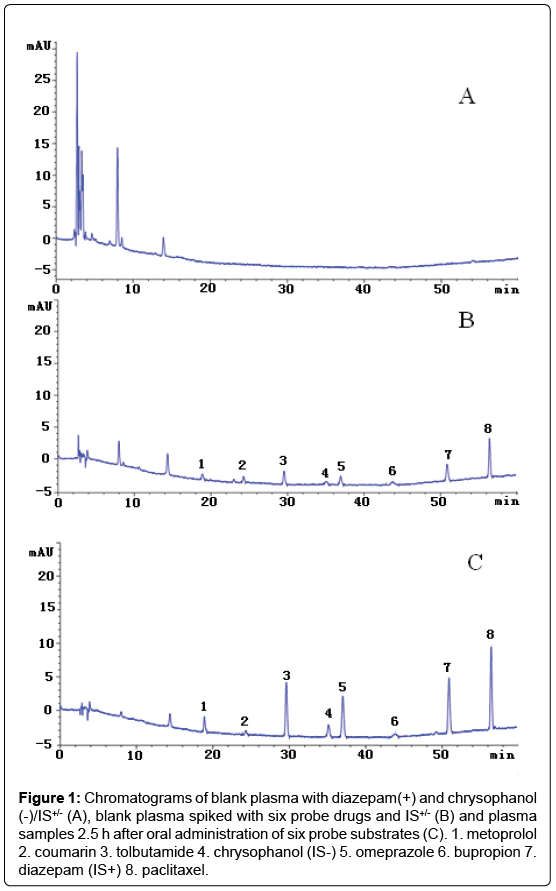
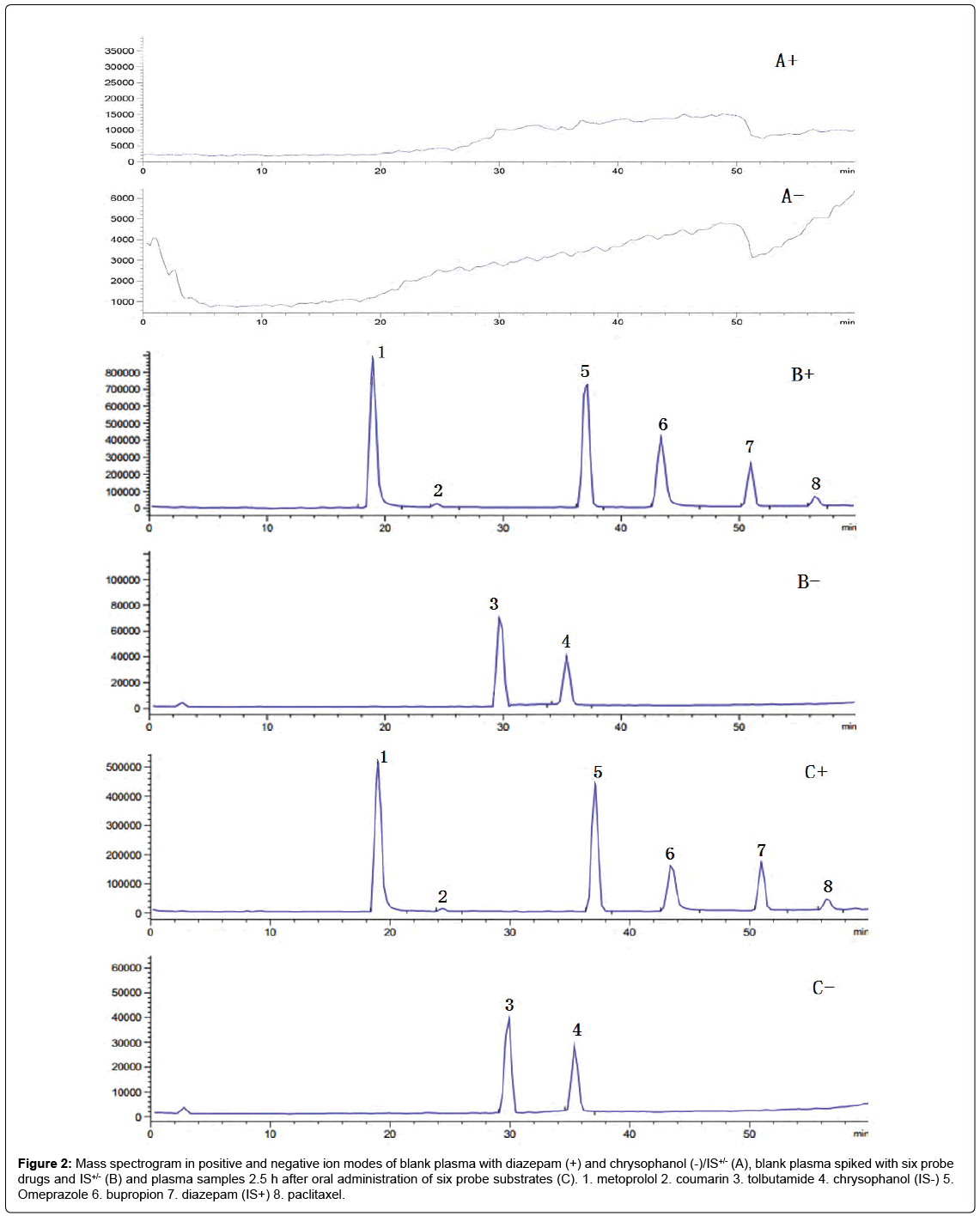
Under optimized chromatographic and mass spectrometry conditions, the retention times of metoprolol, coumarin, omeprazole, bupropion, paclitaxel,tolbutamide and the internal standards, diazepam(IS+), chrysophanol(IS-) were 18.88, 24.35, 37.00, 43.85, 56.42, 29.60, 50.90 and 35.13 min, respectively (Figures1 and 2). These results indicate that the assay had adequate specificity.
Figure 1: Chromatograms of blank plasma with diazepam(+) and chrysophanol (-)/IS+/- (A), blank plasma spiked with six probe drugs and IS+/- (B) and plasma samples 2.5 h after oral administration of six probe substrates (C). 1. metoprolol 2. coumarin 3. tolbutamide 4. chrysophanol (IS-) 5. omeprazole 6. bupropion 7. diazepam (IS+) 8. paclitaxel.
Figure 2: Mass spectrogram in positive and negative ion modes of blank plasma with diazepam (+) and chrysophanol (-)/IS+/- (A), blank plasma spiked with six probe drugs and IS+/- (B) and plasma samples 2.5 h after oral administration of six probe substrates (C). 1. metoprolol 2. coumarin 3. tolbutamide 4. chrysophanol (IS-) 5. Omeprazole 6. bupropion 7. diazepam (IS+) 8. paclitaxel.
Linearitd lower limit of quantification
Each calibration curve contained eight different concentrations of standard was constructed by plotting the peak area ratio of IS versus the concentrations of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide using linear regression. The regression equation was Y=147.9250 X-1.0124 (r=0.9996) for metoprolol, Y=0.5654 X-0.0849 (r=0.9991) for coumarin, Y=35.7308X-6.8690 (r=0.9990) for omeprazole, Y=25.6944 X-0.7178 (r=0.9992) for bupropion, Y=2.0537 X-0.0109 (r=0.9993) for paclitaxel and Y=24.5452X-1.2452 (r=0.9991) for tolbutamide, where X was the concentration of the analyte in rat plasma and Y was the ratio of the analyte peak area to that of the internal standard. Based on a signal-to-noise ratio (S/N=10), the lower limits of quantification (LLOQ) for metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide were 0.4241, 0.2058, 0.1384, 0.1014, 0.1974 and 0.1201µg/mL, respectively.
Recovery
Table 1 presents the recoveries of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide. As shown in Table 1, the mean recoveries were 97.53%, 106.97%, 89.26%, 107.73%, 95.35% and 88.53%, respectively.
| Analytes | Concentration(µg/ml) | Recovery | |
|---|---|---|---|
| Mean(%) | RSD(%) | ||
| Metoprolol | 0.0848 | 95.28±8.96 | 9.40 |
| 1.0602 | 97.53±5.91 | 6.06 | |
| 2.5445 | 91.52±7.27 | 7.94 | |
| Coumarin | 0.4115 | 92.78±9.03 | 9.74 |
| 5.1440 | 104.65±6.58 | 6.29 | |
| 12.3456 | 106.97±3.40 | 3.18 | |
| Omeprazole | 0.2768 | 89.15±9.96 | 11.17 |
| 3.4600 | 98.67±8.71 | 8.82 | |
| 8.3040 | 89.26±1.54 | 1.72 | |
| Bupropion | 0.2029 | 106.90±6.01 | 5.63 |
| 2.5360 | 106.02±4.93 | 4.65 | |
| 6.0864 | 107.73±3.35 | 3.11 | |
| Paclitaxel | 0.3948 | 95.35±2.74 | 2.87 |
| 4.9350 | 101.89±5.13 | 5.04 | |
| 11.8440 | 88.52±4.33 | 4.90 | |
| Tolbutamide | 0.2402 | 76.09±4.02 | 5.29 |
| 3.0030 | 88.53±3.42 | 3.86 | |
| 7.2072 | 78.23±4.07 | 5.20 | |
Table 1: Recovery of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide in rat plasma (mean ± SD, n=5).
Precision and accuracy
Table 2 shows the intra-day precision, inter-day precision and accuracy of the three probe drugs. The precisions for the measurement of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide were calculated as the relative standard deviation (RSD) at three concentrations and were lower than 15% for intra-day and inter-day assays, and the accuracy was within 10% for the QC samples. The results demonstrate that the precision and accuracy of this method were acceptable.
| Analytes | Concentration(µg/mL) | Intra-dayassay | Inter-dayassay | Accuracy(%) | ||
|---|---|---|---|---|---|---|
| Measuredquantity(µg/mL) | RSD(%) | Measuredquantity(µg/mL) | RSD(%) | |||
| Metoprolol | 0.0848 | 0.0755±0.0080 | 10.56 | 0.0787±0.0056 | 7.16 | 96.10±7.04 |
| 1.0602 | 1.0820±0.1068 | 9.87 | 1.0252±0.0835 | 8.15 | 88.29±5.23 | |
| 2.5445 | 2.4245±0.0880 | 3.63 | 2.2846±0.1260 | 5.52 | 94.47±7.43 | |
| Coumarin | 0.4115 | 0.3720±0.0493 | 13.25 | 0.3972±0.0336 | 8.45 | 99.45±5.63 |
| 5.1440 | 4.8215±0.4832 | 10.02 | 4.8687±0.3568 | 7.33 | 85.72±5.13 | |
| 12.3456 | 14.5056±0.6941 | 4.78 | 13.2173±1.5038 | 11.38 | 99.00±3.09 | |
| Omeprazole | 0.2768 | 0.2429±0.0101 | 4.16 | 0.2509±0.0136 | 5.40 | 91.47±6.82 |
| 3.4600 | 2.9733±0.4246 | 14.28 | 3.1382±0.1735 | 5.53 | 92.42±7.87 | |
| 8.3040 | 7.1799±0.7783 | 10.84 | 7.2219±0.1126 | 1.56 | 87.73±1.49 | |
| Bupropion | 0.2029 | 0.2252±0.0132 | 5.86 | 0.2026±0.0269 | 13.25 | 93.36±4.71 |
| 2.5360 | 2.6825±0.1723 | 6.42 | 2.3998±0.2602 | 10.84 | 86.28±3.98 | |
| 6.0864 | 6.5486±0.7326 | 11.19 | 6.3143±0.2069 | 3.28 | 91.17±2.82 | |
| Paclitaxel | 0.3948 | 0.3777±0.0473 | 12.53 | 0.3759±0.0255 | 6.78 | 93.57±0.77 |
| 4.9350 | 5.0259±0.1971 | 3.92 | 5.0353±0.3613 | 7.18 | 89.06±3.78 | |
| 11.8440 | 11.1878±0.8951 | 8.00 | 11.3772±1.1201 | 9.84 | 92.94±4.18 | |
| Tolbutamide | 0.2402 | 0.2161±0.0125 | 5.80 | 0.2277±0.0216 | 9.50 | 92.58±7.99 |
| 3.0030 | 3.0935±0.2425 | 7.84 | 3.1061±0.2209 | 7.11 | 98.32±4.96 | |
| 7.2072 | 7.2642±0.2199 | 3.03 | 7.2143±0.9034 | 12.52 | 95.99±3.76 | |
Table 2: Precision and accuracy of the measurement of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide levels in rat plasma (mean ± SD, n=5).
Stability
Table 3 shows the stability of the three probe drugs. The metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide stabilities were measured by analyzing QC samples at three concentrations that had been exposed to sample storage conditions. The results of these studies demonstrated that there was no significant degradation of metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide occurred in plasma under our experimental conditions.
| Analytes | Parameter | Groups | ||
|---|---|---|---|---|
| Controlgroup | SCgroup | VSCgroup | ||
| Metoprolol | AUC0-t(µg·h/mL) | 5.1497±0.2786 | 8.7300±0.2408# | 11.8568±0.3074#* |
| AUC0-∞(µg·h/mL) | 5.2854±0.3338 | 8.8647±0.3044# | 12.2354±0.4896#* | |
| Cmax(µg/mL) | 2.3784±0.3072 | 3.0277±0.2002# | 3.2104±0.2076# | |
| MRT0-t(h) | 4.32±0.24 | 3.82±0.09 | 4.83±0.11 | |
| t1/2(h) | 5.06±0.76 | 5.31±0.72 | 5.47±0.71 | |
| Tmax(h) | 0.58±0.20 | 1.42±0.20 | 1.58±0.20 | |
| CL/F(L·kg/h) | 3.7960±0.2300 | 2.2580±0.0790# | 1.6370±0.0650# | |
| Coumarin | AUC0-t(µg·h/mL) | 54.1152±0.9331 | 38.1747±2.0796# | 25.2455±2.5606# |
| AUC0-∞(µg·h/mL) | 54.4397±1.0428 | 38.5815±2.1143# | 27.8311±2.1492# | |
| Cmax(µg/mL) | 16.6153±0.1487 | 13.9790±0.8891# | 12.9528±1.2166# | |
| MRT0-t(h) | 4.60±0.04 | 5.17±0.10# | 5.65±0.46# | |
| t1/2(h) | 3.84±0.54 | 4.14±0.17 | 6.60±0.80 | |
| Tmax(h) | 0.47±0.77 | 0.32±0.03# | 0.26±0.03## | |
| CL/F(L·kg/h) | 0.3670±0.0070 | 0.5200±0.0270 | 0.7202±0.0590 | |
| Omeprazole | AUC0-t(µg·h/mL) | 23.1121±0.4006 | 48.1232±2.6523## | 51.4295±0.5609## |
| AUC0-∞(µg·h/mL) | 24.9804±1.1371 | 48.1304±2.6518## | 51.7283±0.7926## | |
| Cmax(µg/mL) | 8.3489±0.2343 | 10.6587±0.4038## | 11.4450±0.2216## | |
| MRT0-t(h) | 3.89±0.51 | 4.55±0.11# | 4.64±0.02# | |
| t1/2(h) | 10.96±1.13 | 1.72±0.09## | 1.42±0.05## | |
| Tmax(h) | 1.42±0.20 | 1.42±0.20 | 1.42±0.20 | |
| CL/F(L·kg/h) | 0.8020±0.0380 | 0.4170±0.0240 | 0.3870±0.0060 | |
| Bupropion | AUC0-t(µg·h/mL) | 18.7938±0.3026 | 14.8704±1.7092# | 10.3694±0.1785# |
| AUC0-∞(µg·h/mL) | 19.8732±1.8555 | 21.8965±3.0423 | 14.0738±1.9375# | |
| Cmax(µg/mL) | 8.3693±0.0850 | 7.6907±0.2917 | 6.8112±0.2250 | |
| MRT0-t(h) | 4.58±0.05 | 5.23±0.35# | 6.05±0.08## | |
| t1/2(h) | 5.00±0.57 | 14.34±0.72## | 12.41±1.36## | |
| Tmax(h) | 0.47±0.07 | 0.32±0.03# | 0.26±0.03## | |
| CL/F(L·kg/h) | 1.0130±0.0830 | 0.9270±0.1180 | 1.3945±0.1991 | |
| Paclitaxel | AUC0-t(µg·h/mL) | 68.4310±2.9991 | 105.0422±2.6293## | 118.8637±3.9188## |
| AUC0-∞(µg·h/mL) | 69.5099±2.6129 | 105.3187±2.6830## | 126.1533±4.8135##* | |
| Cmax(µg/mL) | 14.0298±0.8402 | 15.1230±0.4196# | 16.1395±0.2970#* | |
| MRT0-t(h) | 4.73±0.18 | 6.16±0.19## | 6.63±0.19##* | |
| t1/2(h) | 3.72±0.28 | 4.57±0.13## | 6.03±0.80##* | |
| Tmax(h) | 1.42±0.20 | 1.58±0.20 | 1.92±0.20 | |
| CL/F(L·kg/h) | 0.2880±0.0110 | 0.1900±0.0050 | 0.1590±0.0060 | |
| Tolbutamide | AUC0-t(µg·h/mL) | 30.5098±0.9816 | 19.5317±0.3343# | 13.9170±0.2846## |
| AUC0-∞(µg·h/mL) | 31.7177±2.6762 | 21.1236±1.1203## | 24.5130±3.5061## | |
| Cmax(µg/mL) | 9.3479±0.3533 | 7.6528±0.2226# | 6.3227±0.6508## | |
| MRT0-t(h) | 5.02±0.14 | 5.49±0.06 | 6.42±0.12 | |
| t1/2(h) | 6.21±0.14 | 5.38±0.44# | 5.90±0.80# | |
| Tmax(h) | 0.47±0.07 | 0.32±0.03## | 0.26±0.03## | |
| CL/F(L·kg/h) | 0.6340±0.0480 | 0.9168±0.1248 | 0.9352±0.0344 | |
#Significantly different from control, P<0.05; ##Significantly different from control, P<0.01; *Significantly different from SC group, P<0.05; **Significantly different from SC group, P<0.01
Table 3: Pharmacokinetic parameters of the six probe drugs after a single dose of SC or VSC (mean ± SD, n=6).
Application of the method in pharmacokinetics studies
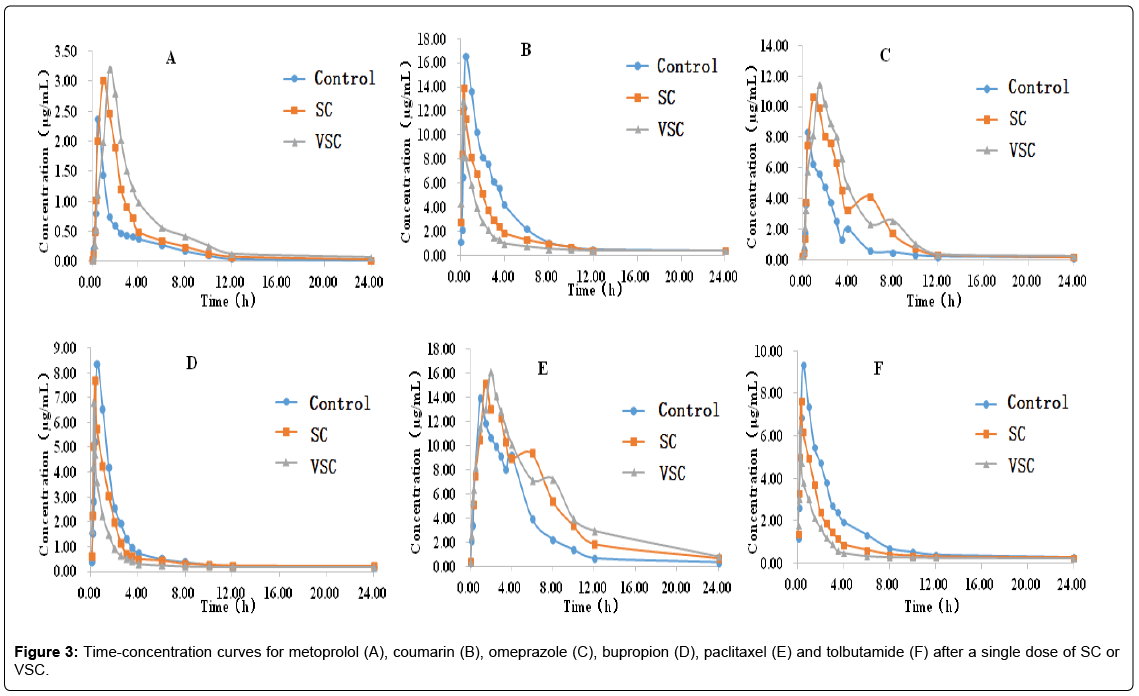
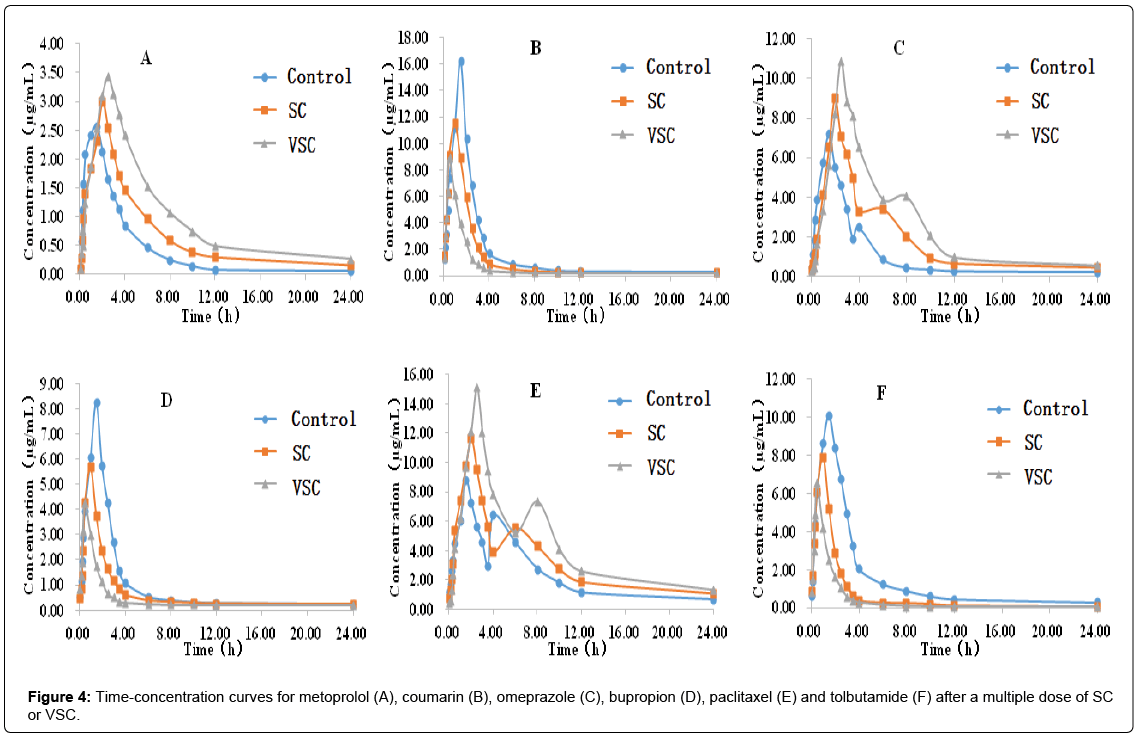
A developed and validated HPLC-MS method was used to determine the levels of the six probe drugs (metoprolol, coumarin, omeprazole, bupropion, paclitaxel and tolbutamide) in rat plasma after single (Figure 3 and Table 4) and multiple (Figure 4 and Table 5) doses of the SC and VSC extracts.
| Analytes | parameter | Groups | ||
|---|---|---|---|---|
| Controlgroup | SCgroup | VSCgroup | ||
| Metoprolol | AUC0-t(µg·h/mL) | 10.3598±0.3400 | 16.2178±0.5100## | 23.6497±0.8887##** |
| AUC0-∞(µg·h/mL) | 10.4838±0.3388 | 17.7865±1.6032## | 24.9862±2.0744##** | |
| Cmax(µg/mL) | 2.5842±0.0865 | 2.9983±0.1476# | 3.4268±0.0126#* | |
| MRT0-t(h) | 4.20±0.10 | 6.19±0.15## | 6.81±0.15## | |
| t1/2(h) | 2.39±0.22 | 3.07±0.38# | 3.15±0.41## | |
| Tmax(h) | 1.42±0.20 | 1.92±0.20# | 2.42±0.20##* | |
| CL/F(L·kg/h) | 1.9095±0.0619 | 1.1322±0.1041## | 0.8048±0.0644##** | |
| Coumarin | AUC0-t(µg·h/mL) | 38.1262±2.7814 | 27.2828±1.6539## | 17.2058±0.4214##** |
| AUC0-∞(µg·h/mL) | 41.8130±4.8695 | 28.0507±1.4121## | 32.2422±3.0508## | |
| Cmax(µg/mL) | 16.2025±0.4166 | 11.5455±0.5385# | 8.9066±1.1289##* | |
| MRT0-t(h) | 4.65±0.10 | 3.34±0.42# | 2.92±0.36#* | |
| t1/2(h) | 6.30±0.82 | 4.37±0.52# | 3.44±0.31##* | |
| Tmax(h) | 1.42±0.20 | 1.42±0.20 | 0.47±0.07##** | |
| CL/F(L·kg/h) | 0.4837±0.0546 | 0.6853±0.0920 | 0.5065±0.0545 | |
| Omeprazole | AUC0-t(µg·h/mL) | 25.2848±1.7822 | 43.5765±1.2493## | 61.2688±1.2511##** |
| AUC0-∞(µg·h/mL) | 27.6797±3.8446 | 43.6245±1.2469## | 62.0482±2.2777##** | |
| Cmax(µg/mL) | 7.1822±0.4498 | 9.0223±0.3645## | 10.9018±0.2667##** | |
| MRT0-t(h) | 4.50±0.12 | 6.03±0.06## | 6.47±0.10##** | |
| t1/2(h) | 1.89±0.16 | 2.28±0.11# | 5.63±0.78##** | |
| Tmax(h) | 1.58±0.20 | 1.92±0.20# | 2.42±0.20##* | |
| CL/F(L·kg/h) | 0.7335±0.0947 | 0.4587±0.0132 | 0.3227±0.0118 | |
| Bupropion | AUC0-t(µg·h/mL) | 23.1380±0.6240 | 15.6132±0.1894## | 10.3997±0.0903#* |
| AUC0-∞(µg·h/mL) | 26.4663±3.4714 | 17.7547±0.2591## | 17.7032±1.6471## | |
| Cmax(µg/mL) | 8.2620±0.1792 | 5.6692±0.1404# | 4.2780±0.1001#** | |
| MRT0-t(h) | 6.49±0.04 | 5.60±0.05# | 4.56±0.08## | |
| t1/2(h) | 11.90±0.98 | 11.80±1.05 | 9.54±0.96#* | |
| Tmax(h) | 1.42±0.20 | 1.42±0.20 | 0.47±0.07##* | |
| CL/F(L·kg/h) | 0.7660±0.0933 | 1.1267±0.0164 | 1.2602±0.0319 | |
| Paclitaxel | AUC0-t(µg·h/mL) | 57.9555±1.3349 | 78.1832±1.3803## | 103.5613±2.9980##** |
| AUC0-∞(µg·h/mL) | 62.0760±4.0296 | 86.6640±8.2491## | 104.1307±2.7055##** | |
| Cmax(µg/mL) | 8.7937±0.4556 | 11.6614±0.7876## | 15.1624±0.2706##** | |
| MRT0-t(h) | 6.73±0.14 | 7.46±0.09## | 7.59±0.15##** | |
| t1/2(h) | 2.42±0.27 | 3.75±0.52# | 6.80±0.93##* | |
| Tmax(h) | 1.58±0.20 | 1.92±0.20# | 2.42±0.20##* | |
| CL/F(L·kg/h) | 0.3233±0.0209 | 0.2323±0.0220 | 0.1923±0.0050 | |
| Tolbutamide | AUC0-t(µg·h/mL) | 37.3023±0.5294 | 15.6508±0.5180## | 9.8770±0.2150##** |
| AUC0-∞(µg·h/mL) | 40.1537±2.2192 | 16.0920±0.6699## | 11.4573±1.7082##** | |
| Cmax(µg/mL) | 10.1065±0.1606 | 7.9201±0.5725## | 6.5617±0.5267##** | |
| MRT0-t(h) | 4.70±0.07 | 2.84±0.11## | 2.51±0.06##** | |
| t1/2(h) | 7.95±1.17 | 7.14±0.57 | 6.87±0.84 | |
| Tmax(h) | 1.42±0.20 | 1.42±0.20 | 0.47±0.07##** | |
| CL/F(L·kg/h) | 0.4992±0.0276 | 1.2447±0.0532 | 1.7773±0.2588 | |
#Significantly different from control, P<0.05; ##Significantly different from control, P<0.01; *Significantly different from SC group, P<0.05; **Significantly different from SC group, P<0.01
Table 4: Pharmacokinetic parameters of the six probe drugs after multiple doses of SC or VSC (mean ± SD, n=6).
| Analytes | Parameter | Singledose | Multipledoses | ||
|---|---|---|---|---|---|
| SC | VSC | SC | VSC | ||
| Metoprolol | AUC0–t | + | + | ++ | ++ |
| AUC0-∞ | + | + | ++ | ++ | |
| Coumarin | AUC0–t | | - | -- | -- |
| AUC0-∞ | - | - | -- | -- | |
| Omeprazole | AUC0–t | ++ | ++ | ++ | ++ |
| AUC0-∞ | ++ | ++ | ++ | ++ | |
| Bupropion | AUC0–t | - | - | -- | - |
| AUC0-∞ | - | -- | -- | ||
| Paclitaxel | AUC0–t | ++ | ++ | ++ | ++ |
| AUC0-∞ | ++ | ++ | ++ | ++ | |
| Tolbutamide | AUC0–t | - | -- | -- | -- |
| AUC0-∞ | -- | -- | -- | -- | |
+: AUC increased; -: AUC decreased; ++: AUC significantly increased; --: AUC significantly decreased
Table 5: The analysis results for the six probe drugs.
Table 4 showed that after a single dose of SC or VSC, there was no change (P>0.05) in t1/2, Tmaxand MRT0–tfor metoprolol; no change in t1/2and MRT0–t for coumarin; no change in t1/2, Tmaxand CL/F for omeprazole; no change in AUC0-∞, Cmax, CL/F and MRT0–t for bupropion; no change in Tmax and CL/Ffor paclitaxel and no change in CL/F and MRT0–t for tolbutamide. Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞and Cmax and decreases in CL/F for metoprolol. When compared with the SC group, the VSC group had a higher AUC0–t and AUC0-∞and a lower CL/Ffor metoprolol (P<0.05), it was indicated that SC could inhibit CYP2D6 and the inhibitory effect was more pronounced in the VSC group. Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, AUC0-∞, Tmax and Cmax(P<0.05) and increases in CL/F for coumarin (P<0.05),that was indicated that SC could induce the activity of CYP2A6. Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞, MRT0-t and Cmax for omeprazole (P<0.01). Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, Tmax and t1/2 for bupropion (P<0.05). Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞, t1/2, Cmax and MRT0-t for paclitaxel (P<0.05 or P<0.01). When compared with the SC group, the VSC group had a higher AUC0-∞, t1/2, Cmax and MRT0-t for paclitaxel (P<0.05), it was indicated that SC could inhibit CYP2C8 and the inhibitory effect was more pronounced in the VSC group. Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, AUC0-∞,Cmax, Tmax and t1/2 for tolbutamide (P<0.05 or P<0.01).
In general, after a single dose of the SC or VSC extract, CYP2D6, CYP2C19 and CYP2C8 enzyme activities were inhibited and CYP2A6, CYP2B6 and CYP2C9 enzyme activity was induced. The inhibitory effect of CYP2D6 and CYP2C8 were more pronounced in the VSC group.
The results inTable 5 showed that after multiple doses of SC or VSC, there was no change in the CL/F for coumarin, omeprazole, bupropion, paclitaxel and tolbutamide and t1/2 fortolbutamide (P>0.05); however, there were significant differences in the MRT0-t, t1/2, Tmax for metoprolol, MRT0-t, t1/2 for coumarin, t1/2, Tmax for omeprazole, AUC0-∞, Cmax, MRT0-t for bupropion, Tmax for paclitaxel and MRT0-t for tolbutamide (P <0.05).
Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞,Cmax, MRT0-t, t1/2 andTmaxand decreases in CL/F for metoprolol. When compared with the SC group, the VSC group had a higher AUC0–t, AUC0-∞,Cmax and Tmaxand a lower CL/F for metoprolol (P<0.05), it was indicated that SC could inhibit CYP2D6 and the inhibitory effect was more pronounced in the VSC group. Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, AUC0-∞, MRT0-t, t1/2,Tmax and Cmax for coumarin (P<0.05 or P<0.01).When compared with the SC group, the VSC group had a lower AUC0–t, Cmax, MRT0-t, t1/2and Tmax for coumarin (P<0.05 or P<0.01). Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞, t1/2, MRT0-t,Tmaxand Cmax for omeprazole (P<0.05 or P<0.01). When compared with the SC group, the VSC group had a higher AUC0–t, AUC0-∞, t1/2, MRT0-t, Tmax and Cmax for omeprazole (P<0.05 or P<0.01), it was indicated that SC could inhibit CYP2C19 and the inhibitory effect was more pronounced in the VSC group. Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, AUC0-∞, t1/2, MRT0-t, Tmax and Cmax for bupropion (P<0.05 or P<0.01). When compared with the SC group, the VSC group had a lower AUC0–t, t1/2, Tmax and Cmax for bupropion (P<0.05 or P<0.01). Compared with the control group, the SC and VSC groups displayed increases in AUC0–t, AUC0-∞, t1/2, MRT0-t, Tmax and Cmax for paclitaxel (P<0.05 or P<0.01). When compared with the SC group, the VSC group had a higher AUC0–t, AUC0-∞, t1/2, MRT0-t, Tmax and Cmax for paclitaxel (P<0.05 or P<0.01), it was indicated that SC could inhibit CYP2C8 and the inhibitory effect was more pronounced in the VSC group. Compared with the control group, the SC and VSC groups displayed decreases in AUC0–t, AUC0-∞,Cmax, Tmax and MRT0-t for tolbutamide (P<0.05 or P<0.01). When compared with the SC group, the VSC group had a lower AUC0–t, AUC0-∞,Cmax, Tmax and MRT0-t for paclitaxel (P<0.05 or P<0.01).
In general, after multiple doses of the SC or VSC extract, CYP2D6, CYP2C19 and CYP2C8 enzyme activities were inhibited. This inhibitory effect was more potent in the VSC group. However, multiple-dose SC or VSC treatment increased CYP2A6, CYP2B6 and CYP2C9 enzyme activity. And this induction effect was more potent in the VSC group.The analysis results for the six probe drugs are shown in Table 5.
Discussion
Modern research has shown that CYP450 enzymes can be induced or inhibited by exogenous materials such as drugs or herbs. Changes in CYP450 levels or activities can affect the concentration of drug in the blood, the pharmacokinetic process and biological medicinal properties [16,36]. Currently, Europe and the United States require that drug screens and metabolic research based on the CYP450 system should be included in new drug evaluations. This methodology is also required by the Chinese SFDA for pharmacokinetic research in new chemical drug development.
The concomitant administration of herbal supplements and synthetic drugs has become increasingly popular. Chinese herbal medicines contain a variety of biologically active ingredients. As a result, herb-drug interactions have become a common clinical problem. According to recent studies, the mechanisms underlying the interaction between herbal medicines and conventional drugs mainly involve induction or inhibition of the activities of metabolic enzymes and drug efflux proteins. Therefore, potential herb-drug interactions involving Chinese herbal medicines are worthy of study based on the CYP450 system.
SC and VSC are widely used in TCM practice. Previous research has suggested that vinegar processing enhances the increase ordecrease in CYP450 enzyme (CYP1A2, CYP2E1 and CYP3A4) levels elicited by SC and VSC[26]. No new lignan compounds were found after vinegar processing, but the amounts of lignan components were changed [37]. Other studies showed that the hepatoprotective potency of SC is significantly increased after vinegar processing [38]. This effect may be attributable to the fact that vinegar-processed SC increases the dissolution rate of the active principles, thus enhancing the curative effects [39]. In view of their well-known effects, we aimed to investigate the effects of SC and VSC on metabolism of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 enzymes in rats. We used probe cocktail methods to predict interactions between the herb and drugs. Single dose administration only affected metabolic enzyme, eliciting obviousinhibition or induction; after long-term administration, there were variouscomprehensive effects.
Interestingly, the inhibitory effect of SC on CYP2D6, 2C19 and 2C8 and the induced effect of SC on CYP2A6, 2B6 and 2C9 were more potent after vinegar processing, possibly due to changes in the pharmacological activities and levels of its components. The differences between a single dose and multiple doses of SC and VSC suggest that the subtypes of enzymes affected did not correlate with the length of administration. However, the mechanisms underlying this effect are not yet documented. In this study, investigation of six CYP450enzymes activity showed that, as the administration time increased, the effect of SC and VSC changed from weak to strong.
To investigate the metabolic mechanisms of SC and VSC treatment, further study is required. In vitro methods, such as liver microsome incubation, gene recombination CYP450 enzyme system, hepatocyte culture and liver tissue slice explant culture may be used. The data obtained will serve as a reference to evaluate possible herb–drug interactions when SC and VSC are prescribed in clinical. Interactions between SC and VSC and other drugs require further study.
Conclusions
This study investigated the potential differences between SC and VSC in their effects on CYP450 enzyme activities, including those of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP2D6 in rats after single and multiple doses of extract. SC and VSC significantly inhibited CYP2D6, 2C19 and 2C8 activity and induced CYP2A6, 2B6 and 2C9activity. The effect of VSC was more potent than that of SC. The results provide a scientific basis for the safe clinical application of SC and VSC in combination with other drugs, potentially preventing possible side effects induced by herb-drug interactions.
Conflict of Interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All of the protocols on living animals used in this paper were come from the Experimental animal center of Nanjing University of Chinese Medicine, license No. SYXK (Su) 2014-0001.
Acknowledgements
This study was supported by the Major Program of State Commission of Science Technology of China (NO. 2009ZX09308-004), the Educational Commission of Jiangsu Province of China (NO. 09KJA360001), The Key Laboratory of Acupuncture of Jiangsu Province of China (NO. KJA200906), the Technology Project of Nanjing University of Chinese Medicine of China (NO. 10XJC06) and the Preponderant discipline of Jiangsu province (NO. 2011ZYX2-003).
References
- Smejkal K, Slapetova T, Krmencik P (2010) Evaluation of cytotoxic activity of Schisandra chinensis Lignans. Planta Medica 76: 1672-1677.
- Li XG, Gao Q, Zhang P (2005) The research of Fructus schisandrae effective parts and its pharmacological. Journal of Chinese Medicinal Materials 28:156-159.
- Shi L, Wang ZC, Feng XQ (2011) Advances in studies on chemical constituents and pharmacological activities of Schisandrae Chinensis. Drug Evaluation Research 34: 208-212.
- Hu JY, Mao CQ, Gong XD (2013) Simultaneous determination of eleven characteristic lignans in Schisandra chinensis by high-performance liquid chromatography. Pharmacognosy Magazine 34: 155-161.
- Zhu LJ, Li B, Liu XY (2015) Purification of six lignans from the stems of Schisandra chinensis by using high-speed counter-current chromatography combined with preparative high-performance liquid chromatography. Food Chemistry 186: 146-152.
- LeiC, Huang SX, Xiao WL (2010) Schisanartane nortriterpenoids withdiverse post- modifications from Schisandra propinqua. Journal of NaturalProducts 8: 1337-1343.
- Panossian A, Wikman G, Sarris J (2010) Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 17: 481-493.
- Ding Z, Lu W, Li H (2010) Simultaneous determination of five lignans in Schisandra chinensis by HPLC. China Journal of Chinese Materia Medica 35:1728-1730.
- Gnabre J, Unlu I, Chang TC (2010) Isolation of lignans from Schisandrachinensis with anti-proliferative activity in human colorectal carcinoma: structure-activity relationships. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 878: 2693-2700.
- Guo Z, Xu L (2000) The basis of clinical pharmacology of Schizandrachinensis. Foreign Medicine 15: 139-145.
- Liu H, Yao Z (2005) Antioxidant research and clinical application prospect of Schizandra chinensis. Xinjiang Medical Journal 35: 137-139.
- Jin SJ (2007) Progress of the hepatoprotective effect of Schizandra chinensis. Journal of Changchun College of Traditional Chinese Medicine 23: 28.
- Iwata H, Tezuka Y, Kadota S (2004) Identification and characterization of potent CYP3A4 inhibitors in schisandra fruit. Drug Metab Dispos 32: 1351-1358.
- Jiang YM, Fan XM, Wang Y (2015) Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associatedwith the inhibition of CYP-mediated bioactivation. Chemico-Biological Interactions 231: 83-89.
- Xie Y, Hao HP, Wang H (2014) Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. Journal of Ethnopharmacology 155: 213-221.
- Wang B, Yang S, Hu J, Yan L (2014) Multifaceted interaction of the traditional Chinese medicinal herb Schisandra chinensis with cytochrome P450-mediated drug metabolism in rats. Journal of Ethnopharmacology 155: 1473-1482.
- Venkatakrishnan K, Von Moltke LL, Greenblatt DJ (2001) Human drug metabolism and the cytochromes P450: application and relevance of in vitro models. Journal of Clinical Pharmacology 41: 1149.
- Han YL, Li D, Meng XL (2009) The review of TCM compound to theregulation of the cytochrome P450 enzyme. Journal of Chinese Medicinal Materials 32: 1638-1642.
- Lonsdale R, Olah J, Mulholland AJ (2011) Does Compound I VarySignificantly between Isoforms of Cytochrome P450? Journal of the American Chemical Society 133: 15464-15474.
- Ball SE, Ahern D, Scatina J (1997) Venlafaxine: in vitro inhibition of CYP2D6 dependent imipramine and desipramine metabolism; comparative studies with selected SSRIs and effects on human hepatic CYP3A4, CYP2C9 and CYP1A2. British Journal of Clinical Pharmacology 43: 619-626.
- Liu GF, Guo XL (2008) Advances in studies on regulation of Chinese material medica on cytochrome P450 system. Chinese Traditional and Herbal Drugs 39: 139-143.
- Slaughter RL, Edwards DJ (1995) Recentadvances: Thecytochrome P450enzymes.J Ann Pharmacother 29:619.
- Tian J, Kristopher W, Yang S (1999) Eight inhibitoory monoclonal antibodies define the role of individual P450 in human liver microsomal diazepam, 7-Ethoxycoumarin and imipramine metabolism.J Drug Metab Dispos 27:102.
- Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activitiesand impact of genetic variation. J Pharacol Ther 138:103-141.
- Liu Y, Wang HX (2011) Effects of Traditional Chinese Medicine Preparations on Rat CYP 3A4 of Liver Microsomes in Vitro. Herald of Medicine 30: 285-289.
- Su T, Mao CQ, Yin FZ (2013) Effects of unprocessed versus vinegar-processed Schisandra chinensis on the activity and mRNA expression of CYP1A2, CYP2E1 and CYP3A4 enzymes in rats. Journal of Ethnopharmacology 146: 734-743.
- Bruce MA, Hall SD, Haehner-Daniels BD (2001) In vivo effect of clarithromycin on multiple cytochrome P450s. Drug Metab Dispos 29: 1023-1028.
- Chow HHS, Hakim IA, Vining DR (2006) Effects of repeated green tea catechin administration on human cytochrome P450 activity. Cancer Epidemiology Biomarkers & Prevention 15: 2473-2476.
- Tomalik-Scharte D, Jetter A, Kinzig-Schippers M (2005) Effect of propiverine on cytochrome P450 enzymes: a cocktail interaction study in healthy volunteers. Drug Metab Dispos 33: 1859-1866.
- Smith D, Sadagopan N, Zientek M (2007) Analytical approaches todetermine cytochrome P450 inhibitory potential of new chemical entities in drug discovery. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 850: 455-463.
- Xu AX, Jia H, Yuan JY (2010) Effects of Zhenyuan Capsule on Cytochrome P450 Enzymes CYP1A2, CYP3A4 and CYP2E1. China Pharmacy 21: 3290-3292.
- Lu TL, Su LL (2015) The research progress of the interaction between CYP450 enzymes and medicine metabolism as well as enzyme activity assay. China Journal of Chinese Materia Medica 40: 3524-3529.
- Chinese Pharmacopoeia Commission(2015) Pharmacopoeia of the People’s Republic of China.Vol 1. Chinese Medicine and Technology Publishing House, Beijing.
- Xu MJ, Wang GJ, Xie HT (2008) Pharmacokinetic comparisons ofschizandrin after oral administration of schizandrin monomer, Fructus Schisandrae aqueous extract and Sheng-Mai-San to rats. Journal of Ethnoharmacology 115: 483-488.
- Gao Y, Ma M (2003) Rapid determination of γ-schizandrin in hugan tablet byHPLC. Shandong Journal of Traditional Chinese Medicine 22: 40.
- Arbus C, Benyamina B, Llorca PM (2007) Characterization of human cytochrome P450 enzymes involved in the metabolism of cyamemazine. European Journal of Pharmaceutical Sciences 32: 357-366.
- Huang X, Song FR, Liu ZQ (2008) Lignans from different processedproducts of Schisandra chinensis fruits. Acta Pharmaeeutica Sinica 43: 630-633.
- Lu TL, Ying FZ (2005) The processing influence on the function of Fructus Schisandrae. Journal of Chinese Medicinal Materials 28: 933-935.
- Zhou Y, Qi YJ, Yan XY (2011) Determination of six lignan components in crude and different processed products of Schisandra chinensis. China Journal of Chinese Materia Medica 36: 3449-3452.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 3057
- [From(publication date):
August-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 2406
- PDF downloads : 651