Research Article Open Access
Effects of Tricyclic Compounds on the Transport of Anti-migraine Triptans through Human Organic Anion Transporting Polypeptide 1A2 (OATP1A2)
Jennifer Lu1,2, Alexia Grangeon1,2, Fleur Gaudette1, Jacques Turgeon2 and Veronique Michaud1*,21Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CRCHUM), Montreal, QC, Canada
2Faculty of Pharmacy, Université de Montréal, Montreal, QC, Canada
- Corresponding Author:
- Michaud V
Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CRCHUM)
900 Rue St.-Denis, Room R08-480, Montreal, QC, H2X 0A9, Canada
Tel: 514-890-8000/5812
Fax: 514-412-7978
E-mail: veronique.michaud.chum@ssss.gouv.qc.ca
Received Date: November 11, 2016; Accepted Date: November 30, 2016; Published Date: December 9, 2016
Citation: Lu J, Grangeon A, Gaudette F, Turgeon J, Michaud V (2016) Effects of Tricyclic Compounds on the Transport of Anti-migraine Triptans through Human Organic Anion Transporting Polypeptide 1A2 (OATP1A2). J Pharmacokinet Exp Ther 1:106.
Copyright: © 2016 Lu J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pharmacokinetics & Experimental Therapeutics
Abstract
OATP1A2 is a membrane drug-transporter expressed at the human blood-brain barrier (BBB) that may potentially mediate penetration of drugs in the brain. Triptans, hydrophilic antimigraine drugs, are substrates of OATP1A2. It is believed that triptans should cross the BBB to reach their site of action. Thus, OATP1A2 can limit brain penetration of triptans and may consequently influence their antimigraine drug action. We have previously demonstrated that compounds composed of a tricyclic ring with a short aliphatic amine chain, such as tricyclic antidepressants and carvedilol, inhibited OATP1A2-mediated rosuvastatin uptake. The main objective of this study was to determine whether triptans transport via OATP1A2 is affected by tricyclic compounds. First, we confirmed that triptans were substrates of OATP1A2 but not OATP2B1 using HEK293 stable cell lines. The tricyclic drugs evaluated were able to inhibit OATP1A2-mediated uptake of triptans. carvedilol was the most potent inhibitor. Potential inhibition was assessed with a range of total plasma concentrations of the drugs. Carvedilol and nortriptyline lowered the uptake of both almotriptan and zolmitriptan whereas clomipramine diminished the uptake of almotriptan only. Our data suggest that these three drugs may limit the penetration of triptans to the brain by modulating OATP1A2 transport at clinically relevant concentrations.
Keywords
Drug transporter; OATP1A2; drug-drug interaction; triptans; blood-brain barrier (BBB)
Introduction
Migraines are an important cause of disability in Canada, affecting 8.3% of the population (2.7 millions) [1]. Triptan drugs are typically used in the treatment of acute migraine attacks. Triptans are selective agonists of the serotonin receptors 5-HT1B and 5-HT1D located on smooth muscle cells of intracranial and extracerebral blood vessels as well as on trigeminal sensory neurons [2-4]. Their mechanisms of action are believed to imply inhibition of activated trigeminal neurons, inhibition of neuropeptides release, interruption of pain transmission, and perhaps selective vasoconstriction of cranial blood vessels [5]. It appears that triptans are required to cross the blood-brain barrier (BBB) to reach their target site in the central nervous system (CNS). However, these drugs are hydrophilic, limiting their penetration through the BBB. Thus, transport mechanisms must exist to facilitate their entrance into the brain. Many membrane drug transporters are expressed at the BBB to limit or facilitate the access of drugs to the brain. Among those involved in drug influx, OATP1A2 and OATP2B1 proteins are expressed on the luminal membrane of the endothelial cells making up the BBB [6-9]. Their physiological roles at the BBB may implicate the distribution of thyroid hormones (triiodothyronine and thyroxine) to the CNS by OATP1A2 and the transport of conjugated neuroactive steroids (pregnenolone sulfate and dehydroepiandrosterone-3-sulfate) to the brain by OATP2B1 [10,11].
OATP1A2 and OATP2B1 transport a wide spectrum of endogenous compounds and xenobiotics while having overlapping substrate selectivity. Recently, a study screened 36 CNS-active drugs for transport through OATP1A2 and has shown that triptans are OATP1A2 substrates [12]. Using triptan structural analogs, a structure-activity relationship was established where an amine residue was essential for transport through OATP1A2 and the uptake rate was the highest for tertiary amine followed by secondary and then primary amines. These findings are interesting as it would suggest that OATP1A2 may facilitate permeation of triptans to the brain.
We have previously demonstrated that the transport of rosuvastatin through OATP1A2 can be inhibited by compounds composed of a tricyclic ring and a short aliphatic amine chain, such as tricyclic antidepressants and carvedilol [13].
The objectives of this study were to:
• Confirm triptans as OATP1A2 substrates in our human embryonic kidney (HEK293)-OATP1A2 stable cell line.
• Determine whether triptans are OATP2B1 substrates using a HEK293-OATP2B1 stable cell line.
• Determine whether compounds composed of a tricyclic ring and a short aliphatic amine chain inhibit the transport of triptans through OATP1A2.
• Determine whether tricyclic compounds can inhibit OATP1A2- mediated uptake of triptans at total plasma concentrations. The consequence of such an interaction in humans would be a diminishment or abolishment in antimigraine efficiency by a limited delivery of triptans into the brain.
Materials and Methods
Reagents
Amitriptyline hydrochloride, carbamazepine, carbazole, chlorpromazine hydrochloride, clomipramine hydrochloride, desipramine hydrochloride, imipramine hydrochloride, naratriptan hydrochloride, nortriptyline hydrochloride, phenothiazine, rizatriptan benzoate, sumatriptan succinate, trimipramine maleate salt, zolmitriptan were purchased from Sigma-Aldrich (St-Louis, MO, USA). Almotriptan hydrochloride, carazolol hydrochloride, carvedilol, doxepin hydrochloride, eletriptan hydrobromide were purchased from Toronto Research Chemicals (Toronto, ON, Canada). All chemicals and solvents were obtained from Sigma-Aldrich, Fisher Scientific (Fair Lawn, NJ, USA) or J.T. Baker (Center Valley, PA, USA).
Cell culture
HEK293-OATP1A2, HEK293-OATP2B1, and HEK293-VC cells were kindly provided by Dr. Markus Keiser and Dr. Werner Siegmund (Department of Clinical Pharmacology, Center of Drug Absorption and Transport, University Medicine Greifswald, Greifswald, Germany). The cells were cultured in minimum essential medium supplemented with 10% fetal bovine serum, 1X nonessential amino acids, and 1X sodium pyruvate at 37°C and 5% CO2. Cell culture media and supplements were purchased from Multicell Wisent Inc. (St-Jean- Baptiste, QC, Canada); whereas, fetal bovine serum was obtained from HyClone Thermo Scientific (Logan, UT, USA).
Uptake assays and competition assays
Reproducibility of our HEK293-OATP1A2 cell model was assessed with 2-3 different cell batches and comparable Km values were obtained. The uptake assays were performed as previously described [13]. Briefly, tissue culture plates (6-well or 12-well) were first treated with poly-L-lysine (Sigma-Aldrich, St-Louis, MO, USA) before seeding the HEK293-OATP1A2, HEK293-OATP2B1, and HEK293-VC cells. The number of cells seeded in 6-well and 12-well plates was 1.5 × 106 cells/well and 7.5 × 105 cells/well, respectively. After 24 h, the culture media was replaced with warm transport buffer (142 mM NaCl, 5 mM KCl, 1 mM K2HPO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM glucose, and 12.5 mM HEPES, pH 7.3) and pre-incubated at 37°C for 5 min. Following the pre-incubation period, the cells were incubated with warm transport buffer containing the substrate in the presence or absence of an inhibitor at 37°C. After incubation, the cells were washed twice with phosphate-buffered saline (PBS) containing 10% acetonitrile followed by a final wash with PBS. Time-dependent uptake experiments through OATP1A2 were done in six-well plates by incubating HEK293-OATP1A2 and HEK293-VC cells with drugs at determined Km, i.e., almotriptan (5 μm), eletriptan (1 μm), naratriptan (20 μm), rizatriptan (43 μm), sumatriptan (94 μm), or zolmitriptan (21 μm). The Km and Vmax of the different triptans transport through OATP1A2 was determined by incubating HEK293-OATP1A2 and HEK293-VC cells in six-well plates with almotriptan (0.375-25 μm), eletriptan (0.125-5 μm), naratriptan (0.625-100 μm), rizatriptan (0.75-250 μm), sumatriptan (1.5-500 μm), and zolmitriptan (0.75-250 μm). To determine whether a compound can block OATP1A2- mediated transport of triptans, HEK293-OATP1A2 and HEK293-VC cells were seeded in 12-well plates and co-incubated with almotriptan (15 μm), eletriptan (3 μm), naratriptan (60 μm), rizatriptan (130 μm), sumatriptan (300 μm), or zolmitriptan (65 μm) in the absence or presence of different tricyclic compounds (0.15-150 μM). In the inhibition studies, a concentration of triptan at three times the Km value was selected in order to saturate the OATP1A2 transporter with the substrate. An incubation time of 2 min was chosen for almotriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan; whereas 1 min was chosen for eletriptan. Time-dependent uptake of triptans at clinically relevant concentrations was done in six-well plates by incubating HEK293-OATP1A2 and HEK293-VC cells with almotriptan (50 ng/mL) or zolmitriptan (3 ng/mL). To determine whether clinically relevant concentrations of tricyclic compounds can inhibit OATP1A2-mediated transport of triptans, HEK293-OATP1A2 and HEK293-VC cells were seeded in six-well plates and co-incubated with either almotriptan (50 ng/mL) or zolmitriptan (3 ng/mL) for 1 or 2 min, respectively, in the absence or presence of different tricyclic compounds (10-200 ng/mL).
To determine whether triptans are transported by OATP2B1, HEK293-OATP2B1 and HEK293-VC cells were seeded in six-well plates and incubated with almotriptan (5 and 25 μM), eletriptan (1 and 3.75 μM), naratriptan (20 and 60 μM), rizatriptan (40 and 120 μM), sumatriptan (100 and 300 μM), or zolmitriptan (21 and 65 μM). The concentrations chosen for each substrate correspond to its Km and 3 times Km value determined in HEK293-OATP1A2 cells. The protein concentration was measured using the Pierce BCA protein assay kit from Thermo Scientific (Rockford, IL, USA). Three wells of each cell line were lyzed with 1% SDS+0.2 N NaOH and the average value were used to normalize intracellular triptan concentrations.
Quantification of triptans by high-performance liquid chromatography-UV
The quantity of triptans transported in the cells was measured by high performance liquid chromatography with UV detection. The instrumentation consisted of a SpectraSystem P4000 pump, SpectraSystem AS3000 autosampler, Finnigan SpectraSystem UV6000 ultraviolet detector and SpectraSystem SN4000 system controller from Thermo Electron Corporation (San Jose, CA, USA). ChromQuest Version 4.2.34 software was used for data acquisition (Thermo Electron Corporation). The samples were separated on a Phenomenex Luna 3 μm PFP (2) column (150 × 4.6 mm, 3 μM; Phenomenex, Torrance, CA, USA). Table 1 summarizes the details for each method.
| Almotriptan | Eletriptan | Naratriptan | Rizatriptan | Sumatriptan | Zolmitriptan | |
|---|---|---|---|---|---|---|
| Buffer | 10 mM AF pH3.0 | 10 mM AF pH3.0 | 10 mM AF pH3.0 | 10 mM AF pH 3.0 | 10 mM AF pH 3.0 | 10 mM AF pH 3.0 |
| Solvent | Methanol | Methanol | Methanol | Methanol | Methanol | Methanol |
| % Buffer/Solvent | 65/35 | 48/52 | 68/32 | 71/29 | 68/32 | 80/20 |
| Run time (min) | 22 | 30 | 20 | 21 | 20 | 24 |
| Flow (ml/min) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Wavelength (nm) | 283 | 272 | 284 | 282 | 283 | 283 |
| Temperature (°C) | 40 | 50 | 40 | 50 | 40 | 50 |
| tR (min) | 19 | 15.7 | 18.1 | 9 | 8.9 | 21.7 |
| tR IS (min) | 15.2 (IS: Naratriptan) | 27.5 (IS: Doxepin) | 8.8 (IS: Sumatriptan) | 17.9 (IS: Naratriptan) | 18.5 (IS: Naratriptan) | 17.8 (IS: Rizatriptan) |
| AF: Ammonium Formate; tR: Retention Time; IS: Internal Standard | ||||||
Table 1: HPLC-UV quantification methods details.
Since the cell lysate affected the absorbance of the analytes, calibration curves and quality controls samples were prepared in the cell lysate. Linear regressions (weighted 1/concentration) were judged to produce the best fit for the concentration-detector relationship for all triptans. The coefficients of correlation (r2) were greater than 0.997 for all compounds in all batches. The reproducibility of each method was evaluated by analyzing six replicates of lysate samples fortified at LLOQ, low, mid and high concentrations in three individual runs. Precisions were better than 11.3% and accuracies were in the 96.0-110% range. The inter- and intra-batch precision and accuracy statistical results for all compounds are shown in Table 2.
| Compound | Concentration (ng/mL) | Intra (n=6) | Inter (n=18) | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | CV (%) | Nominal (%) | Mean ± SD (ng/mL) | CV (%) | Nominal (%) | ||
| Almotriptan | 25 | 22 ± 4.5 | 20 | -11.9 | 23.9 ± 2.7 | 11.3 | -4.5 |
| 100 | 97.5 ± 2.8 | 3 | -7 | 96.1 ± 3.4 | 3.5 | -3.9 | |
| 500 | 484 ± 9.7 | 2 | -3.2 | 494 ± 12.9 | 2.6 | -1.2 | |
| 5000 | 5229 ± 77.7 | 1.5 | 4.6 | 5102 ± 124 | 2.4 | 2 | |
| Eletriptan | 100 | 106 ± 14.4 | 13.5 | 14.8 | 111 ± 9 | 8.1 | 11.1 |
| 250 | 268 ± 10 | 3.7 | 7 | 258 ± 9.3 | 3.6 | 3.4 | |
| 500 | 508 ± 16.3 | 3.2 | -2.1 | 496 ± 16 | 3.2 | -0.8 | |
| 5000 | 5326 ± 163 | 3.1 | 6.5 | 5123 ± 188 | 3.7 | 2.5 | |
| Naratriptan | 50 | 54.9 ± 2.4 | 4.4 | 15.8 | 55 ± 2.4 | 4.4 | 10 |
| 100 | 103 ± 5.8 | 5.6 | 4.8 | 104 ± 3.9 | 3.8 | 4 | |
| 500 | 491 ± 20.6 | 4.2 | -5.4 | 480 ± 15.8 | 3.3 | -4 | |
| 5000 | 5162 ± 170 | 3.3 | 3.2 | 5089 ± 130 | 2.6 | 1.8 | |
| Rizatriptan | 25 | 27.5 ± 0.9 | 3.4 | 12.2 | 26.8 ± 1.6 | 6.2 | 7.1 |
| 100 | 98.5 ± 3.7 | 3.7 | -1.7 | 99.2 ± 2.7 | 2.8 | -0.8 | |
| 500 | 510 ± 10.5 | 2.1 | -6.5 | 484 ± 21 | 4.3 | -3.1 | |
| 5000 | 5173 ± 97.3 | 1.9 | 3.4 | 5024 ± 130 | 2.6 | 0.5 | |
| Sumatriptan | 25 | 26 ± 2 | 7.5 | 13.8 | 26.6 ± 2 | 7.4 | 6.3 |
| 100 | 103 ± 2.9 | 2.8 | -5.2 | 99.2 ± 4.2 | 4.2 | -0.8 | |
| 500 | 498 ± 15 | 3 | -3.9 | 490 ± 14.4 | 2.9 | -2.1 | |
| 2500 | 2480 ± 93 | 3.8 | -2.2 | 2480 ± 70.4 | 2.8 | -0.8 | |
| Zolmitriptan | 50 | 47 ± 2.9 | 6.1 | 8.7 | 51.5 ± 4.2 | 8.1 | 3 |
| 100 | 103 ± 9.4 | 9.2 | 5.7 | 102 ± 7 | 6.8 | 2.3 | |
| 500 | 498 ± 12.8 | 2.6 | 3.8 | 492 ± 11.8 | 2.4 | -1.5 | |
| 5000 | 5113 ± 110 | 2.2 | 2.3 | 5052 ± 91.9 | 1.8 | 1 | |
| SD: Standard Deviation; CV: Coefficient of Variation | |||||||
Table 2: Validation of HPLC-UV quantification methods of triptans.
After the final wash with PBS, the samples were processed as previously described [13]. Briefly, the cells were lyzed with methanol containing the IS (100 ng/ml). The cell lysate was transferred to a 1.7 mL microtube and the samples were spun down at maximum speed for 10 min at room temperature. The supernatant was transferred to a culture borosilicate glass tube, evaporated to dryness, and reconstituted in 100 μl of reconstitution solution. The reconstitution solution consisted of a mixture of ddH2O and methanol in the following proportions: almotriptan (70:30 v/v), eletriptan (50:50 v/v), naratriptan (70:30 v/v), rizatriptan (70:30 v/v), sumatriptan (70:30 v/v), and zolmitriptan (70:30 v/v). A volume of 20 μl per sample was injected.
Quantification of almotriptan and zolmitriptan by liquid chromatography-tandem mass spectrometry
The quantity of almotriptan and zolmitriptan transported in the cells when incubated at clinically relevant concentrations was measured by liquid chromatography-tandem mass spectrometry. The instrumentation consisted of a TSQ Quantiva Triple Quadrupole mass spectrometer interfaced with an Ultimate 3000XRS UHPLC system using pneumatic assisted heated electrospray ion source from Thermo Scientific (San Jose, CA, USA). Xcalibur 3.0.63 software was used for data acquisition and analysis (San Jose, CA, USA). The samples were separated on a Phenomenex Luna PFP (2) column (150 × 3.0 mm, 3 μM; Phenomenex, Torrance, CA, USA) coupled with a Phenomenex PFP security guard cartridge (4 × 2.0 mm; Phenomenex, Torrance, CA, USA). The mobile phase consisted of 10 mM ammonium formate, pH 3, and acetonitrile in the following proportions: almotriptan (70:30 v/v) and zolmitriptan (80:20 v/v). The flow rate was set at 0.3 ml/min and the column was heated at 40°C for almotriptan. The flow rate was set at 0.4 ml/min and the column was heated at 50°C for zolmitriptan. 2H6-almotriptan and 2H6-zolmitriptan were used as IS and the retention times are 4.4 and 2.8 min for almotriptan and zolmitriptan, respectively. MS detection was performed in positive ion mode, using selected reaction monitoring. The precursor-ion reactions for the analytes were set at 336.2 → 291.1 for almotriptan and 288.3 → 167.1 for zolmitriptan.
The analytical range was set at 37.5-25,000 pg/ml for almotriptan and set at 75.0-25,000 pg/ml for zolmitriptan. A linear regression (weighted 1/concentration) was judged to produce the best fit for the concentration-detector relationship for almotriptan and zolmitriptan. The r2 was greater than 0.998 for almotriptan and 0.996 for zolmitriptan. The reproducibility of the method was evaluated by analyzing three replicates of lysate samples fortified at low, mid and high concentrations in three individual runs. Precisions were better than 13% and accuracies were in the 92-103% range. The intra and inter batch precision and accuracy statistical results are shown in Supplemental Table 1.
After the final wash with PBS, the samples were processed as follows. The cells were lyzed with 1 ml methanol containing the IS (2 ng/ml 2H6-almotriptan or 0.5 ng/ml 2H6-zolmitriptan). The cell lysate was transferred to a 1.7 ml microtube and the samples were spun down at maximum speed for 10 min at room temperature. The supernatant was transferred to a culture borosilicate glass tube, evaporated to dryness at 10 psi with N2 at 40°C, and reconstituted in 200 μl of reconstitution solution. The reconstitution solution consisted of a mixture of 10 mM ammonium formate, pH 3, and methanol (70:30 v/v) for almotriptan and H2O and methanol (95:5 v/v) for zolmitriptan. A volume of 10 μl per sample for almotriptan and 5 μl per sample for zolmitriptan was injected.
Data Analysis
The net transport of triptan through OATP1A2 was calculated by subtracting the value in the VC cells from the value in the OATP1A2 cells. Data were analyzed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Each data point is expressed as the mean ± S.D. Km and Vmax were calculated by fitting the data to the Michaelis-Menten equation. IC50 values were calculated by fitting the data to the log (inhibitor) versus response equation, and the range given represents the 95% confidence interval.
Results
Transport of triptans through OATP1A2
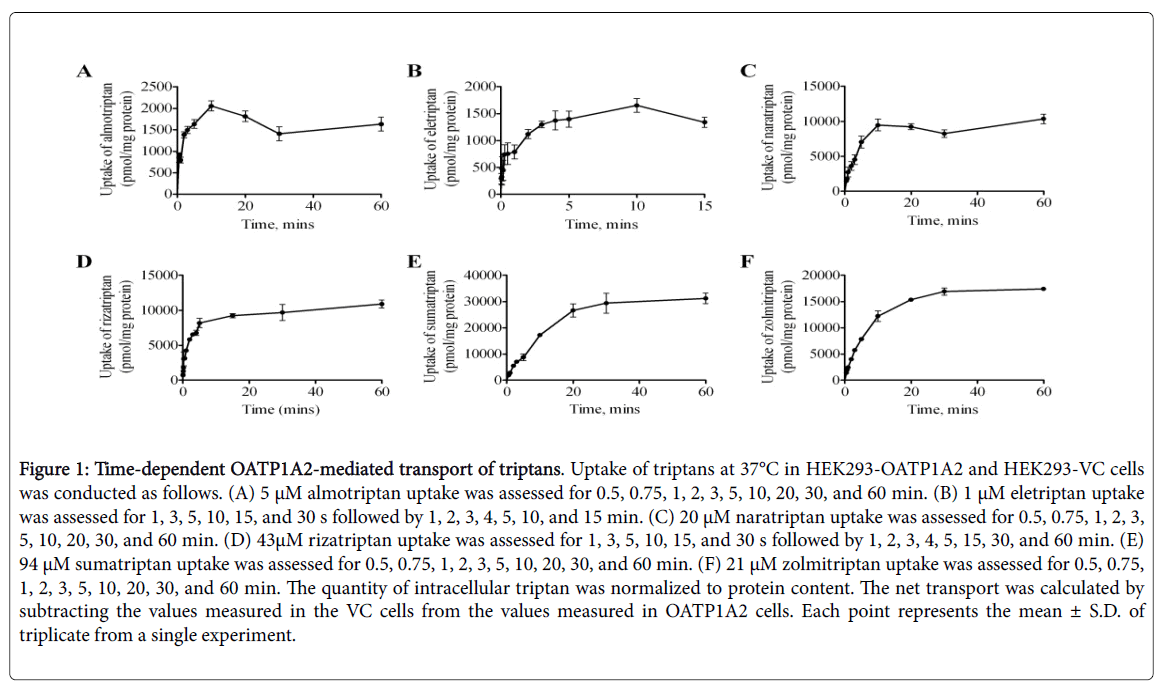
A cell model stably expressing OATP1A2 in HEK293 cells was used to study OATP1A2-mediated transport of the different triptans. Timedependent uptake was assessed up to 60 min with the exception of eletriptan, up to 15 min, due to its higher lipophilicity compared to other triptans (Figure 1).
Figure 1: Time-dependent OATP1A2-mediated transport of triptans. Uptake of triptans at 37°C in HEK293-OATP1A2 and HEK293-VC cells was conducted as follows. (A) 5 μM almotriptan uptake was assessed for 0.5, 0.75, 1, 2, 3, 5, 10, 20, 30, and 60 min. (B) 1 μM eletriptan uptake was assessed for 1, 3, 5, 10, 15, and 30 s followed by 1, 2, 3, 4, 5, 10, and 15 min. (C) 20 μM naratriptan uptake was assessed for 0.5, 0.75, 1, 2, 3, 5, 10, 20, 30, and 60 min. (D) 43μM rizatriptan uptake was assessed for 1, 3, 5, 10, 15, and 30 s followed by 1, 2, 3, 4, 5, 15, 30, and 60 min. (E) 94 μM sumatriptan uptake was assessed for 0.5, 0.75, 1, 2, 3, 5, 10, 20, 30, and 60 min. (F) 21 μM zolmitriptan uptake was assessed for 0.5, 0.75, 1, 2, 3, 5, 10, 20, 30, and 60 min. The quantity of intracellular triptan was normalized to protein content. The net transport was calculated by subtracting the values measured in the VC cells from the values measured in OATP1A2 cells. Each point represents the mean ± S.D. of triplicate from a single experiment.
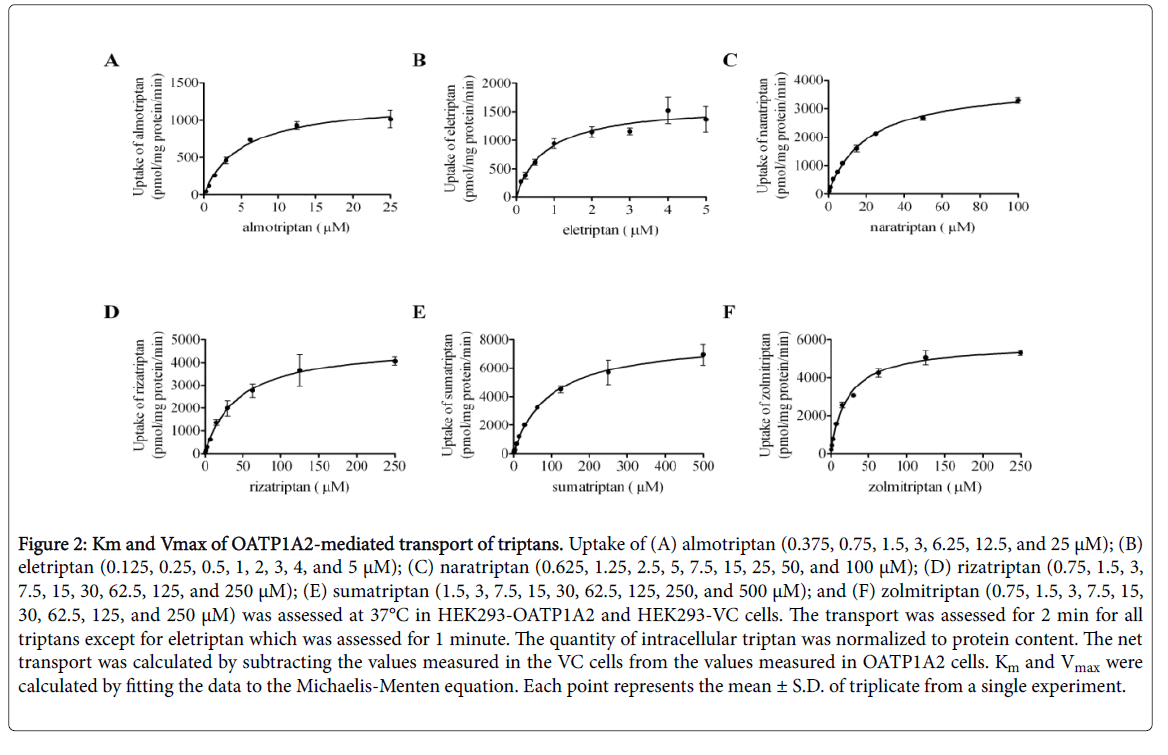
All triptans evaluated showed time-dependent saturable transport by OATP1A2. An incubation time of 2 min was chosen for further experiments with almotriptan, naratriptan, rizatriptan, sumatriptan, and zolmitriptan and 1 min was chosen for eletriptan as these timepoints remain in their linear range. All triptans evaluated also showed concentration-dependent saturable transport via OATP1A2 (Figure 2 and Table 3).
Figure 2: Km and Vmax of OATP1A2-mediated transport of triptans. Uptake of (A) almotriptan (0.375, 0.75, 1.5, 3, 6.25, 12.5, and 25 μM); (B) eletriptan (0.125, 0.25, 0.5, 1, 2, 3, 4, and 5 μM); (C) naratriptan (0.625, 1.25, 2.5, 5, 7.5, 15, 25, 50, and 100 μM); (D) rizatriptan (0.75, 1.5, 3, 7.5, 15, 30, 62.5, 125, and 250 μM); (E) sumatriptan (1.5, 3, 7.5, 15, 30, 62.5, 125, 250, and 500 μM); and (F) zolmitriptan (0.75, 1.5, 3, 7.5, 15, 30, 62.5, 125, and 250 μM) was assessed at 37°C in HEK293-OATP1A2 and HEK293-VC cells. The transport was assessed for 2 min for all triptans except for eletriptan which was assessed for 1 minute. The quantity of intracellular triptan was normalized to protein content. The net transport was calculated by subtracting the values measured in the VC cells from the values measured in OATP1A2 cells. Km and Vmax were calculated by fitting the data to the Michaelis-Menten equation. Each point represents the mean ± S.D. of triplicate from a single experiment.
| Km (µM) | Vmax (pmol/mg protein per minute) | CLint (µl/mg protein per minute) | |
|---|---|---|---|
| Almotriptan | 5.1 ± 0.6 | 1265 ± 54 | 248 |
| Eletriptan | 0.8 ± 0.2 | 1634 ± 93 | 2042 |
| Naratriptan | 20.3 ± 1.0 | 3871 ± 70 | 191 |
| Rizatriptan | 42.9 ± 5.7 | 4798 ± 234 | 112 |
| Sumatriptan | 94.5 ± 9.9 | 8072 ± 300 | 85 |
| Zolmitriptan | 21.4 ± 1.4 | 5764 ± 110 | 269 |
Table 3: Km, Vmax and CLint values for the transport of triptans through OATP1A2.
The values were calculated by fitting the data to the Michaelis- Menten equation (± S.D.). CLint was calculated by dividing the Vmax by Km. Eletriptan showed the highest affinity for OATP1A2 and sumatriptan has the lowest affinity as the Km were calculated to be 0.8 ± 0.2 μM and 94.5 ± 9.9 μM, respectively.
OATP1A2 transport velocity was the lowest for almotriptan and the highest was observed with sumatriptan as the Vmax were 1265 ± 54.4 pmol/mg proteins per minute and 8072 ± 300.2 pmol/mg proteins per minute, respectively. The intrinsic clearance (CLint) was the lowest for sumatriptan (85.4 μl/mg protein per minute) and the highest for eletriptan (2042.5 μl/mg protein per minute).
Transport of Triptans through OATP2B1
Transport of triptans through OATP2B1 was evaluated, using a HEK293 cell model stably expressing this transporter, as OATP2B1 is also found at the BBB and has overlapping substrates with OATP1A2. Two concentrations of each triptan were assessed. The concentrations chosen for each substrate correspond to its Km and 3-times Km value determined for OATP1A2. A slightly greater intracellular concentration of eletriptan and sumatriptan was observed in HEK293- OATP2B1 cells compared to HEK293-VC cells (Figure 3).
Figure 3: OATP2B1-mediated transport of triptans. Uptake of (A) almotriptan (5 and 25 μM); (B) eletriptan (1 and 3.75 μM); (C) naratriptan (20 and 60 μM); (D) rizatriptan (40 and 120 μM); (E) sumatriptan (100 and 300 μM); and (F) zolmitriptan (21 and 65 μM) was assessed at 37°C in HEK293-OATP2B1 and HEK293-VC cells. The transport was assessed for 2 min for all triptans except for eletriptan which was assessed for 1 minute. The quantity of intracellular triptan was normalized to protein content. Each point represents the mean ± S.D. of triplicate from a single experiment.
No transport by OATP2B1 was noticed when incubations were performed with almotriptan, naratriptan, rizatriptan, and zolmitriptan. The small difference, less than 21%, observed between the two cell lines with eletriptan and sumatriptan was considered non-significant and likely due to the variability of experiments.
Effect of tricyclic compounds on OATP1A2-mediated uptake of triptans
To determine whether compounds composed of a tricyclic ring and a short aliphatic amine chain inhibit OATP1A2-mediated uptake of triptans, competition studies were performed (Supplemental Figures 1-4; Table 4). Carvedilol showed the strongest inhibition on the uptake of all six triptans with an IC50 of 0.5, 0.7, 2.1, 2.2, 3.5, and 3.8 μM for eletriptan, almotriptan, sumatriptan, zolmitriptan, rizatriptan, and naratriptan, respectively. Carazolol was the second strongest inhibitor with an IC50 of 1.6, 4.6, and 5.5 μM for almotriptan, zolmitriptan, and naratriptan, respectively. amitriptyline, chlorpromazine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, and trimipramine demonstrated slightly lower inhibition potencies than carvedilol and carazolol. Carbamazepine, carbazole, and phenothiazine exerted no significant effect on the transport of almotriptan, naratriptan, and zolmitriptan.
| Inhibitors | Almotriptan µM | Naratriptan | Zolmitriptan |
|---|---|---|---|
| Amitriptyline | 4.6 (2.4−8.9) | 13.2 (7.2−24.2) | 6.4 (4.0−10.1) |
| Carazolol | 1.6 (0.9−2.8) | 5.5 (3.3−9.2) | 4.6 (3.1−6.8) |
| Carvedilol | 0.7 (0.3−1.4) | 3.8 (3.0−4.8) | 2.2 (1.6−2.9) |
| Chlorpromazine | 8.7 (4.9−15.6) | 20.3 (12.1−34.1) | 16.9 (10.2−27.8) |
| Clomipramine | 6.1 (3.7−10.2) | 19.6 (11.4−33.9) | 13.5 (8.8−20.9) |
| Desipramine | 16.2 (7.5−35.0) | 19.8 (11.1−35.3) | 18.4 (12.1−28.1) |
| Doxepin | 2.5 (1.7−3.9) | 12.9 (8.4−19.8) | 6.8 (2.9−16.1) |
| Imipramine | 4.3 (2.8−6.7) | 7.4 (3.2−17.1) | 10.3 (4.7−22.4) |
| Nortriptyline | 4.5 (2.6−7.8) | 19.1 (6.8−53.7) | 13.0 (9.4−17.9) |
| Trimipramine | 7.6 (3.5−16.4) | 20.0 (12.2−32.6) | 13.6 (9.4−19.7) |
| Carbamazepine | No effect | No effect | No effect |
| Carbazole | No effect | No effect | No effect |
| Phenothiazine | No effect | No effect | No effect |
| Inhibitors | Eletriptan µM | Rizatriptan | Sumatriptan |
| Amitriptyline | N/A | 12.6 (6.8−23.4) | 9.5 (5.2−17.4) |
| Carvedilol | 0.5 (0.2−1.6) | 3.5 (2.2−5.6) | 2.1 (1.2−3.6) |
| Doxepin | N/A | 4.8 (2.2−10.8) | 5.9 (3.4−10.2) |
| Imipramine | 11.1 (2.7−46.1) | N/A | N/A |
| Nortriptyline | 81.0 (9.9−662.2) | N/A | N/A |
| N/A: Not Available (The inhibition assay was not evaluated) | |||
Table 4: IC50 values from the inhibition of triptans uptake through OATP1A2 by different tricyclic compounds. The values in parentheses represent the 95% confidence interval (Supplemental Figures 1-4).
Studies in the range of total plasma concentrations
As the IC50 studies were carried out with the concentration of substrates at saturation, it does not reflect the interaction at the BBB in clinical settings. Thus, a study using range of total plasma concentrations of triptans and inhibitors were carried out with almotriptan and zolmitrptan. These two triptans were selected based on their greater hydrophilic profile in the cell model used. In Canada, almotriptan is typically given in a 12.5 mg dose tablet and zolmitriptan is typically given in a 2.5 mg dose tablet, 2.5-5 mg dose nasal spray or 2.5 mg dose orally disintegrating tablet [14]. Pharmacokinetic studies have shown that a single dose of almotriptan results in a Cmax of 50 ng/mL and a single dose of zolmitriptan in any of the dosage forms corresponds to a Cmax of 3-7 ng/mL [15-19].
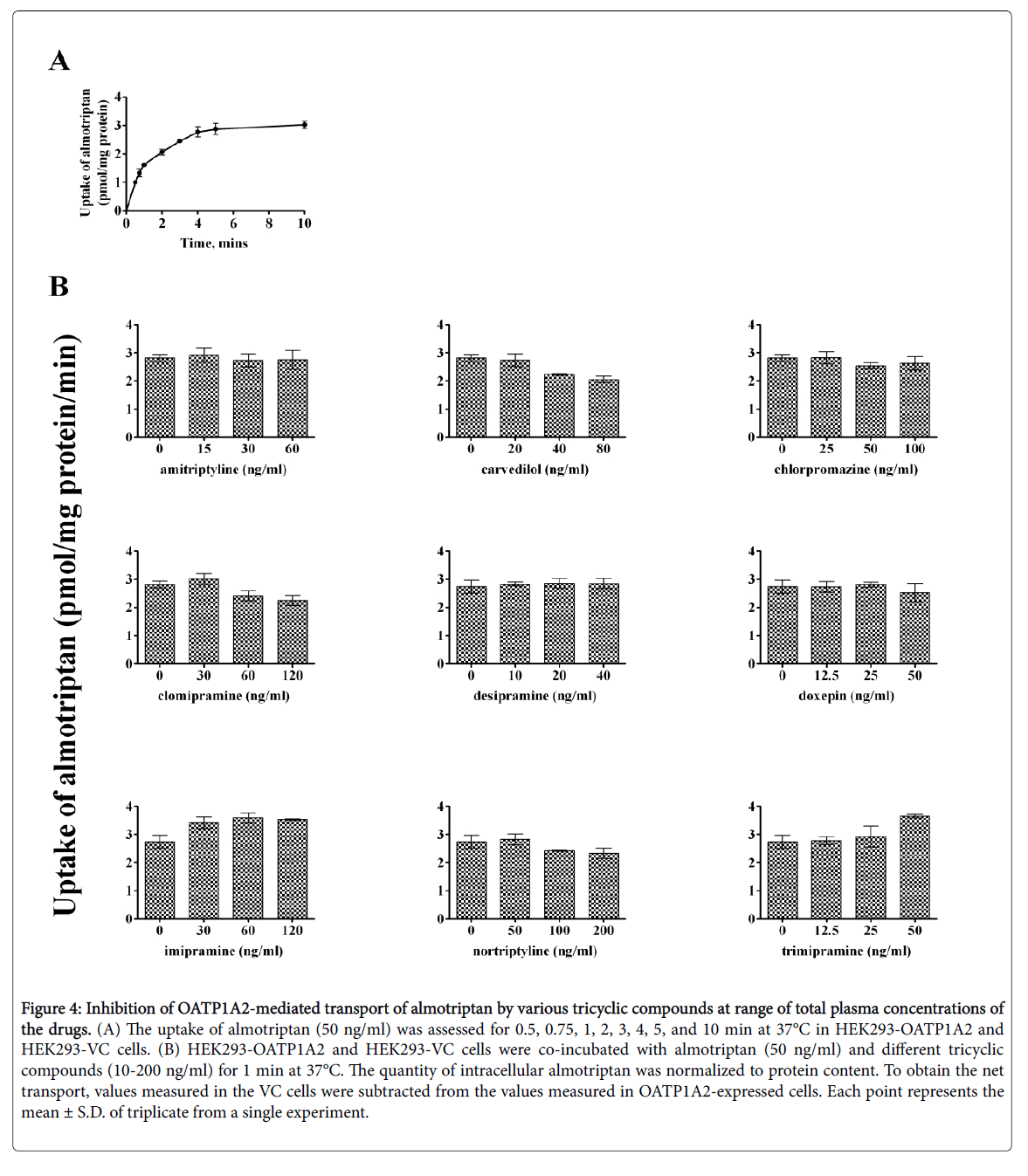
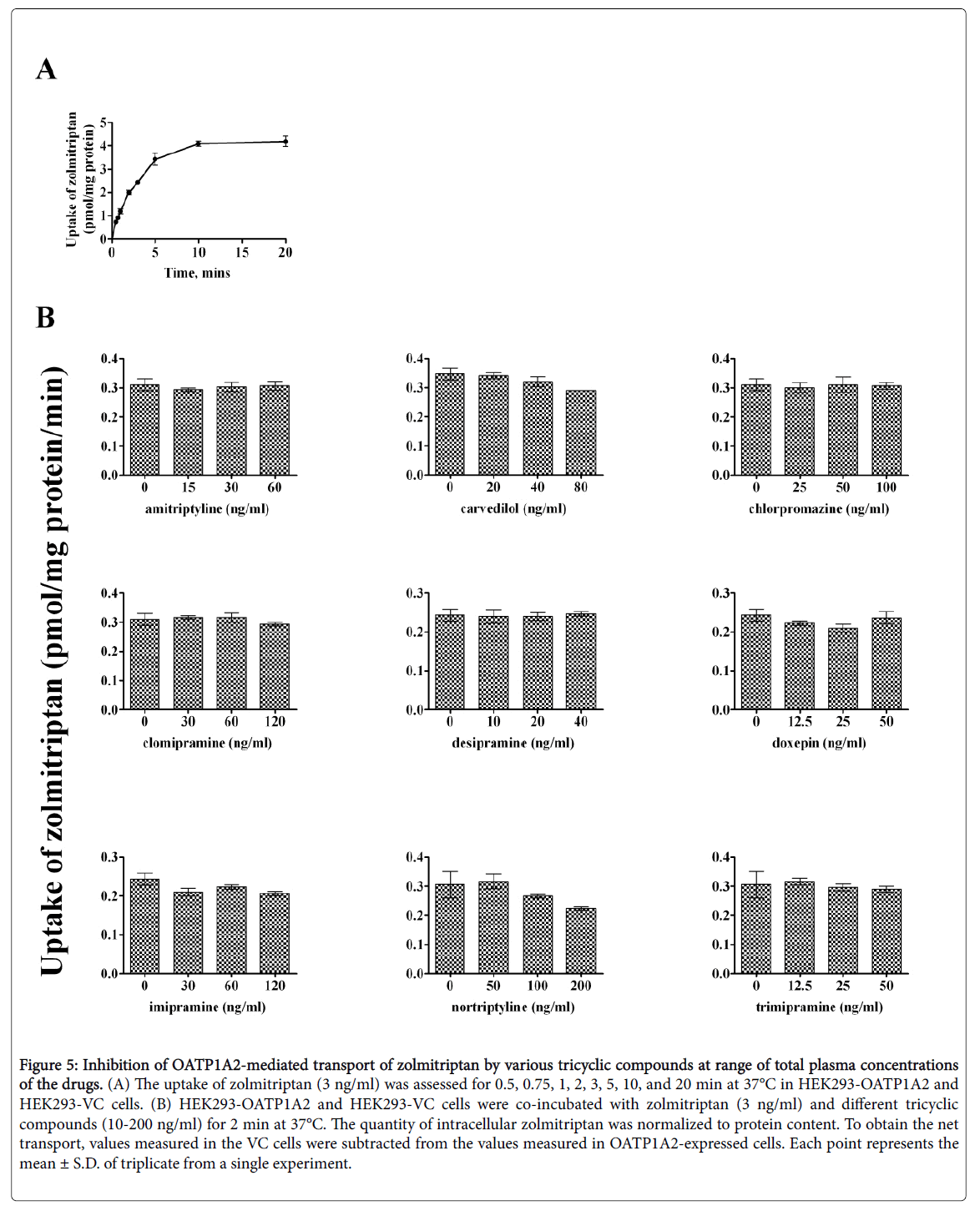
Time-dependent uptake of almotriptan and zolmitriptan was reassessed as lower concentrations might affect the kinetics. almotriptan (50 ng/mL) and zolmitriptan (3 ng/mL) showed a time-dependent saturable transport with a similar profile as when incubated at Km (Figures 4 and 5).
Figure 4: Inhibition of OATP1A2-mediated transport of almotriptan by various tricyclic compounds at range of total plasma concentrations of the drugs. (A) The uptake of almotriptan (50 ng/ml) was assessed for 0.5, 0.75, 1, 2, 3, 4, 5, and 10 min at 37°C in HEK293-OATP1A2 and HEK293-VC cells. (B) HEK293-OATP1A2 and HEK293-VC cells were co-incubated with almotriptan (50 ng/ml) and different tricyclic compounds (10-200 ng/ml) for 1 min at 37°C. The quantity of intracellular almotriptan was normalized to protein content. To obtain the net transport, values measured in the VC cells were subtracted from the values measured in OATP1A2-expressed cells. Each point represents the mean ± S.D. of triplicate from a single experiment.
Figure 5: Inhibition of OATP1A2-mediated transport of zolmitriptan by various tricyclic compounds at range of total plasma concentrations of the drugs. (A) The uptake of zolmitriptan (3 ng/ml) was assessed for 0.5, 0.75, 1, 2, 3, 5, 10, and 20 min at 37°C in HEK293-OATP1A2 and HEK293-VC cells. (B) HEK293-OATP1A2 and HEK293-VC cells were co-incubated with zolmitriptan (3 ng/ml) and different tricyclic compounds (10-200 ng/ml) for 2 min at 37°C. The quantity of intracellular zolmitriptan was normalized to protein content. To obtain the net transport, values measured in the VC cells were subtracted from the values measured in OATP1A2-expressed cells. Each point represents the mean ± S.D. of triplicate from a single experiment.
An incubation time of 1 and 2 min was chosen for the competition experiments for almotriptan and zolmitriptan, respectively. The concentrations of inhibitors correspond to the plasma concentrations measured at half Cmax, Cmax, and 2-times Cmax for a given dose. The reported peak plasma concentrations (Cmax) after an oral dose are: 33.5 ng/ml for amitriptyline 50 mg; 35 ng/ml for carvedilol 12.5 mg; 50 ng/ml for chlorpromazine 100 mg; 63 ng/ml for clomipramine 25 mg; 18 ng/ml for desipramine 50 mg; 25 ng/ml for doxepin 75 mg; 63 ng/ml for imipramine 100 mg; 50-150 ng/ml for nortriptyline 25-50 mg; and 22 ng/ml for trimipramine 75 mg [20-28]. Among the inhibitors evaluated, carvedilol and nortriptyline lowered the uptake of both almotriptan and zolmitriptan whereas clomipramine diminished the uptake of almotriptan only. The other tricyclic compounds had no significant effects on OATP1A2-mediated uptake of almotriptan and zolmitriptan at clinically relevant concentrations.
Discussion
Using stable cell lines overexpressing selected transporters, we confirmed that triptans are substrates for OATP1A2 but not OATP2B1. Inhibition studies demonstrated that compounds composed of a tricyclic ring and a short aliphatic amine chain inhibited OATP1A2- mediated uptake of triptans. The IC50 values of the inhibitors determined in this study followed the same pattern as those previously published when using rosuvastatin as the probe substrate for OATP1A2 [13]. Carvedilol and carazolol were the strongest inhibitors followed by amitriptyline, chlorpromazine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, and trimipramine. Inhibition studies conducted in the range of total plasma concentrations showed that carvedilol, clomipramine and nortriptyline were able to diminish the transport of triptans through OATP1A2.
The Km values of almotriptan, eletriptan and zolmitriptan for OATP1A2 determined in this study (5.1, 0.8, and 21.4 μM, respectively) are in line with those previously published (4.8, 1.3, and 15.1 μM, respectively) [12]. However, the Km values of rizatriptan and sumatriptan are higher in this study (42.9 and 94.5 vs. 6.0 and 27.0 μM). The drug’s solubility in the solvent used to dissolve or the incubation buffer may account for this discrepancy. In fact, when a drug is incompletely dissolved, the shape of the Km Vmax curve is changed when compared to the situation where the drug is completely dissolved at all concentrations. The Vmax values for the substrates are higher in this study. This variability may be explained by the differences in the in vitro model used: the quantity of OATP1A2 protein expressed at the cell surface, the quantity of functional proteins expressed, or the quantity of transporters exposed to the media and available for drug uptake. Although the CLint values (Vmax/Km) are different in the two studies, they both follow the same order of magnitude: eletriptan>zolmitriptan>almotriptan>rizatriptan> sumatriptan. Naratriptan could not be compared as the previous publication did not assess it.
There are evidences supporting a mechanism of action in the CNS for triptans in addition to their peripheral effects: 1) 5-HT1B and 5- HT1D receptors proteins are found on trigeminal sensory neurons; and 2) Activation of the trigeminal nucleus neurons by electrical stimulation is inhibited after administration of a triptan in animal models [2,3,29-31]. CNS adverse events, such as dizziness, vertigo, and ataxia are indirect indications that triptans have the potential to access the brain [32]. Using positron emission tomography (PET), two studies demonstrated that zolmitriptan can penetrate the brain at therapeutic doses and can bind to their receptors located in the CNS [33,34]. As triptans are hydrophilic, thus cannot cross the BBB by passive diffusion, OATP1A2 may play a role in facilitating the transport of triptans to their site of action. Our data suggest that the coadministration of carvedilol, clomipramine, or nortriptyline with a triptan may limit the entrance of triptans to the CNS by inhibiting OATP1A2. The drug concentration might fall below its therapeutic window in the brain. Consequently, the antimigraine activity may be abolished.
Of interest, one third of migraineurs receiving triptan therapy do not achieve headache relief and the most common reason for the discontinuation of these medications is the lack efficacy [32,35,36]. With the purpose of understanding the causes behind this lack of efficacy, a few studies have looked at polymorphisms found in genes involved in the pharmacokinetic and pharmacodynamic response to triptans. Associations have been reported for the genes encoding the serotonin transporter, monoamine oxidase A, and CYP1A2 [37]. Polymorphisms in the gene encoding for OATP1A2 and their ability to transport triptans should also be investigated. However, pharmacogenomics alone may not explain the lack of efficacy of triptans in all non-responders. Drug-drug interactions may explain inter-subject variability in antimigraine efficacy for cases where gene polymorphisms are not involved. Interestingly, migraine is often diagnosed in patients with mood disorders, such as depression, anxiety, panic disorder, and bipolar disorder [38,39]. As a result, treatments for both conditions are commonly prescribed. Tricyclic antidepressants are not only prescribed for depression but also for other off-label uses such as obsessive-compulsive disorder, panic disorder, chronic pain, insomnia, premenstrual symptoms and bulimia. In addition, β-blockers and antidepressants, especially amitriptyline, are occasionally prescribed for the prevention of migraine attacks [40]. These observations indicate that the coprescription of a triptan with a tricyclic antidepressant is not unusual.
Taken together, we demonstrated that compounds composed of a tricyclic ring and a short aliphatic amine chain inhibited the OATP1A2-mediated uptake of triptans. Our data suggest that carvedilol, clomipramine, and nortriptyline may limit the penetration of triptans to the brain by modulating OATP1A2 transport. Although an in vitro cell model permits to study the transport of a drug through a specific transporter, this experimental model is also associated with limitations when extrapolating in vitro findings to in vivo settings. Thus, emphasizing the need to confirm these results in humans. Indeed, the impact of concomitant administration of triptans with a potent OATP1A2 inhibitor on their antimigraine efficiency needs to be investigated further in clinical studies.
References
- Ramage-Morin PL, Gilmour H (2014) Prevalence of migraine in the Canadian household population. Statistics Canada: Health Reports 25.
- Longmore J, Shaw D, Smith D, Hopkins R, McAllister G, et al. (1997) Differential distribution of 5HT1D- and 5HT1B-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new antimigraine drugs. Cephalalgia 17:833-842.
- Hou M, Kanje M, Longmore J, Tajti J, Uddman R, et al. (2001) 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 909:112-120.
- Razzaque Z, Pickard JD, Ma QP, Shaw D, Morrison K, et al. (2002) 5-HT1B-receptors and vascular reactivity in human isolated blood vessels: assessment of the potential craniovascular selectivity of sumatriptan. Br J Clin Pharmacol 53:266-274.
- Tepper SJ, Rapoport AM, Sheftell FD (2002) Mechanisms of action of the 5-HT1B/1D receptor agonists. Arch Neurol 59:1084-1088.
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, et al. (2005) ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res 65:11419-11428.
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, et al. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73-79.
- Gao B, Vavricka SR, Meier PJ, Stieger B (2015) Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch 467:1481-1493.
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, et al. (2005) Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem 280:9610-9617.
- van der Deure WM, Peeters RP, Visser TJ (2010) Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol 44:1-11.
- Grube M, Kock K, Karner S, Reuther S, Ritter CA, et al. (2006) Modification of OATP2B1-mediated transport by steroid hormones. Mol Pharmacol 70:1735-1741.
- Cheng Z, Liu H, Yu N, Wang F, An G, et al. (2012) Hydrophilic anti-migraine triptans are substrates for OATP1A2 a transporter expressed at human blood-brain barrier. Xenobiotica 42:880-890.
- Lu J, Michaud V, Guilarte Moya LG, Gaudette F, Leung YH, et al. (2015) Effects of beta-blockers and tricyclic antidepressants on the activity of human organic anion transporting polypeptide 1A2 (OATP1A2). J Pharmacol Exp Ther 352:552-558.
- Gomes T, Camacho X, Martins D, Yao Z, Paterson M, et al. (2014) Triptans for Migraine Therapy: A Pharmacoepidemiologic Analysis. Ontario Drug Policy ResearchNetwork.
- Dixon R, Warrander A (1997) The clinical pharmacokinetics of zolmitriptan. Cephalalgia 18:15-20.
- Seaber EJ, Peck RW, Smith DA, Allanson J, Hefting NR, et al. (1998) The absolute bioavailability and effect of food on the pharmacokinetics of zolmitriptan in healthy volunteers. Br J Clin Pharmacol 46:433-439.
- Yates R, Nairn K, Dixon R, Seaber E (2002) Preliminary studies of the pharmacokinetics and tolerability of zolmitriptan nasal spray in healthy volunteers. J Clin Pharmacol 42:1237-1243.
- Lionetto L, Casolla B, Mastropietri F, D'Alonzo L, Negro A, et al. (2012) Pharmacokinetic evaluation of zolmitriptan for the treatment of migraines. Expert Opin Drug Metab Toxicol 8:1043-1050.
- Baldwin JR, Fleishaker JC, Azie NE, Carel BJ (2004) A comparison of the pharmacokinetics and tolerability of the anti-migraine compound almotriptan in healthy adolescents and adults. Cephalalgia 24:288-292.
- https://www.gsk-clinicalstudyregister.com/files2/595.pdf
- Patel DP, Sharma P, Sanyal M, Singhal P, Shrivastav PS (2013) UPLC-MS/MS assay for the simultaneous quantification of carvedilol and its active metabolite 4'-hydroxyphenyl carvedilol in human plasma to support a bioequivalence study in healthy volunteers. Biomed Chromatogr 27:974-986.
- Borges NC, Rezende VM, Santana JM, Moreira RP, Moreira RF, et al. (2011) Chlorpromazine quantification in human plasma by UPLC-electrospray ionization tandem mass spectrometry. Application to a comparative pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 879:3728-3734.
- Herrera D, Mayet L, Galindo MC, Jung H (2000) Pharmacokinetics of a sustained-release dosage form of clomipramine. J Clin Pharmacol 40:1488-1493.
- Yan JH, Hubbard JW, McKay G, Korchinski ED, Midha KK (2002) Absolute bioavailability and stereoselective pharmacokinetics of doxepin. Xenobiotica 32:615-623.
- Albers LJ, Reist C, Vu RL, Fujimoto K, Ozdemir V, et al. (2000) Effect of venlafaxine on imipramine metabolism. Psychiatry Res 96:235-243.
- Murphy GM Jr, Pollock BG, Kirshner MA, Pascoe N, Cheuk W, et al. (2001) CYP2D6 genotyping with oligonucleotide microarrays and nortriptyline concentrations in geriatric depression. Neuropsychopharmacology 25:737-743.
- Kirchheiner J, Muller G, Meineke I, Wernecke KD, Roots I, et al. (2003) Effects of polymorphisms in CYP2D6, CYP2C9, and CYP2C19 on trimipramine pharmacokinetics. J Clin Psychopharmacol 23:459-466.
- Cruz HG, Hay JL, Hoever P, Alessi F, te Beek ET, et al. (2014) Pharmacokinetic and pharmacodynamic interactions between almorexant, a dual orexin receptor antagonist, and desipramine. Eur Neuropsychopharmacol 24:1257-1268.
- Goadsby PJ, Knight Y (1997) Inhibition of trigeminal neurones after intravenous administration of naratriptan through an action at 5-hydroxy-tryptamine (5-HT(1B/1D)) receptors. Br J Pharmacol 122:918-922.
- Cumberbatch MJ, Hill RG, Hargreaves RJ (1997) Rizatriptan has central antinociceptive effects against durally evoked responses. Eur J Pharmacol 328:37-40.
- Cumberbatch MJ, Hill RG, Hargreaves RJ (1998) The effects of 5-HT1A, 5-HT1B and 5-HT1D receptor agonists on trigeminal nociceptive neurotransmission in anaesthetized rats. Eur J Pharmacol 362:43-46.
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB (2002) Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia 22:633-658.
- Varnas K, Jucaite A, McCarthy DJ, Stenkrona P, Nord M, et al. (2013) A PET study with [11C]AZ10419369 to determine brain 5-HT1B receptor occupancy of zolmitriptan in healthy male volunteers. Cephalalgia 33:853-860.
- Wall A, Kagedal M, Bergstrom M, Jacobsson E, Nilsson D, et al. (2005) Distribution of zolmitriptan into the CNS in healthy volunteers: a positron emission tomography study. Drugs R D 6:139-147.
- Holland S, Fanning KM, Serrano D, Buse DC, Reed ML, et al. (2013) Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 326:10-17.
- Wells RE, Markowitz SY, Baron EP, Hentz JG, Kalidas K, et al. (2014) Identifying the factors underlying discontinuation of triptans. Headache 54:278-289.
- Gentile G, Borro M, Simmaco M, Missori S, Lala N, et al. (2011) Gene polymorphisms involved in triptans pharmacokinetics and pharmacodynamics in migraine therapy. Expert Opin Drug Metab Toxicol 7:39-47.
- Hamelsky SW, Lipton RB (2006) Psychiatric comorbidity of migraine. Headache 46:1327-1333.
- Torelli P, D'Amico D (2004) An updated review of migraine and co-morbid psychiatric disorders. Neurol Sci 3: 234-235.
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, et al. (2012) Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 78:1337-1345.
Relevant Topics
- Adalimumab Pharmacokinetics
- ADME Studies
- Atorvastatin Pharmacokinetics
- Clinical Pharmacokinetics
- Clopidogrel Pharmacokinetics
- DMPK Studies
- Esomeprazole Pharmacokinetics
- Etanercept Pharmacokinetics
- Fluticasone Pharmacokinetics
- Infliximab Pharmacokinetics
- Linear Pharmacokinetics
- Morphine Pharmacokinetics
- Nonlinear Pharmacokinetics
- Olanzapine Pharmacokinetics
- Pharmacokinetics Modelling
- Quetiapine Pharmacokinetics
- Rosuvastatin Pharmacokinetics
Recommended Journals
Article Tools
Article Usage
- Total views: 2043
- [From(publication date):
January-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 1166
- PDF downloads : 877