Research Article Open Access
Effects of Surfactant and a Hyperthermostable Protease on Infectivity of Scrapie-Infected Mouse Brain Homogenate
Azumi Hirata1,2, Akikazu Sakudo3, Kazufumi Takano1, Shigenori Kanaya4 and Yuichi Koga4*1Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, 1-5 Hangi-cho, Shimogamo, Sakyo-ku, Japan
2Departments of Anatomy and Cell Biology, Faculty of Medicine, Osaka Medical College, 2-7 Daigaku-machi, Takatsuki, Japan
3Laboratory of Biometabolic Chemistry, School of Health Science, Faculty of Medicine, University of the Ryukyus, 207 Uehara, Nishihara, Japan
4Department of Material and Life Science, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Japan
- Corresponding Author:
- Yuichi Koga
Department of Material and Life Science
Graduate School of Engineering, Osaka University
2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
Tel: +81-6-6879-7443
Fax: +81-6-6879-7443
E-mail: kogay@mls.eng.osaka-u.ac.jp
Received date: July 22, 2015; Accepted date: August 25, 2015; Published date: August 31, 2015
Citation: Hirata A, Sakudo A, Takano K, Kanaya S, Koga Y (2015) Effects of Surfactant and a Hyperthermostable Protease on Infectivity of Scrapie-Infected Mouse Brain Homogenate. J Biotechnol Biomater 5:194. doi:10.4172/2155-952X.1000194
Copyright: © 2015 Hirata A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
PrPSc is thought to be the infective agent of TSE, and inactivating the infectivity of PrPSc without using strong reagents is difficult. Although PrPSc is a protease resistant protein, it can be degraded in vitro by the hyperthermophilic protease (Tk-subtilisin) at temperatures above 65ºC through the synergistic effect of heat destabilization of PrP and the high proteolytic activity of the thermostable protease. However, the change in infectivity of the proteasedigested PrPSc is still unknown. Therefore, we used mouse brain homogenate containing PrPSc (SBH) in a bioassay to investigate the loss of infectivity after Tk-subtilisin digestion. Surprisingly, the Tk-subtilisin digested SBH retained a high level of infectivity. Despite this, Tk-subtilisin could still be used for decontamination in highly protein denaturing condition such as in the presence of SDS.
Keywords
Protease; Thermostable enzyme; Prion; Scrapie; Tksubtilisin
Abbreviations
PrP: Prion Protein; PrPSc: Scrapie-Associated PrP; PrPC: Cellular PrP; TSE: Transmissible Spongiform Encephalopathy; CJD: Creutzfeldt - Jakob disease; SBH: Mouse Scrapie (Strain Chandler) Brain Homogenate; PK: Proteinase K; SDS: Sodium Dodecyl Sulfate; GdnHCl: Guanidine Hydrochloride; DTT: Dithiothreitol; SDW: Sterilized Distilled Water
Introduction
An abnormal prion protein with a β-sheet-rich conformation, designated scrapie-associated prion protein (PrPSc) [1], is the major protein component of an infective prion associated with transmissible spongiform encephalopathies (TSEs) [2]. PrP is distinguished to PrPC and PrPSc by its infectivity and they have different physical properties such as Proteinase K sensitivity and solubility. PrPSc is partially resistant to Proteinase K digestion and favors to form various oligomers [1,3]. PrPSc is formed from PrPC through its structural change from an α-rich conformation to a β-rich conformation [4]. PrPSc induces the structural change of PrPC by binding and being a template of new PrPSc molecule. Thus PrPSc is a proteinaceous, self-propagating molecule [5-7].
Since PrPSc is resistant to heat denaturation at 121ºC and many chemical decontamination methods, PrPSc inactivation is an important research target with the objective of TSE prevention. The World Health Organization recommends a combination of cleaning, chemical treatment, and heat sterilization of medical equipment [8]. More specifically, the guidelines recommend the use of an autoclave and strong chemical treatments, such as high concentration sodium hydroxide or sodium hypochlorite, for reusable instruments. Although these procedures are effective for eliminating infectivity, some surgical and complex instruments such as fiber optic endoscopes cannot be decontaminated using these methods because they could be damaged [9]. Furthermore, the safety risks associated with the use of strong chemicals are of concern to the medical community [10]. Therefore, prion decontamination procedures with sufficient potency and improved safety are required [11].
Subtilisin family proteases from Bacillus sp. are often used in cleaning reagents for medical equipment as an active element for removing contaminating protein. Okoroma et al. found that keratinase from Bacillus licheniformis can degrade PrPSc [12], and other subtilisin variants are in practical use as prion decontamination agents that can be used in moderate conditions such as at 50ºC. In addition, hyperthermostable proteases have been tested for PrPSc degradation. Snaider et al. used an extracellular extract of Aeropyrum pernix for in vitro digestion of PrPSc in CJD infected brain homogenate [13]. Takano and Koga found that two subtilisin family proteases from Thermococcus kodakarensis KOD1, Tksubtilisin and Tk-SP, degrade PrPSc in mouse scrapie (Chandler strain)- infected brain homogenate (SBH) to a level undetectable by western blot analysis [14]. Tk-subtilisin is a subtilisin-like serine protease which has significant heat stability, with its highest specific activity at 90ºC [15,16]. Tk-subtilisin can digest proteins under severe physical conditions in which most proteins are denatured. Since PrPSc is thought to be destabilized at high temperature, hyperthermophilic proteases have promising potential as active ingredients of medical detergents for prion decontamination. However, there is no report evaluating the loss of infectivity of protease digested PrPSc. In this study, we used a bioassay to evaluate the infectivity of SBH treated with Tk-subtilisin under various conditions.
Materials and Methods
Preparation of proteases
Proteinase K (PK) was purchased from Wako Pure Chemicals Ltd, Osaka, Japan. Tk-subtilisin was prepared from recombinant E. coli BL21 (DE3) harboring the Tk-subtilisin gene expression vector, as described previously [14,17].
Preparation of mouse brain homogenate and western blotting
Brain homogenate of terminally-diseased mice infected with the Chandler strain of scrapie prion (SBH) was prepared at 10% (w/v) in sterile PBS. The protein concentration of the homogenate was measured using a DC protein assay kit (BioRad). To prepare the sample for PrP degradation, an appropriate amount of the homogenate, equivalent to 60 μg of protein, was mixed with 0.5 M Tris-HCl (pH 8.0) and the required concentrations of Tk-subtilisin and distilled water, such that the total volume was 50 μL for each condition. Sodium dodecyl sulfate (SDS) at 3% (w/v) was added as necessary. The resultant samples were incubated at 100ºC for the specified time. When inactivation of Tk-subtilisin was required, 50 mM diisopropylfluorophosphate was added prior to sample preparation for SDS polyacrylamide gel electrophoresis (SDS-PAGE). Two-times loading buffer (150 mM Tris-HCl (pH 6.8), 6% (w/v) SDS, 30% (w/v) glycerol, and 0.03% (w/v) bromophenol blue) was added and the samples were boiled for 5 min. SDS-PAGE was performed using a 15% polyacrylamide gel, and PrP was detected by western blotting with an anti-PrP antibody, SAF83 (SPI bio, Montigny le Bretonneux, France).
Bioassay of infectivity
All animal studies were carried out in accordance with the guidelines for animal experiments of the School of Health Science, Faculty of Medicine, University of the Ryukyus.
A 10% w/v suspension of SBH from Chandler strain-infected mice was used to test the effect of Tk-subtilisin degradation or SDS treatment on the infectivity of PrPSc. The 10% SBH suspension was diluted to 1% with 200 mM Tris-HCl buffer (pH 8.0). Either 2.0 μg/mL of Tksubtilisin or 1% SDS, or both, was added to each aliquot of the SBH. SBH without Tk-subtilisin and SDS was prepared as a control. Each aliquot was incubated at 100ºC for 60 min to inactivate PrPSc. Each resultant aliquot was intracerebrally inoculated into 11-week-old male C57BL6/ JJmsSlc mice. A total of 20 μl of 1% SBH suspension was injected into the cerebral ventricular system of mice using a micro syringe. Six mice from each inoculation group were examined for the indicated period.
Immunohistochemistry
Two mice from each inoculation group were euthanized with ether after 155 days of incubation. Brains and spleens were dissected and immersed in 4% paraformaldehyde in phosphate buffered saline for 2 days at 4ºC. For light microscopy, specimens were dehydrated in a graded ethanol series and embedded in paraffin. The specimens were sliced into sections and immobilized on glass slides. The sections were deparaffinized using xylene and washed with a series of ethanol and distilled water. For histological observation, some sections were stained with hematoxylin and eosin. For immunohistochemistry, serial sections were prepared by autoclaving at 121ºC for 15 min, immersing in 1 mM HCl, and then washing with 3% hydrogen peroxide solution. The sections were incubated overnight at 4ºC with SAF83 (SPI bio) anti-mouse PrP monoclonal antibody diluted to 1:1000. After washing, the sections on the slides were incubated with a secondary antibody (Dako EnVision; Dako Japan Co., Tokyo, Japan) at room temperature for 30 min. Immunoreactivity was visualized by immersion in a DAB-H2O2 solution (Nichirei Biosciences, Tokyo, Japan). Sections were then counterstained with hematoxylin and observed under an All-in-one Type Fluorescence Microscope (BZ-8000; Keyence, Osaka, Japan) using BZ Analyzer Software (Keyence).
Results
As shown in our previous study [14], PrPSc in brain homogenate from scrapie-infected mice was degraded by a hyperthermophilic protease, Tk-subtilisin, to levels undetectable by western blot. The results show that 2.0 μg/mL of Tk-subtilisin can degrade the PrP to a level undetectable by western blotting. However, it was not known whether the degraded PrPSc had lost its infectivity. To clarify this, the infectivity of SBH treated with Tk-subtilisin under various conditions was evaluated using a bioassay. In total, six groups of mice were subjected to different inoculants, as shown in Table 1. The clinical symptoms and weight loss for each mouse from each group were observed until they died, or until 453 days post-inoculation.
| Inoculum | N/N0a | Survival rate (%) | Mean time of survival (days) |
| Sterilized distilled water | 0/6 | 100 | >453 |
| 1% SBH without heat treatment | 4/4 | 0 | 168.5 |
| 1% SBH* | 6/6 | 0 | 255.7 |
| 1% SBH* + 2 μg/mL Tk-subtilisin | 6/6 | 0 | 262.7 |
| 1% SBH* + 1 % SDS | 1/6 | 83.3 | >420 |
| 1% SBH* + 2 μg/mL Tk-subtilisin + 1% SDS | 0/6 | 100 | >453 |
aN/N0, number of mice that developed infection per number of inoculated mice.
*Samples were heat treated at 100°C for 1 hr.
Table 1: Survival times for mice infected with scrapie (Chandler strain).
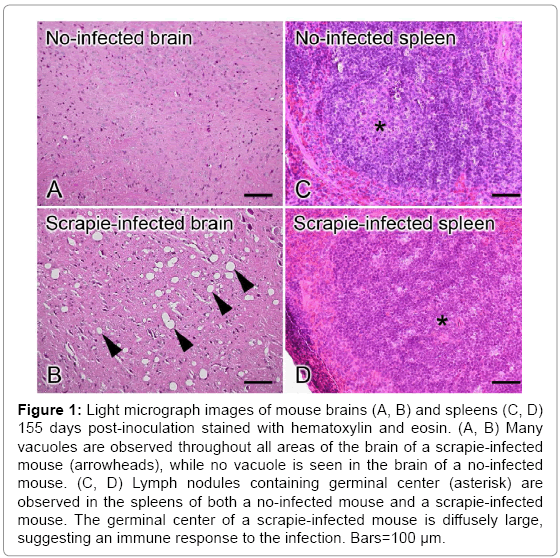
Two mice from each inoculation group were euthanized at 155 day post-inoculation to compare PrPSc accumulation in their tissues. Tissue specimens were prepared from brains and spleens of each mouse, and the pathological changes in the brains and accumulation of PrPSc in the spleens was observed. Brain and spleen tissue from a non-infected and infected mouse are shown in Figure 1. The number and extent of vacuoles observed in the brain of the infected animal indicated that it was at the terminal stage of TSE disease at 155 day post-inoculation while the spleen tissues from both infected and non-infected mouse shows slight difference. The brains and spleens of mice from each test group were also observed at 155 day post-inoculation, as shown in Figure 2. To identify the mouse at the terminal stage of Scrapie, the brain tissue of each test group has been observed whether it has vacuoles or not. Brain tissues of each mouse inoculated SBH treated with heat, 1% SDS, Tk-subtilisin and both 1% SDS and Tk-subtilisin respectively (Figure 2C-2F), did not show vacuoles as shown in Figure 2B. However, the immunohistochemistry results showed PrPSc accumulation in the spleens of mice that were inoculated with heat-treated SBH or Tksubtilisin- treated SBH (Figure 2I and 2K) while the other brain tissue has no PrPSc accumulation (Figure 2J and 2L). Since the spleen is the organ that accumulates the PrPSc earlier than brain, infection of scrapie can detect before the final stage of the disease.
Figure 1: Light micrograph images of mouse brains (A, B) and spleens (C, D) 155 days post-inoculation stained with hematoxylin and eosin. (A, B) Many vacuoles are observed throughout all areas of the brain of a scrapie-infected mouse (arrowheads), while no vacuole is seen in the brain of a no-infected mouse. (C, D) Lymph nodules containing germinal center (asterisk) are observed in the spleens of both a no-infected mouse and a scrapie-infected mouse. The germinal center of a scrapie-infected mouse is diffusely large, suggesting an immune response to the infection. Bars=100 μm.
Figure 2: Light micrograph images of mouse brains (A-F) and spleens (G-L) 155 days post-inoculation. (A-F) Neuropil vacuolation is observed throughout all areas of the brain of (B) a scrapie-infected mouse (arrowheads), whereas no vacuolation is seen in the brain of (A) a no-infected mouse, (C) a mouse inoculated with SBH incubated at 100°C, (D) a mouse inoculated with SBH incubated at 100°C with 1% SDS, (E) a mouse inoculated with SBH digested with Tk-subtilisin, and (F) a mouse inoculated with SBH incubated with Tk-subtilisin and 1% SDS. (G-L) Light micrograph images showing the localization of PrP in the spleen of (G) a no-infected mouse, (H) a scrapieinfected mouse, (I) a mouse inoculated with SBH incubated at 100ºC, (J) a mouse inoculated with SBH incubated at 100°C with 1% SDS, (K) a mouse inoculated with SBH digested with Tk-subtilisin, and (L) a mouse inoculated with SBH incubated with Tk-subtilisin and 1% SDS. Immunoreactivity for PrP (brown signal) is detected around the center of the lymph nodule in the spleen of (H) a scrapie-infected mouse, (I) a mouse inoculated with SBH incubated at 100ºC and (K) a mouse inoculated with SBH digested with Tksubtilisin. No labeling for PrP is observed in the lymph nodules (G, J, L). Bars: A-F=100 μm; G-L=50 μm.
The number of mice that survived until the end of the experiment, the survival rate, and the mean time of survival for each group are summarized in Table 1. All four mice injected with the untreated 1% SBH (positive control) lost weight from 144–155 days post-inoculation, and abnormal behaviors such as tremors and ataxia were observed in this group. The incubation period matched that of a typical scrapieinfected mouse. Because the described symptoms indicated that the mice were at the terminal stage of TSE, the mice were euthanized at 155 or 182 days post-injection (mean incubation time of 168.5 days). The mice from the groups injected with heat-treated SBH developed infections from 239–265 days post-inoculation (mean time 255.7 days), indicating that 1 h of heat treatment at 100ºC decreased the infectivity of SBH. The mice injected with Tk-subtilisin-treated SBH developed infections from 253–280 days post-inoculation (mean time 262.7 day), indicating that enzymatic digestion did not improve the heat treatment. 17 mice in the other groups survived until the end of the experimental period, indicating that the inoculant used in these groups had lost significant infectivity. However, there was one exception: a mouse from the group injected with SBH treated with 1% SDS developed TSE at 255 days post-inoculation, indicating that this treatment method did not completely eliminate infectivity.
Discussion
Based on the results, we conclude that Tk-subtilisin-treated SBH maintains its infectivity, despite the fact that western blot analysis did not show any PrP signal. It is possible that the Tk-subtilisin degraded the epitope region of the PrPSc, but that the undigested parts of PrPSc remained as infectious as the native protein. However, a similar result was observed when another anti-PrP antibody, SAF32, was used for the western blot analysis (data not shown). The epitope of SAF83 is amino acid residues 142–160 of PrP, while that of SAF32 is residues 51–91. The other possibility is that the degradation of PrPSc is quantitatively insufficient to eliminate infectivity even though it cannot be detected by western blot analysis. Further experiments are required to investigate the potential reasons for PrPSc infectivity after Tk-subtilisin digestion.
Additionally, it is likely that SDS plays an important role in the degradation of PrPSc by Tk-subtilisin. SBH includes many biogenic substances, such as nucleic acids and fatty acids, which are thought to interact with PrPSc [18], and are likely to interfere with the interaction between Tk-subtilisin and PrPSc. Furthermore, PrPSc is known to form oligomers and insoluble particles. These features may hinder proteolysis of PrPSc in SBH. Because SDS is presumed to function by enabling Tksubtilisin to access the insoluble PrPSc particles, it is possible that the presence of SDS allows Tk-subtilisin to degrade the infective core of PrPSc, which cannot be achieved by Tk-subtilisin alone.
From the bioassay results (Table 1), it is clear that SDS treatment significantly decreases the infectivity of SBH, likely by denaturing PrPSc in SBH. However, as shown by the results of the survival test, the infectivity loss is not complete. However, treating SBH with both Tk-subtilisin and SDS decreases SBH infectivity more effectively than SDS alone as a result of the cooperative effect of denaturation by SDS and degradation by Tk-subtilisin. Thus, Tk-subtilisin may be a useful ingredient to include in reagents designed for the decontamination of PrPSc. To probe this apparent cooperation, further quantitative analysis of Tk-subtilisin degradation of PrPSc is necessary.
Effective decontamination procedures for PrPSc in the medical field are of great importance. The use of a protease as an ingredient in medical detergents is a very attractive option for establishing a simple prion decontamination procedure. Further quantitative evaluation of the effects of Tk-subtilisin on PrPSc infectivity in less stringent conditions is imperative.
Acknowledgements
The authors wish to acknowledge Dr. Kazuyoshi Ikuta, Professor of The Research Foundation for Microbial Disease of Osaka University, for his help in interpreting the significance of the results of this study. This work was supported in part by a Grant from the Senri Life Science Foundation, Japan, by the Ministry of Health, Labour and Welfare, Japan, and by an Industrial Technology Research Grant Program from the New Energy and Industrial Technology Development Organization (NEDO) of Japan. The authors have no conflicts of interest to declare.
References
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, et al. (1993) Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90: 10962-10966.
- Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95: 13363-13383.
- Hay B, Barry RA, Lieberburg I, Prusiner SB, Lingappa VR (1987) Biogenesis and transmembrane orientation of the cellular isoform of the scrapie prion protein.Mol Cell Biol 7: 914-920.
- Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339-1347.
- Castilla J, Saá P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121: 195-206.
- Deleault NR, Harris BT, Rees JR, Supattapone S (2007) Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA 104: 9741-9746.
- Wang F, Wang X, Yuan CG, Ma J (2010) Generating a prion with bacterially expressed recombinant prion protein. Science 327: 1132-1135.
- World Health Organization WHO infection Control Guidelines for Transmissible Spongiform Encephalopathies, 2000.
- Brown SA, Merritt K, Woods TO, Busick DN (2005) Effects on instruments of the World Health Organization--recommended protocols for decontamination after possible exposure to transmissible spongiform encephalopathy-contaminated tissue. J Biomed Mater Res-A 72 186–190.
- McDonnell G, Burke P (2003) The challenge of prion decontamination. Clin Infect Dis 36: 1152-1154.
- Baier M, Schwarz A, Mielke M (2004) Activity of an alkaline 'cleaner' in the inactivation of the scrapie agent. J Hosp Infect 57: 80-84.
- Okoroma EA, Purchase D, Garelick H, Morris R, Neale MH, et al. (2013) Enzymatic formulation capable of degrading scrapie prion under mild digestion conditions. PLoS One 8: e68099.
- Snajder M, Vilfan T, Cernilec M, Rupreht R, PopoviÄM, et al. (2012) Enzymatic degradation of PrPSc by a protease secreted from Aeropyrum pernix K1. PLoS One 7: e39548.
- Koga Y, Tanaka S, Sakudo A, Tobiume M, Aranishi M, et al. (2014) Proteolysis of abnormal prion protein with a thermostable protease from Thermococcuskodakarensis KOD1. Appl Microbiol Biotechnol 98: 2113-2120.
- Kannan Y, Koga Y, Inoue Y, Haruki M, Takagi M, et al. (2001) Active subtilisin-like protease from a hyperthermophilicarchaeon in a form with a putative prosequence. Appl Environ Microbiol 67: 2445-2452.
- Pulido M, Saito K, Tanaka SI, Koga Y, Morikawa M, et al. (2006) Ca2+-dependent maturation of subtilisin from a hyperthermophilic archaeon, Thermococcuskodakaraensis: The propeptide is a potent inhibitor of the mature domain but is not required for its folding.Appl Environ Microbiol 72: 4154–4162.
- Tanaka S, Saito K, Chon H, Matsumura H, Koga Y, et al. (2007) Crystal structure of unautoprocessed precursor of subtilisin from a hyperthermophilic archaeon: Evidence for Ca2+-induced folding. J Biol Chem 282: 8246-8255.
- Diaz-Espinoza R, Soto C (2012) High-resolution structure of infectious prion protein: The final frontier. Nat Struct Mol Biol 19: 370-377.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14783
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10267
- PDF downloads : 4516