Research Article Open Access
Effects of Soaked Pigeon Peas on the Growth of Nile Tilapia (Oreochromis niloticus L) Fingerlings
Leonard Jonas Ndau* and Amos Nazael Madalla
Department of Animal Science and Production, Faculty of Agriculture, Sokoine University of Agriculture, P.O. Box 3004, Chuo Kikuu, Morogoro, Tanzania
- *Corresponding Author:
- Leonard Jonas Ndau
Department of Animal science and production
Faculty of agriculture Sokoine
University of Agriculture Morogoro
Tanzania
Tel: +4550195887;
E-mail: ndaumash@gmail.com
Received Date: September 01, 2014; Accepted Date: November 03, 2014; Published Date: November 10, 2014
Citation: Ndau LJ, Madalla AN (2015) Effects of Soaked Pigeon Peas on the Growth of Nile Tilapia (Oreochromis niloticus L) Fingerlings. J Fisheries Livest Prod 3:125. doi:10.4172/2332-2608.1000125
Copyright: © 2015 Ndau LJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The demand to substitute fishmeal in Fish feed has necessitated with the use of plant-derived Feedstuffs. Though, problems of anti-nutritional factors in these feedstuffs have limited their usage and assimilation into fish feed formulation. Different processing approaches have been applied to remove or reduce the level of these anti nutrition factors in these plant- derived feedstuffs. In this study Pigeon peas (Cajanus Cajan L) were soaked in cold water (1:3 w/v) for 24 hours. Effect of soaked Pigeon peas on the growth of Nile tilapia; Oreochromis niloticus was then evaluated for a period of 54 days. Seven diets (three diets of raw pigeon peas and soaked pigeon peas and one control diet) were formulated to contain about 30% crude protein. Fishmeal was replaced by 0% (control), 15%, 30% and 45% for both raw and soaked pigeon peas. Results obtained in this study showed that growth and feed utilization of fish fed diets containing raw Pigeon peas seed meal decreased significantly (P>0.05) with increased dietary levels of raw Pigeon peas While significant results (P<0.05) were are shown for fish fed soaked Pigeon peas seed meal with 45% level inclusion performing best next to control. It was therefore concluded that whenever the cost of Pigeon peas is lesser than fish meal, Pigeon peas can be soaked for 24 hours with a ratio of 1:3 w/v to replace up to 45% fish meal in fish diets as a way of reducing the current demand pressure on fish meal
Keywords
Tilapias; Cajanus cajan; Galactosidase; Feed utilization
Introduction
Tilapias are tropical cichlid teleosts native to Africa, the Mediterranean, and the Middle East. Tilapias are some of the oldest cultured fishes, as illustrated in Line drawings found in Egyptian tombs that date back to 2000 BC. Tilapia is the second mainly important farmed fish after the carps [1].
This group consists of three important genera, which are taxonomically distinguished according to their reproductive behaviors: Tilapia, Oreochromis, and Sarotherodon, all are commonly known as “tilapia.” Tilapias are biparental caring-substrate spawners; Oreochromis are generally maternal mouth brooders; and Sarotherodon are usually paternal or biparental mouth brooders. Currently, Nile tilapia (Oreochromis niloticus) various hybrids are the most commonly produced tilapine species worldwide. Other less commonly cultured species include Blue tilapia (O. aureus), Mozambique tilapia (O. mossambicus), Zanzibar tilapia (O. urolepis hornorum), and red tilapia (T. rendalli and T. zill.
The Growth of Tilapia in different parts of the world has increased exponentially in recent years. Many factors explain to why tilapia is globally cultured. These are: adaptive capability to a variety of environmental conditions, acceptance of a wide broad spectrum of diets and are low on the food chain, palatability, ability to reproduce in captivity and low expense relative to other finfishes.
High nutritional value due to a well-balanced amino acid profile, low in fat and saturated fat, omega-3 fatty acids, calories, carbohydrates high amount of vitamins and minerals has been accounted as a major factor which makes Tilapia to be liked by people all around the world [2,3].
Likewise, fish meat is usually affectionate because it contains less connective protein tissue than other meats from beef, mutton, pork, and poultry. For this reason it is easy to chew up, particularly for children and the elderly [4].
More than 93% of the total fish production in developing countries has been reported to be contributed by omnivorous and herbivorous fish species such as carps and tilapia. As production of these fish species step up, feed inputs and nutritionally balanced diets are required. Fishmeal has been used as a source of protein in formulating the balanced fish diets. The estimations that have done by many researchers suggest that the amount of feeds for fish will increase at a rate of 93% by 2012 to give the massive production of farmed fish especially in developing countries. This suggests that fishmeal demand, as a source of protein will increase. To reduce this increase in fishmeal demand, different fish feed ingredients have been evaluated. El-Sayed and Tacon; El-Sayed [5,6]; reported that ingredients can either be animal protein sources – by products such as blood meal, bone meal and hydrolyzed or Plant protein sources such as soybean meal, cottonseed meal, Pigeon peas and groundnut meal. Based on affordability, local availability and good nutritional profile, Pigeon peas (Cajanus cajan L) have been acknowledged as a potential source of protein in aqua feed formulation.
Nevertheless the use of Pigeon peas is limited by the presence of ant- nutritional factors such as protease inhibitors, Amylase inhibitors, Oligosaccharides and poly phenol.
Therefore, the current study was designed to assess the growth performance and nutrient utilization of Nile tilapia (Oreochromis niloticus) fed diet containing soaked Pigeon as replacer of fishmeal.
Materials and Methods
The experiment was conducted at Magadu farm located at Sokoine University of Agriculture, (SUA), Morogoro, Tanzania. The farm belongs to the Department of Animal Science and Production of Sokoine University of Agriculture. Sokoine University of Agriculture is situated at 2.5 km south of Morogoro municipality at an altitude of 550 m above sea level and gets an average annual rainfall of approximately 880 mm with temperature vary between 27°C and 31°C.
Collection of ingredients
Pigeon peas, maize brain, and fishmeal were acquired from Morogoro Municipal Market and were manually sorted to remove impurities. Wheat flower was obtained from local shop while premix was obtained from livestock shop. Manufactured by ABCON CHEMICALLTD P.O BOX 60098- DAR-ES-SALAAM (Table 1).
| Mineral | Amount (%) |
|---|---|
| Calcium | 3 |
| Phosphorus | 7 |
| Salt | 12.5 |
Table 1: Composition of mineral used in the experimental diets.
Nile tilapia (Oreochromis niloticus) fingerlings were collected from Magadu fish pond.
Processing of ingredients
In attempting to remove anti- nutritional factors, seeds were soaked by dipping them in cold water in plastic container for 24 hours with soaking ratio of 100 g/300 ml (1:3w/v) at room temperature. After soaking, the seeds were oven dried at 60°C for 24 hours. Maize brain and Pigeon peas were milled by using milling machine and stored in name labelled containers prior to analysis.
Chemical composition
Chemical composition to determine composition of raw and soaked Pigeon peas seeds was carried out according to standard method adopted by Association of Official Analytical Chemists (AOAC) [7]. Moisture content was determined by drying samples in an oven at 70°C for 48 hours.
Crude protein was determined according to Kjeldah method. Samples were digested in concentrated sulphuric acid by a digestor, distillated and titrated to obtained nitrogen. Crude protein was obtained by multiplying nitrogen content with conversion factor of 6.25. Crude fibre was determined by digesting a sample with weak base preceded by weak acid. Ankom 220 Fiber analyzer was used to determine the crude fibre.
Crude lipid was determined after soxhlet extraction of dried samples with 1.25% H2SO4 and 1.25% NaOH Ash content was determined by calcinations method. Samples which were previously oven dried were put in crucible and heat in a muffle furnace at 550°C for 3 hours and then cooled before weighing.
Experimental diet formulation
Diets were formulated to replace 0% (control), 15%, 30%, and 45% of fishmeal. Diets were iso-nitrogenous (30 g 100 g-1), isolipidic (10 g 100 g-1) and gave up to 30% crude protein.
During compounding of diets, the weighed proportions of dry milled ingredients were carefully mixed by hand before adding water. The mixture was then passed through meat micer of 0.4 mm sieve size to make pellets.
During compounding of diets, the weighed proportions of dry milled ingredients were carefully mixed by hand before adding water. The mixture was then passed through meat micer with 0.4 mm sieve size to make pellets. Pellets were then dried by passing in the oven for 6 hours at 40°C and then spread on the floor under room temperature for one week prior to packaging. Composition of experimental Diets (g/100 g diet) is shown in Table 2.
| Ingredient | ||||||
|---|---|---|---|---|---|---|
| Diet | Fish Meal | Pigeon peas | Maize bran | Premix | Binder | Total |
| RPPSM 0 | 37 | 0.0 | 59 | 2 | 2 | 100 |
| RPPSM 15 | 33 | 22 | 41 | 2 | 2 | 100 |
| RPPSM 30 | 29.5 | 45 | 21 | 2 | 2 | 100 |
| RPPSM 45 | 26 | 66 | 4 | 2 | 2 | 100 |
| SPPSM 15 | 33 | 21 | 42 | 2 | 2 | 100 |
| SPPSM 30 | 29 | 45 | 22 | 2 | 2 | 100 |
| SPPSM 45 | 26 | 66 | 4 | 2 | 2 | 100 |
RPPSM = Raw Pigeon peas seed meal, SPPSM = Soaked Pigeon peas seed meal
Table 2: Typical dietary inclusion used for the formulation of the experimental diets (g 100 g-1).
Experimental design and feeding regime
The experimental design was a completely randomized design (CRD). 105 Nile tilapia fingerlings with initial average body weight of 15 g were randomly distributed in plastic buckets with a capacity of 20 litres at a stocking density of 5 Nile tilapia per bucket.
Fish were hand fed twice a day according their apatite; diets were given between 9.00 hours and 17:00 hours at 5% of body weight throughout the experiment. The quantity of feed was adjusted weekly based on the weight of fish to avoid feed wastage and deterioration of water quality parameters. A few remaining feeds at the end of the week were weighed to and used to estimate feed intake.
Handling of experimental fish
Fish were weighed prior to the start of each experiment to obtain fish of consistent size and weight. A top pan balance was used in weighing procedures. Fish were acclimated to Experimental environment for one week and fed control diet. After the acclimation time fish were netted from each plastic bucket using a fine mesh hand net and total weighed. The dietary treatments were at random assigned to the plastic buckets. Total weighing of experimental fish was done weekly throughout the experiment.
Data collection and computation
The study lasted for 8 weeks during which fish were fed the experimental diets containing Pigeon pea seed meal. Parameters measured were bulk initial weight, bulk final weight and feed intake. Weekly weight gain was measured to monitor growth while other parameters were calculated as described below:
Specific Growth
Specific growth rate (SGR) (% day-1) % = {In Wt2 - InWt1 /t2 – t 1} × 100
Average Daily weight gain
Average Daily weight gain (ADWG) (day-1) = (Wt2 – Wt1)/ t.
Where:
Wt2 = final weight (g) at time t2 (end of experiment)
Wt1 = Initial weight (g) at time t1 (beginning Of experiment) and In = Natural logarithm.
t= time (day)
Feed utilization
Feed conversion ratio (FCR) = Weight of food fed (g) /increased weight (g)
Feed intake (FI) (g day-1)) = Total feed intake /number of days
Survival rate (SR)= {N2 /N1}× 100
Where:
N2 -Number of fish at the end of the experiment
N1 - Number of fish at the beginning of the experiment
Data analysis
Data collected from this experiment were subjected to one -way analysis of variance (ANOVA). Comparisons of treatment means were done by Turkey’s Honest at 5% level of significance. Analyses were performed using SPSS software versioN16.
Results
This part presents the results of the experiment. First, the proximate composition results for both Raw and Soaked Pigeon Peas are presented followed by Growth Performance and Feed Utilization which include
Proximate composition
The biochemical composition of unprocessed and processed Pigeon peas is shown in Table 3. In general soaking method led to reduction of dry matter, crude lipid, crude fibre and ash content. However there was slightly improvement of protein content.
| Parameter | Feed ingredient | |
|---|---|---|
| Raw Pigeon peas | Soaked Pigeon peas | |
| Dry matter | 96.72 | 95.45 |
| Crude protein | 20.34 | 21.02 |
| Crude lipid | 2.10 | 1.94 |
| Crude fibre | 10.08 | 10.1 |
| Ash | 4.18 | 3.94 |
Table 3: Proximate composition (%) of raw and soaked Pigeon peas used for diet Formulation.
Growth performance and feed utilization
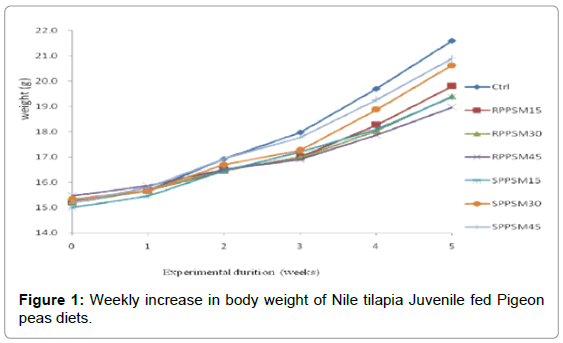
The dissimilarity in body weight between the control diet and the other experimental diets containing raw and soaked Pigeon peas is shown in Figure 1. Increased body weight was examined during the first week. However decline in body weight for fish fed diets containing SPPSM 30, RPPSM 30 and RPPSM 45 was noted during the third week.
Table 4 describes the decreased performance with increased inclusion level of RPPSM. The decline in intake was accompanied by a considerable decline in growth in terms of final weights, average daily gain, and specific growth rate as well as feed utilization in terms of feed conversion ratio. No significant difference in fish survival between different dietary treatments was observed.
Discussion
Proximate compositions
Processing of Pigeon peas using cold water led to the reduction of nutrients due to Nutritional change which caused losses of water soluble components such as carbohydrates and nitrogen. Valvearde et al., [8] detailed decrease in dry matter of beans after soaking. Low dry matter in soaked Pigeon peas than in raw Pigeon peas was reported by Apata and Kadam et al. [9] due to leaching during soaking. Contrasting these results is Akingbade et al. [10] who detailed that protein content of Jack Bean (Carnavalia ensiformis) Seeds increased from 88-92% after 96 hours of soaking in water
Slightly modification of crude protein (increased crude protein) may be explained by the fact that during soaking mobilization of both true and non-protein compound has occurred [11]. Badifu attributed increased protein when kernels were soaked in cold water for 48 hours. However crude protein obtained in this study contrary to results reported by Sopade et al. [12] who reported decreased crude protein as a result of soaking sorghum cultivars in cold water.
Moreover, processing of Pigeon peas by using cold water led to the reduction of crude lipid. Nevertheless it is within the value reported by Ravindran and Ravindran [13]; Siddhuragu et al., when two groups (A and B) of Mucuna seeds were soaked in water for 12 and 24 hours.
Low content a of ash in the soaked Pigeon peas could be explained by leaching of minerals despite the fact that it is still within the values reported by earlier studies [14,15]. Pigeon peas are known to contain mineral such Iron, Calcium, Manganese, magnesium and Zinc which tend to dissolve and hence leaching into soaking medium during the whole process of soaking.
Growth performance and feed utilization
Figure 1 shows the weekly change in body weight of Nile tilapia Juvenile fed diets containing both raw and soaked Pigeon peas while Table 4 elaborates feed utilization of Nile tilapia Juvenile. Growth performance (Final weight, ADG, SGR), and feed utilization Feed intake).
| Initial weight (g) | final weight (g) | weight gain (g) | ADG (g fish-1 day-1) | feed intake (g fish-1) | FCR | SGR (% fish-1) | Survival (%) | |
|---|---|---|---|---|---|---|---|---|
| RPSM 0 | 3.05 ± 0.76a | 4.32± 0.18a | 1.27 ±0.19a | 0.03 ± 0.06a | 2.52 ± 0.36a | 2.01 ± 0.34a | 0.99 ± 0.07a | 100a |
| RPSM 15 | 3.04 ± 0.11a | 3.96 ± 0.26a | 0.92 ± 0.15ab | 0.03 ± 0.07ab | 2.04 ± 0.17ab | 2.24 ± 0.19a | 0.75 ± 0.05ab | 100a |
| RPSM 30 | 3.05 ± 0.15a | 3.88 ± 0.31ab | 0.83 ± 0.17ab | 0.03 ± 0.04ab | 1.97 ± 0.27ab | 2.39 ± 0.23a | 0.69 ± 0.06ab | 100a |
| RPSM 45 | 3.09 ±0.10a | 3.09 ± 0.10b | 0.70 ± 0.03b | 0.02 ± 0.05b | 1.59 ±0.14b | 2.28 ± 0.09a | 0.58 ± 0.02b | 100a |
| SPSM 15 | 3.00 ± 0.13a | 3.87 ± 0.25ab | 0.87 ± 0.28ab | 0.02 ± 0.06ab | 2.16 ±0.65ab | 2.77 ± 0.59a | 0.73 ± 0.12ab | 100a |
| SPSM 30 | 3.07 ± 0.08a | 4.13 ±0.05a | 1.0 6 ± 0.22ab | 0.03 ± 0.00ab | 2.16 ± 0.63ab | 2.08 ± 0.79a | 0.85 ± 0.04ab | 100a |
| SPMS 45 | 3.05 ± 0.06a | 4.18 ± 0.19a | 1.14 ± 0.24ab | 0.03 ± 0.05ab | 2.87 ±0.09ab | 2.57± 0.44ab | 0.91 ± 0.09ab | 100a |
Means with the same superscripts in the same column are not significantly different (P>0.05).RPPSM = Raw Pigeon peas seed meal; SPPSM = soaked Pigeon peas seed meal .PPSM = Pigeon peas seed meal.ADG =Average Daily Gain, FCR = Feed Conversion Ratio, SGR =Specific Growth Rate
Table 4: Growth performance and nutrient utilization of Nile tilapia (Oreochromis niloticus) fingerlings fed diet containing PPSM (mean ± SD, n=3).
Decreased significantly with increased dietary of Raw Pigeon pea Seed Meal diets while the reverse was true for Soaked Pigeon peas Seed Meal diets.
Declined in feed utilization in terms of feed in intake for fish fed Raw Pigeon peas diets was perhaps caused by poor palatability of raw Pigeon peas which eventually affect the growth performance of the fish. Raw Pigeon peas thought to contain Poly-phenols ant nutritional factors (tannins and phenol), which are responsible for poor palatability of raw Pigeon peas. Poor palatability accountable for feed rejection and or poor feed intake. Bureau et al., madalla [3,16] explained that Poor feed intake led to starvation of Chinook salmon fed diets reached in ant nutritional.
Afuang et al. and Dongmeza et al. [17,18] reported poor intake in Nile tilapia (Oreochromis niloticus) fed diets containing tannin while Becker and Makkar [19] reported similar phenomenon, which has occurred in common, carps.
Poor Performance in terms of growth and feed conversion ratio for fish fed diets containing raw Pigeon peas could also be attributed by presence. Protease inhibitors such as trypsin and chymotrypsin inhibitors. Faris and Singh [20] reported that these ant nutritional factors have ability to inhibit the activity of protein digestive enzymes within the gastro intestinal tract by binding to and precipitating these protein digestive enzymes thereby causing digestive losses. Again Antnutritional factors have been reported to have deleterious effects and can interfere with food utilization, health and production of catfish fed diets containing raw Pigeon peas [21]. Liener [22,23] recorded that protease inhibitors including trypsin are potential anti-nutrients and are known to decrease growth performance
Fish fed SPPSM 45 diets recorded high growth and feed intake next to those fed the control diet. But significantly different from those fed SPPSM 30 and SPPSM 15 diets. That is to say that fish fed diets containing soaked PPSM conferred better growth and feed utilization (feed intake) than those fed diets containing RPPSM.
Conclusion
Based on the results obtained from dietary composition of Pigeon peas, processing of Pigeon peas in cold water (1:3w/v) for 24 hours under room temperature has no significant influence on the nutritive value of Pigeon peas. However slightly reduction in dry matter, crude lipid and ash content has occurred.
The results of this study show that processed Pigeon peas seed can be included in Nile tilapia diets at 45% level without harmful effect in terms of growth performance and feed utilization of Nile tilapia fingerlings. It is therefore concluded that whenever the cost of Pigeon peas is lesser than fish meal, fish farmers can use soaked Pigeon peas to replace up to 45% fish meal in Nile tilapia diets as a way of reducing the current demand pressure on fish meal.
Acknowledgements
We wish to acknowledge the Department of Animal science and production for generously providing experimental fish for this study.
We are also grateful to Mr. Mfui; Alute and Haji for their assistance they offered during the laboratory operations. We are thankful to Sharon (Editorial Assistant) and his panel for assistance, manuscript reviewing and preparation.
References
- FAO (2009).
- Edwards P (1997) Sustainable food production through aquaculture. Aquaculture Asia 2: 4-7.
- Madalla N (2008) Novel Feed Ingredients for Nile Tilapia (Oreochromisniloticus L.) Institute of Aquaculture University of Stirling Scotland United Kingdom.
- Tibbets J (2001) Satisfying the global appetite. Environmental Health Perspectives 109: A318-A323.
- El-Sayed AFM, Tacon AGJ (1997) Fishmeal replacers for tilapia: A review. Cahiers Options Mediterranées 22: 205-224.
- El-Sayed AFM (1999) Alternative dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture 179:149-168.
- AOAC (1990) Official Methods of Analysis of the Association of Official Analytical Chemists. Helrich, K., (Edn. 15), Arlington, VA, USA.
- Vidal-Valverde C, Frías J, Valverde S (1993) Changes in the carbohydrate composition of legumes after Soaking and cooking. J Am Diet Assoc 93: 547-550.
- Kadam SS, Salunkhe DK (1985) Nutritional composition, processing, and utilization of horse gram and moth bean. Crit Rev Food Sci Nut 22: 1-26.
- Akingbade AA, Sodeind FG, Olaniyi CO, Oyetayo TS, Fadare OR, et al. (2009) Proximate and Mineral Elements Composition of Water Soaked Carnavaliaensiformis Seeds. Pakistan Journal of Nutrition 8: 1401-1403.
- Emenalom OO, Udedibie ABI (2005) Evaluation of different heat processing methods on the nutritive value of Mucunapruriens (Velvet Bean) seed meals for broilers. Int J PoultSci 4: 543-548.
- Sopade PA, Ajisegiri ES, Badau MH (1992) Use of Peleg's equation to model water absorption in some cereal grains during soaking. Journal of Food Engineering 15: 269-283.
- Ravindran V, Ravindran G (1988) Nutritional and anti-nutritional characteristics of Mucunaseeds. J Sci of Food Agric 1: 71-79.
- Nwokolo E (1987) Nutritional evaluation of Pigeon peas meal. Plants Food for Human Nutrition 37: 283-290.
- Amaefule KU, Iheukwumere FC, Nwaokoro CC (2005) A note on the growth performance and carcass characteristics of rabbits fed graded dietary levels of boiled Pigeon peas seed (Cajanuscajan L). Livestock Research for Rural Development.
- Bureau DP, Harris AM, Cho CY (1998) The effects of purified alcohol extracts from soy products on feed intake and growth of chinook salmon (Oncorhynchustshawytscha) and rainbow trout (Oncorhynchusmykiss). Aquaculture 161: 27-43.
- Afuang W, Siddhuraju P, Becker K (2003) Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of moringa (Moringaoleifera Lam.) leaves on growth performance and feed utilization in Nile tilapia (OreochromisniloticusL.). Aquaculture Research 34: 1147-1159.
- Dongmeza E, Siddhuraju P, Francis G, Becker K (2006) Effects of dehydrated methanol extracts of moringa ( Moringaoleifera Lam.) leaves and three of its fractions on growth performance and feed nutrient assimilation in Nile tilapia (Oreochromisniloticus (L.)). Aquaculture 261: 407-422.
- Becker K, Makkar HPS (1999) Effects of dietary tannic acid and quebra cho tannin on growth performance and metabolic rates of common carp (Cyprinuscarpio L.).Aquaculture 175: 327-335.
- Faris DG, Singh U (1990) Pigeon peas: nutrition and products. In: Nene YL et al., (eds). The Pigeon peas Patancheru, India.
- Makkar HPS, Blummel M, Borowy NK, Becker K (1993) Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. JSci of FoodAgric 61: 161-165.
- Liener IE (1994) Anti nutritional factors related to proteins and amino acids. In: Hui YH Gorham, JR Murrel KD, Cliver DOS (eds). Food borne Disease Hand book, Marcel Dekker, New York.
- Liener IE (1994) Implications of anti nutritional components in soybean Feeds. Crit Rev Food Sci Nutrition 34:31-67.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 25669
- [From(publication date):
April-2015 - Dec 22, 2024] - Breakdown by view type
- HTML page views : 21061
- PDF downloads : 4608