Research Article Open Access
Effects of Pyriproxyfen on Viability and Increase of Intracellular Lipids in HepG2 Cell Line
Monica Lamberti1*, Antonietta Stellavato2, Anna Pirozzi2, Antonella D’Agostino2, Gianclaudio Panariello1, Nicola Sannolo1 and Chiara Schiraldi21Department of Experimental Section of Hygiene, Occupational Medicine and Forensic Medicine, School of Medicine, Second University of Naples, Via L. De Crecchio 7, 80138 Naples, Italy
2Department of Experimental Section of Biotechnology, Hystology and Molecular Biology, Second University of Naples, Via L. De Crecchio 7, 80138 Naples, Italy
- *Corresponding Author:
- Monica Lamberi
Department of Experimental Medicine
Section of Hygiene, Occupational Medicine and Forensic Medicine
School of Medicine, Second University of Naples, Naples, Italy
Tel: +39 081 566 5901
Fax: +39 081 566 5898
E-mail: monica.lamberti@unina2.it
Received date: November 10, 2014; Accepted date: December 12, 2014; Published date: December 18, 2014
Citation: Lamberti M, Stellavato A, Pirozzi A, Agostino AD, Panariello G, et al. (2014) Effects of Pyriproxyfen on Viability and Increase of Intracellular Lipids in HepG2 Cell Line. Occup Med Health Aff 2:189. doi: 10.4172/2329-6879.1000189
Copyright: © 2014 Lamberti M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Abstract
Introduction: Pyriproxyfen, (2-[1-methyl-2-(4-phenoxyphenoxy) ethoxy] pyridine) (PPF) is an insecticidal used in household, agricultural, and horticultural applications to control many insect species. We tested its hepatic toxicity in hepatoma HepG2 cell line, we also evaluate if PPF could induce nonalcoholic fatty liver disease.
Materials and methods: The hepatoma HepG2 cell line was exposed for 24-48 hrs with serum-free DMEM to the active principles at different concentrations. The cell viability was assessed by measuring reduction of the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). For the evaluation of in vitro steatosis, the cells were rinsed with cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde. Images of cell were captured using an optic microscope and stained lipid droplets were then extracted with isopropanol (60%) for quantification by measuring its absorbance at 510 nm.
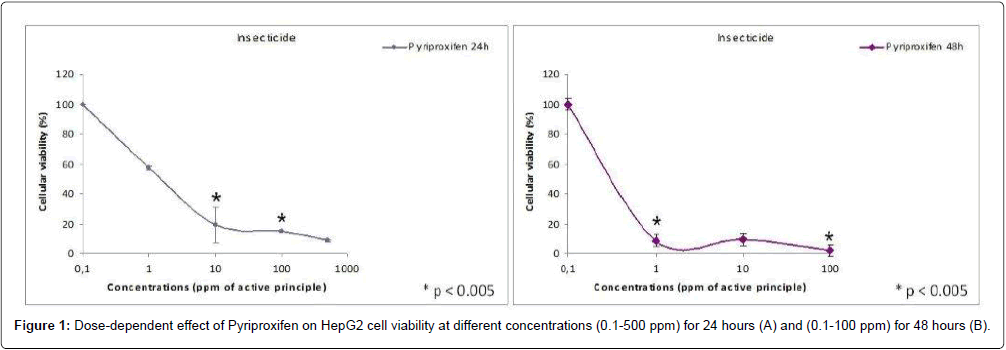
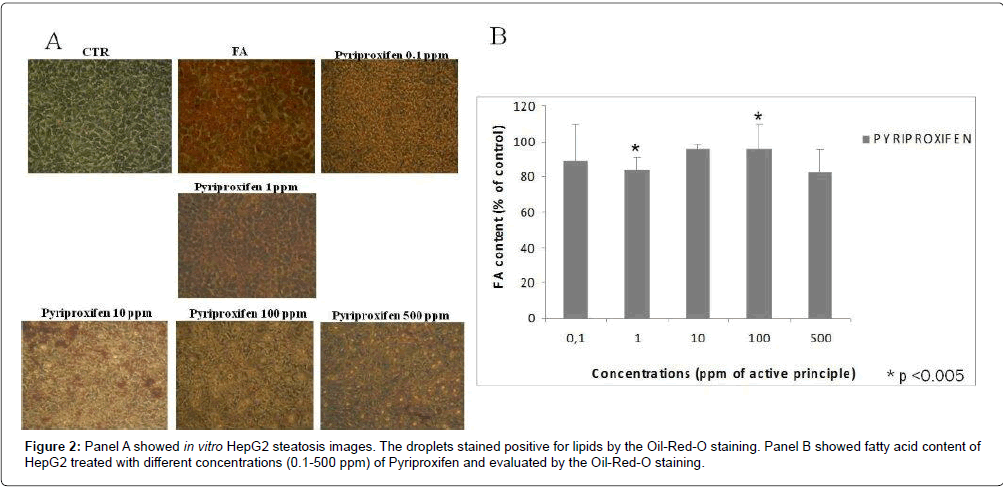
Results: The MTT-test showed that PPF is cytotoxic at all concentrations tested both at 24 h and 48 h. Cell viability is below 50% for concentrations 1-10 ppm while the viability is less than 10% for the concentration 100 ppm. PPF induces the increasing intracellular lipids from 1 ppm concentration. The maximum effect is observed at 100 ppm.
Discussion: In our in vitro study we found a loss of cell viability of about 50% for concentrations from 1-10 ppm by the MTT-Test that measures mitochondrial enzyme activity. Because the mitochondrial enzyme activity affected major changes at the starting/beginning of the apoptotic this condition suggested that PPF is strongly cytotoxic to human hepatocytes in the presented assays. Already at 1 ppm concentration PPF induces the increasing intracellular lipids, in HepG2 in vitro culture.
Keywords
Pyriproxyfen; Agricultural pesticides; HepG2 cell line
Introduction
Agricultural pesticides include those chemicals intended to kill insects, plants, fungi, rodents, and other organisms that interfere with the production, storage, and distribution of agricultural products. Most agricultural pesticides now being used can have detrimental effects on human health. Despite their popularity and extensive use, pesticides are now raising serious concerns about health risks arising from the exposure to farmers when mixing and applying pesticides or working in treated fields and from residues on food and in drinking water for the general population [1].
Several researchers have found associations between pesticide exposure and chronic health effects that include lung cancer, prostate cancer, lymphohemopoietic cancers, pancreatic cancer, and colorectal cancer [2-5]. Other scholars reported a significant association between neurological symptoms and pesticide exposure [6]. Bronchitis, asthma, wheezing, farmer’s lung, and rhinitis have also been reported to be associated with respiratory morbidity especially among farmworkers [7]. In fact, farmworker, pesticide exposure must be considered separately compared to the exposure of the general population because of the extensive exposure which farmers must endure [8,9].
Pyriproxyfen, (2-[1-methyl-2-(4-phenoxyphenoxy) ethoxy] pyridine,) (PPF) is an insecticidal used in household, agricultural, and horticultural applications to control many insect species, including the common housefly (Musca domestica), mosquitos, imported red fire ants (Solenopsis invicta), and silver leaf whitefly (Bemisia argentifolii) [10].
It is used on citrus fruit in Israel, South Africa, Spain and Italy and it has also been considered by WHO for vector control under its Pesticides Evaluation Scheme. PPF is a juvenile hormone analogue (JHA) that interrupts normal development and metamorphosis of targeted mosquitoes [11]. It also alters parthenogenic reproduction in non-target cladoceran species as it induces male production that can lead to a decrease in fecundity, a reduction in population density, and subsequent ecological effects [12]. Highly potent in terms of activity and specificity, PPF degrades rapidly in soil under aerobic conditions, with a half-life of 6.4-36 days and it disappeared from aerobic lake water–sediment systems with half-lives ranging from 16 to 21 days. Pyriproxyfen is also relatively lipophilic as it has an octanol/water partitioning coefficient of Kow 5.6. In short- and long-term studies of the effects of pyriproxyfen in mice, rats and dogs, the liver was assessed as the main toxicological target (increases in liver weight and changes in plasma lipid concentrations, particularly cholesterol) [13].
In this paper, also in consideration of the chemical and physical characteristics of the substance, we tested its hepatic toxicity in hepatoma HepG2 cell line, we also evaluate if PPF can induce nonalcoholic fatty liver disease (NAFLD).
Materials and Methods
Materials
The hepatoma HepG2 cell line was provided by American Type Culture Collection (ATCC HB 8065). Cell viability was performed using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium (MTT-test) (Sigma Aldrich, Milan, Italy). In vitro steatosis was induced by incubating hepatocytes with 6mM of a mixture of oleic (18:1) and linoleic acid (18:2) ratio 1:1. Fat accumulation was determined using Oil red O staining (Sigma Aldrich, Milan Italy).
Methods
Cell culture: The hepatoma HepG2 cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/ml streptomycin and 100 μg/ml antimycotic. All cell culture materials were purchased from Gibco, Invitrogen, Milan Italy. The cells were grown on tissue culture plates (BD Bioscience-Falcon, San Jose, USA), using an incubator with a humidified atmosphere (95% air/5% CO2 v/v) at 37°C during 48 h to 80% confluence, than washed, and exposed for 24-48 hrs with serumfree DMEM to the active principles at different concentrations (1-10- 100-500 ppm). Before treatment, all the pesticides were solubilized in a 100% DMSO solution, then diluted in serum-free medium to reach 0.5% DMSO.
MTT assay: HepG2 (1.0x105) cultured in a 24-well plate in DMEM medium were treated with AP (active principle) for 24-48 hrs at different concentrations. In particular the cells were treated with Pyriproxifen 0.1-1-10-100-500 ppm for 24 hrs and with Pyriproxifen 1-10-100 ppm for 48hrs. The cell viability was assessed by measuring reduction of the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Mitochondrial dehydrogenates of living cells reduce the tetrazolium ring, yielding a blue formazan product which can be measured spectrophotometrically. The optical densities obtained are directly proportional to the number of living cells. The cytotoxic effect of a sample is evaluated by the percentage of living cells present in the sample, in relation to the cells treated only with the solutions [14]. After treatment, the medium was replaced by a solution of MTT 1 mg/ml in DMEM medium without phenol-red. After 3 h of incubation, the liquid was aspirated and the insoluble formazan produced was dissolved in HCl 0.1 M in isopropanol. The optical densities of the obtained solutions were measured at 570 nm using a Beckman DU 640 spectrometer (Beckman, Milan, Italy).
Oil red O staining: For the evaluation of in vitro steatosis, 1.0x105 HepG2 cells were seeded in a 24-well plate and treated for 24 hrs with fat acid. After treatment, the cells were exposed for 24hrs to the pesticides. Than the cells were rinsed with cold phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (Sigma Aldrich, Milan Italy) for 30 min and stained with Oil Red O solution (0.5%) (Sigma Aldrich, Milan Italy). Images of cells were captured using an optic microscope and stained lipid droplets were then extracted with isopropanol (60%) for quantification by measuring its absorbance at 510 nm.
Results
The MTT-test showed that PPF is cytotoxic at all concentrations tested both at 24 hrs and 48 hrs (Figure 1). Cell viability is below 50% for concentrations 1-10 ppm while the viability is less than 10% for the concentration 100ppm and over.
In order to evaluate the levels of in vitro steatosis, Oil red staining was performed. Pyriproxifen induces the increasing intracellular lipids from 1 ppm concentration. The maximum effect is observed at 100 ppm as reported in Figure 2.
Discussion
According to the US Environmental Protection Agency (EPA), approximately five billion pounds of pesticides are used annually worldwide [15] A cross-sectional study of pesticide handling practices among Cambodian farmers suggested that most of the pesticides used in agriculture belonged to the World Health Organization (WHO) class I (extremely and highly dangerous) and class II (moderately dangerous) categories [16]. Therefore, one immediate aim might be to reduce the use of WHO Classes I and II pesticides, and replace these pesticides with less-toxic alternatives, especially in developing countries [17].
Pyriproxyfen is a juvenile hormone mimicking insecticide, used for the control of flies, beetles, midges and mosquitoes that inhibit larval development and maturation as a juvenile hormone III mimic.
The World Health Organization has classified pyriproxyfen as “product that improbably can cause acute risk in normal use” [18]. It is a solid (melting range 48-50°C) of low volatility and only slightly soluble in water. It has no discernible acidic or basic characteristics and is stable to hydrolysis at pH 4-9 at 25°C, but it is prone to slow photolysis.
The toxicity of pyriproxyfen was evaluated, for the first time, in the study WHO/FAO Joint Meeting on Pesticdes Residues [18] that showed low acute oral toxicity of pyriproxyfen, with LD50 values >5000 mg/kg body weight of mice, rats and dogs. The daily dose absorbed (ADI) by man was evaluated between 0 and 0.1 mg of active substance per kg of body weight. This assessment is derived from the determination of a value of NOAEL of 10 mg/kg of body weight per day, in one study with the duration of a year, performed on dogs, this value has been applied a safety factor of 100. The pyriproxyfen in these concentrations has been shown to be neither genotoxic nor carcinogenic.
Although Chang et al. [19] showed that the population of microbial community decreased rapidly after incubation with 10 mg Kg (-1) of pyriproxyfen for 91 days, indicating the toxicity of pyriproxyfen toward bacterial communities in a closed soil ecosystem [19].
Koyama et al. showed a decrease in body rats weight exposed at 10,000 ppm of PPF [20]. In blood tests they found an increase in bilirubin, GOT, triglyceride and total cholesterol. In particular, they showed that the weight of rat liver increased with an exposition of only 2,000 ppm with a higher incidence of blackish brown coloration of the liver [20]. This study aimed to the evaluation of the hepatotoxic effect of PPF on HepG2 cell line.
In our in vitro study we found a loss of cell viability of about 50% for concentrations 1-10 ppm by the MTT-Test that measures mitochondrial enzyme activity. Because the mitochondrial enzyme activity affected major changes at the starting/beginning of the apoptotic process [21], this condition suggested that pyriproxifen is strongly cytotoxic to human hepatocytes in the presented assays.
The outcome of this experimental research showed that PPF induces the increasing intracellular lipids, in HepG2 in vitro culture, already at 1 ppm concentration. Pesticides cause the impairment or inhibition of enzyme responsible for oxidation and synthesis of fatty acids or the receptor molecules controlling these enzymes. The result is the lipid accumulation in the cells [22]. This finding may correlate in vivo with increased liver damage toward the possibility to induce nonalcoholic fatty liver disease (NAFLD). The latter, is characterized by fat accumulation in the liver that start with simple hepatic steatosis and may progress toward inflammation (nonalcoholic steatohepatitis [NASH]) with progressive fibrosis and liver damage and is the most common reason for abnormal level (expression) of hepatic enzymes worldwide. Pyriproxifen may alter the hepatic function and thus can influence a xenobiotic response in the cells. We observed moderate steatosis in the hepatocytes when the cells are treated with 10 and 100 ppm of Pyriproxifen. Microscopic image analyses relative to HepG2 treated at different concentrations of pyriproxifen revealed accumulation of small lipid droplets after 24 hrs of treatment indicating that the doses (1 and 10 ppm) of active principle caused steatosis or abnormal accumulation of lipids.
The morphologic analysis obtained by inverted microscope images capture, confirmed by quantification spectrophotometrically at 510 nm of the vesicles stained positive for lipids (Oil Red O) shows a significant increase in triacylglyceride in the 100 ppm exposed hepatocytes compared to the control.
Conclusion
The current in vitro study indicates that pyriproxifen causes cytotoxicity and steatosis at the concentrations evaluated. Workers in the agricultural sector, or people involved in gardening or disinfestations may inhalate this active principle and even at low doses this may increase the severity of liver disese or even induce the liver cell damage and steatosis in healthy people.
For these reasons pesticide management and regulations, educational programs on safety precautions, reinforcement of safety behaviors, especially the proper use of personal protection equipment (PPE) in the workplace, are effective approaches for preventing diseases related to occupational pesticide exposures regardless of pesticides risk class of which we do not know fully the possible toxicity.
Acknowledgements
We would like to sincerely thank Dr Francesca De Novellis for her technical support.
References
- Damalas CA,Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators.Int J Environ Res Public Health 8: 1402-1419.
- Alavanja MC, Hofmann JN, Lynch CF, Hines CJ, Barry KH, et al. (2014) Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PLoS 9:e109332.
- Lemarchand C,Tual S,Levêque-Morlais N, Perrier S,Guizard AV, et al. (2014) 0206 Prostate cancer risk among French farmers in the AGRICAN cohort.Occup Environ Med 71 Suppl 1: A86-87.
- Lerro C,Koutros S, Andreotti G, Hines C, Lubin J, et al. (2014) 0301 Use of acetochlor and cancer incidence in the Agricultural Health Study Cohort.Occup Environ Med 71 Suppl 1: A104.
- Salem EA,Hegazy MM, El Khouley EA (2014) Pesticide exposure as a risk factor for lymphoproliferative disorders in adults.East Mediterr Health J 20: 363-371.
- Elbaz A,Clavel J, Rathouz PJ, Moisan F, Galanaud JP, et al. (2009) Professional exposure to pesticides and Parkinson disease.Ann Neurol 66: 494-504.
- Ye M, Beach J, Martin JW, Senthilselvan A (2013) Occupational pesticide exposures and respiratory health.Int J Environ Res Public Health 10: 6442-6471.
- LevesqueDL, ArifAA, ShenJ(2012) Association between Workplace and Housing Conditions and Use of Pesticide Safety Practices and Personal Protective Equipment among North Carolina Farmworkers in 2010.Int J Occup Environ Med 3: 53-67.
- Basilicata P,Simonelli A, Silvestre A, Lamberti M, Pedata P, et al. (2013) Evaluation by environmental monitoring of pesticide absorption in farm workers of 18 Italian tomato cultivations.Int J ImmunopatholPharmacol 26: 517-523.
- Bouyer J,Lefrançois T (2014) Boosting the sterile insect technique to control mosquitoes.Trends Parasitol 30: 271-273.
- Mbare O, Lindsay LS, Fillinger U (2014) Pyriproxyfen for mosquito control: female sterilization or horizontal transfer to oviposition substrates by Anopheles gambiaesensustricto and Culexquinquefasciatus. Parasites & Vectors 7:280.
- Ginjupalli GK, Baldwin WS (2013) The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction.Chemosphere 92: 1260-1266.
- World Health Organization (2008) Guidelines for Drinking-Water Quality. 3rd edition, including first and second addenda 1: 431-432.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays.J Immunol Methods 65: 55-63.
- http://www.epa.gov/pesticides/pestsales/07pestsales/table_of_contents2007.htm
- Jensen HK,Konradsen F, Jørs E, Petersen JH, Dalsgaard A (2011) Pesticide Use and Self-Reported Symptoms of Acute Pesticide Poisoning among Aquatic Farmers in Phnom Penh, Cambodia.J Toxicol 2011: 639814.
- Konradsen F, van der Hoek W, Cole DC, Hutchinson G, Daisley H, et al. (2003) Reducing acute poisoning in developing countries--options for restricting the availability of pesticides.Toxicology 192: 249-261.
- WHO/FAO (1999) Joint Meeting on Pesticdes Residues 1:188-200.
- Chang CS, Yen JH, Chen WC, Wang YS (2012) Soil dissipation of juvenile hormone analog insecticide pyriproxyfen and its effect on the bacterial community.J Environ Sci Health B 47: 13-21.
- Koyama Y, Kimura J, Yoshioka K, Watanabe T, Seki T, et al. (1989) [A six-month chronic dietary toxicity study of pyriproxyfen in rats].J ToxicolSci 14: 43-64.
- Kannan K, Jain SK (2000) Oxidative stress and apoptosis.Pathophysiology 7: 153-163.
- Krøvel AV,Søfteland L, Torstensen BE, Olsvik PA (2010) Endosulfan in vitro toxicity in Atlantic salmon hepatocytes obtained from fish fed either fish oil or vegetable oil.Comp BiochemPhysiol C ToxicolPharmacol 151: 175-186.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 14982
- [From(publication date):
December-2014 - Apr 29, 2025] - Breakdown by view type
- HTML page views : 10300
- PDF downloads : 4682