Effects of Pre-Sowing Treatments in Seed Germination and Early Seedling Growth of Grevillea robusta in Bako Agricultural Research Center Nursery Site, Western Oromia, Ethiopia
Received: 02-Oct-2023 / Manuscript No. jescc-23-115340 / Editor assigned: 04-Oct-2023 / PreQC No. jescc-23-115340 (PQ) / Reviewed: 18-Oct-2023 / QC No. jescc-23-115340 / Revised: 23-Sep-2023 / Manuscript No. jescc-23-115340 (R) / Accepted Date: 30-Oct-2023 / Published Date: 30-Oct-2023
Abstract
Determining pre-sowing treatments at the seedling stage is critical to effectively managing seed germination and growth. This study investigated the effects of pre-sowing treatments on Grevillea seed germination and early seedling growth. Grevillea robusta seed germination and seedling growth performance under different water soaking treatments were evaluated for three months under nursery and laboratory conditions. A total of 250 seeds were pre-treated with cold water and soaked for 12, 24, 36, 48, and 72 hours, respectively, while the control was not soaked. Each treatment used a total of 25 seeds. Sow the seeds directly into polyethylene bags filled with a homogeneous growing medium (top forest soil, sand, and fertilizer) mixed in the recommended proportions to avoid disturbing the root system after germination. Seeds were sown using a randomized complete block design (RCBD), with 10 treatments and 3 replicates. The growth parameters evaluated were root collar diameter (cm), seedling height (cm), root depth (cm), survival rate (%), germination presentation, and seed germination rate. The results showed that there were significant differences (p < 0.05) in the seedling growth parameters (root collar diameter, root depth, and seedling height) of different pre-planting treatments. The findings indicated that Grevillea seeds immersed in cold water (T3) had a slightly better survival rate (92%), whereas under T9, root collar diameter (0.44 cm), seedling height (31.05 cm), and root depth (25.62 cm) all showed slightly greater growth performances. Therefore, cold water soaking (T9) is the best pre- sowing treatment when compared to other seed-enhancing pre-treatments for promoting uniform and optimal seed germination and early seedling growth. Seeds sown without any treatment (control) were found to have the poorest growth performance among all parameters studied. The present study showed that seed germination percentage, germination time, and seedling vigor were affected by the cold water soaking of seeds. This indicates that pre-sowing treatments significantly affected seed germination and seedling growth.
Keywords
Grevillea robusta; Germination, Growth parameter; Presowing treatment
Introduction
The largest species in the genus Grevillea in the family Proteaceae is Grevillea robusta, often known as southern silky oak, silk oak, or Australian silver oak (Resch, 1998). It is native to the east coast of Australia and lives in river, subtropical, and dry tropical rainforest environments with an average annual rainfall of more than 1000 mm (Bowman, 2000). Population growth and rapid decline in forest cover, poor increments, low sustainable yields, and increasing demand have resulted in timber and fuel-wood shortages in the country (Lal, 2010). To meet the growing demand for wood, the timber industry is promoting short-term rotations of fast-growing tree species such as Grevillea robusta. Grevillea robusta has been introduced to tropical and subtropical plateaus and warm temperate regions around the world since the mid- to late-19th century (Ciesla, 2001). Grevillea robusta is a fast-growing tree with multiple uses, providing a variety of goods and services, including building materials, power transmission poles, timber, fuel wood, shade, windbreaks, fodder, and soil fertility improvement (Kimaro and Liingilie, 2015; Kuyah et al., 2020; Nyambati and Kioko, 2018) [1].
Many natural fast-growing tropical tree species contain hard seeds that have low/poor germination rates and show seed dormancy (Bezkorowajnyi, 2001). An essential method of woody plant propagation for mass manufacturing is seed germination (Guan et al., 2016). Being able to quickly acquire viable, evenly sized seedlings is one technique to boost the production of healthy seedlings in orchards and plantations. However, because seeds take so long to germinate, this is typically a difficult operation. Especially in leguminous species,a stiff seed coat that inhibits water absorption is the cause of slow germination (Barton, 1965). Large-scale Grevillea robusta plantations are being cultivated in Ethiopia's farmlands, degraded forest lands, roads, railways, community lands and canal belts (Othieno et al., 2016). These plantations produced a lot of wood, telephone poles, and firewood that was extremely beneficial (Kuria et al., 2014). However, the majority of the early plantations had very low production. Therefore, forest nurseries are essential to the success of any planting and restoration initiative. The most fundamental and basic method of plant regeneration, preservation, and proliferation is through the use of seeds (Benson, 2000). Most species' life cycles begin with the seed phase, which is the most crucial for survival, dormancy, and germination (Penfield, 2017). However, for this to happen, seeds must germinate in an environment that promotes the growth of seedlings and future plants. It needs technical expertise to grow quality seedlings, including careful planning of quality seed selection, containers, and required (Kettenring and Tarsa, 2020; Kildisheva et al., 2020). However, during this period various dormancy mechanisms inhibit germination, so the seeds need to be treated. Some species have very poor germination rates due to seed coat dormancy (Kheloufi et al., 2018). The reason for its dormancy is its permeability to water. The seeds are treated to ensure they germinate quickly and evenly. In order to promote the rapid and uniform germination of seeds sown in the nursery, pre-sowing treatment is carried out (Krishnan et al., 2014; Wang et al., 2016) [2].
As Grevillea robusta becomes more important, its systematic cultivation becomes more important for productivity. However, little information is available on the scientific management of this species. Pre-sowing treatments may affect the germination period and germination rate of different tree seed species (Azad et al., 2013). Seed germination rates and germination processes have been reported based on numerous studies on different species of some tropical forest trees (Fischer et al., 2016; Kennedy and Swaine, 1992; Maass and Burgos, 2011). Pre-sowing seed treatments directly impact tree growth and the success of planting activities. Sometimes, healthy seeds will not germinate even with the right temperature, light, and adequate moisture (Tamado et al., 2002). Seed dormancy is the failure of developed seeds to germinate under the right conditions (Bradbeer, 2013). The main problem encountered in the production of afforestation seedlings in arid and semi-arid areas is poor seed germination due to hard seed coats (Bradbeer, 2013). The Grevillea robusta seed dormancy is mainly due to the hard seed coat, which affects seed germination. In order to break dormancy and obtain rapid germination, the seeds of the tree species must undergo some physical treatment if they are to be successfully incorporated into a tree planting program. Pre-sowing treatments of seeds break down the resistant seed coat and allow the embryos to absorb moisture (Veiga-Barbosa et al., 2016) [3].
Therefore, pre-germination seed treatment is one of the most important factors for successful seedling production and is responsible for higher growth performance of plantations. Reforestation and afforestation programs that incorporate these species into their planting plans are expected to receive an abundance of planting material from nursery operators. There is insufficient information on pre-sowing treatments for seed germination and early seedling growth of most tree species in Ethiopia. Unless we master the details of seed germination, there can be no viable saplings. This study evaluated the effects of different pre-sowing treatments on Grevillea germination and growth during nursery and laboratory cultivation. The main purpose of this study was to investigate the effects of nursery and laboratory pre-sowing treatments on grevillea seed germination and early seedling growth [4].
Materials and Methods
Description of the study area
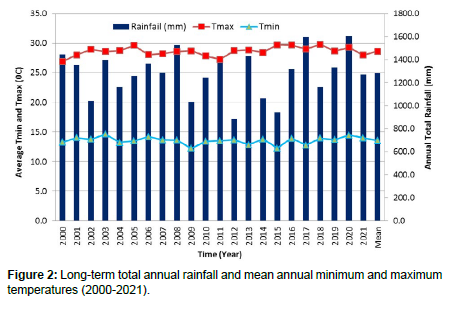
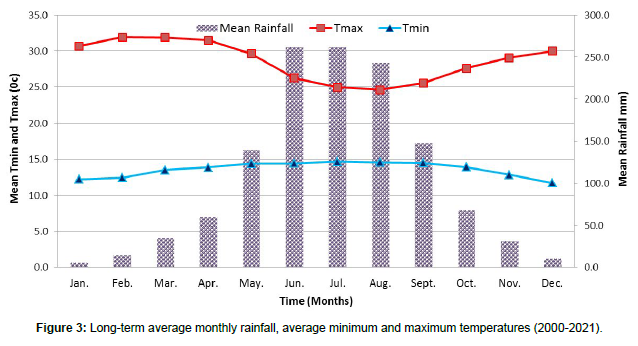
The trial was conducted for a year at the nursery and laboratory of the Bako Agricultural Research Center. The center is located in western Ethiopia, 906’N, 3709’E, 250 km from Addis Ababa and 1,650 m above sea level (Figure 1). The climate is warm and humid, with the annual average minimum temperature, maximum temperature and average temperature from 2000 to 2021 being 13.6, 28.6 and 21.1oC, respectively. The annual rainfall in this area is 1279.6 mm, with the highest rainfall from June to August (meteorological data records of Bako Agricultural Research Center) (Figures 2 and 3). Soils in this region are typically reddish-brown in color and have a pH that ranges from slightly high to acidic [5].
Experimental design and treatments
The research work includes laboratory testing and nursery activities. The study took place over a twelve-week period at the nursery and laboratory of the Bako Agricultural Research Centre. Seeds of Grevillea robusta were collected around the study area. Place collected fresh seeds into perforated plastic bags and exclude, extract and clean seeds that are insect-infested, physically damaged or rotten. Seeds were soaked manually and dried in the shade to study the effects of different pretreatments on germination parameters. Pretreat Grevillea seeds with cold water at different intervals. The pots are made from a mix of local soil, forest soil, sand and animal manure. Sow seeds of Grevillea robusta in polyethylene pots that match the nursery recommended pot size (Figure 4). Three replicates of 250 seeds were taken from each treatment, with 25 seeds in each replicate, and germination experiments were conducted under nursery conditions. The experiment was conducted using a randomized complete block design (RCBD) with ten (10) treatments and each block was randomly assigned to a treatment. Different cold water seed soaking times (12, 24, 36, 48, 72 hours and control (without any treatment)) were used to evaluate the effect of pre-sowing treatments [6-10 ].
Each replicate of seeds were subjected to the following treatments: (T1) - Control (seeds sown without any treatment), (T2) - Soaked in cold water for 12 hours, (T3) - Soaked in cold water for 24 hours, (T4) - Soaked in cold water for 36 hours, (T5) - Soaked in cold water for 48 hours, (T6) - Soaked in cold water for 72 hours, (T7) - Soaked in cold water for 12 hours + dried + again soaked in cold water for 12 hours, (T8) - Soaked in cold water for 24 hours + dried + again soaked in cold water for 24 hours, (T9) - Soaked in cold water for 36 hours + dried + again soaked in cold water for 36 hours, (T10) - Soaked in cold water for 48 hours +dried + again soaked in cold water for 48 hours.
The experiment was conducted in the off-season, with watering every morning and evening. Seed germination and seedling growth parameters were collected to evaluate their impact on seed germination and early seedling growth in nursery and laboratory [11].
Growth parameters including root collar diameter, height, root depth, and survival were evaluated at the nursery stage and destructive sampling method has been used to assess the effects of pre-sowing treatments. The middle seedlings (10 plants per block) were used as measurement samples to minimize boundary effects, while survival counts were performed on the entire seedlings within the block. Use a caliper to measure the root collar diameter, and use a tape measure to measure the root neck height and root depth, as shown in Figure 5 [12-17].
Germination test at laboratory
Finally, the investigation also describes germination of seeds in the laboratory shortly after field experiments. Untreated Grevillea seeds were soaked in cold water for 12, 24, 36, 48, and 72 h and then sown on laboratory Petri dishes (Figure 6). If water vapor is removed from the Petri dish, add distilled water to the Petri dish. Seeds were considered to have germinated when the radicle penetrated the seed coat, and germinated seeds were removed from the Petri dish to avoid double counting of seeds. Continue daily germination counts until no more seeds germinate [18].
Seed germination and seedling growth performance assessment
Nursery data on growth performance of seedlings were recorded at the age of three months. These parameters include root collar diameter (cm), seedling height (cm), seedling root depth (cm) and seedling survival rate (%). So, use a ruler to measure the stem height in centimeters (cm) from the soil surface to the tip of the Grevillea main axis, where new shoots appear. Use a caliper to measure the root collar diameter in millimeters (mm) near the soil surface. In addition, detailed practical observations, photographs, and interpretations are performed regularly, thereby documenting the various morphologies and anatomy of the seedlings. The effects of pre-sowing treatments on seed germination and seedling growth are regularly found by counting germinated seeds and assessing the initial growth performance of the seedlings. Cumulative germination rates were recorded every other day of sowing and continued until germination was complete. All growth parameters were collected to evaluate the growth performance of Grevillea robusta. Seedling vigor index (SVI) was calculated according to (Abdul-baki and Anderson, 1973) as the germination percentage multiplied by the total seedling length (i.e., the sum of shoot and root lengths). Ten (10) seedlings from each replicate were randomly uprooted and root lengths were measured. The seedling vigor index (SVI) was calculated according to (Abdul-Baki and Anderson, 1973) as the percentage of germination multiplied by the total seedling length (i.e. the sum of shoot and root lengths).

Based on the daily number of germination, calculate the germination percentage and germination rate through the formula given below.

Statistical data analysis
Data are presented in the form of percentages, figures and tables. To compare the effects of pre-seeding treatments, statistical analysis of variance (ANOVA) and general linear models (GLM) were performed to determine whether there were any statistical differences between treatments. SAS9.3 was used to conduct statistical tests on the early survival rate and growth performance parameters of seedlings. Mean values were separated using the least significant difference (LSD) test. Statistically significant differences between treatments with p < 0.05 confidence intervals were determined [19].
Results
Climatic condition during the experimental period
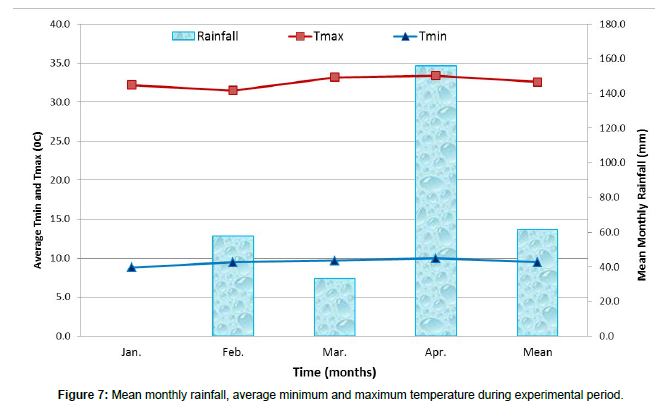
During the experiment period, the temperature in this area was higher and the rainfall was the least. The area received average monthly rainfall; minimum and maximum temperatures were 61.7 mm, 9.5°C and 32.6°C respectively (Figure 7). The highest rainfall of 155.8 mm was recorded in April (end of the trial period) and the lowest rainfall was recorded in January (beginning of the trial period). Rainfall increased slightly at the end of the experiment [20].
Growth parameters
Results of this study on seed germination and early seedling growth showed significant changes (p<0.05) among pre-sowing treatments. The summary results of germination rate, root collar diameter, root depth, height and survival rate of Grevillea robusta at seedling stage are shown in Table 1.
| SV | Root collar diameter | Height | Root depth | Survival rate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | Pr > F | MS | F | Pr > F | MS | F | Pr > F | MS | F | Pr > F | |
| TRT | 9 | 0.016 | 11.63 ** | < 0.0006 | 171.99 | 11.26 ** | < 0.0007 | 58.98 | 9.02 ** | 0.0019 | 18.53 | 2.06ns | 0.8089 |
| REP | 2 | 0.002 | 1.69 ns | 0.1651 | 29.3 | 1.92ns | 0.1148 | 7.89 | 1.21ns | 0.2539 | 56.42 | 0.6505 ns | 0.7388 |
| ERROR | 18 | 0.001 | 15.26 | 6.54 | 86.38 | ||||||||
| Total | 29 | ||||||||||||
| *Significantly different at 5% probability; ns: not significantly different at 5%; SV: Source of variation; MS: Mean square; F: F value | |||||||||||||
Table 1: Summary of variance analysis of Grevillea seedlings under different seed pretreatments.
Root collar diameter (RCD)
Statistical analysis showed that there were significant differences in RCD between different pre-sowing treatments (p<0.05). The largest RCD (0.443 and 0.433 cm) were recorded for treatment 7 (soaked in cold water for 12 h and again dry soaked for 12 h) and treatment 5 (soaked in cold water for 48 h). Nursery field data showed that the minimum and maximum values RCD between different pre-sowing treatments were 0.37 and 0.443 cm, respectively. The results of this study indicate that soaking Grevillea in cold water for 12 and 48 hours breaks the seed coat, thereby promoting germination. Therefore, soaking Grevillea in cold water for 12 hours before sowing (drying and then soaking for 12 hours) or soaking in cold water for 48 hours can ensure the normal growth of the RCD of the seedlings (Table 2) [21].
| Treatments | RCD | Ht | RD | SR |
|---|---|---|---|---|
| TI | 0.383 ± 0.049abc | 23.187 ± 2.794bc | 21.5131.272ab | 77.33 ± 10.07a |
| T2 | 0.377 ± 0.067bc | 21.547 ± 5.306c | 21.913 3.414ab | 88.00 10.58 a |
| T3 | 0.420 ± 0.010abc | 28.080 ± 2.669abc | 23.547 ± 4.790ab | 92.00 ± 6.93 a |
| T4 | 0.403 ± 0.064abc | 24.753 ± 7.804abc | 24.600 ± 2.208a | 82.67 ± 10.07 a |
| T5 | 0.433 ± 0.029abc | 28.987 ± 4.534ab | 23.837 ± 2.973ab | 86.67 ± 6.11 a |
| T6 | 0.370 ± 0.046c | 23.720 ± 5.304bc | 23.193 ± 2.030ab | 82.67 ± 6.11 a |
| T7 | 0.443 ± 0.021a | 29.867 ± 1.358ab | 23.333 ± 4.956ab | 80.67 ± 7.02 a |
| T8 | 0.377 ± 0.071bc | 25.433 ± 9.241abc | 19.920 3.900b | 86.67 ± 11.55 a |
| T9 | 0.440 ± 0.026ab | 31.047 ± 4.083ª | 25.620=3.441a | 80.00 10.58 a |
| T10 | 0.417 ± 0.095abc | 26.0477.267abc | 23.593=3.493ab | 84.00 18.00a |
| CV | 13.3 | 21.2 | 14.9 | 10.6 |
| LSD ( 0.05 ) | 0.092 | 9.474 | 5.848 | 15.2 |
| T1,T2…: Treatments; LSD (0.05): Least Significant Different at P < 5%; CV: Coefficient Variance | ||||
Table 2: Average growth parameters of silver birch seedlings under different cold water treatments.
Seedling height (cm)
The effect of pre-sowing treatments on the average height of Grevillea seedlings was significant (p < 0.05) (Table 1). Compared with normal germination (control), there was no significant difference in the plant height of T3, T4, T8, and T10 soaked seeds. The results of the determination of the height of the Grevillea seedlings indicate that seed soaking is a suitable pre-sowing treatment for the growth of Grevillea seedlings. It was observed that the highest heights (31.04, 29.87 and 28.99 cm) were recorded after the seeds of Grevillea were soaked in cold water for 36 hours, dried and soaked again for 36 hours; soaked in cold water for 12 hours and dried again, soaked for 12 hours and soaked in cold water for 48 hours, respectively [22].
Seedlings root depth (cm)
Statistical analysis shows that there was a significant difference in root depth among different pre-sowing treatment (p < 0.05) (Table 1). The highest root depth (25.62 cm) was recorded in Grevillea robusta seeds soaked in cold water for 36 hours and dried again soaked for 36 hours (T9). Untreated seeds and T8 had comparable values (19.9-21.5 cm) which were lower than all soaking in cold water [23 ].
Seedlings survival rate (%)
The highest survival rate was observed in T3 (92.00 ± 6.93), followed by T2 (88.00 ± 10.58), but the difference between treatments was not significant (Table 1). Thus, water treatment did not have a significant effect on the survival rate of Grevillea seeds. Seedling survival rates recorded at the end of the experiment for different pre-sowing treatments ranged from 77% to 92%. Generally speaking, among different pre-sowing treatments, seeds pretreated with T9 (seeds soaked in cold water for 36 hours and then soaked in dry water for 36 hours) germinate quickly (2 to 8 days), have high collar diameter (0.440 cm), and plant height (31.05 cm), root length (25.620 cm) and higher germination rate (97%) and survival rate (80%) under laboratory conditions). The next best pre-sowing treatments are T7 (soak in cold water for 12 hours, then dry soak for 24 hours), collar diameter (0.443 cm), plant height (29.87 cm), root length (23.33 cm) and T5 (soak in cold water for 48 hours), high collar diameter (0.433 cm), plant height (28.99 cm), root length (23.84 cm) and survival rate under nursery conditions (86.67%). The profitability of a nursery depends largely on the germination, growth and survival of seeds in the nursery until planting (Sohel et al., 2023). Studies on pre-germination treatments of tree seeds conducted by many researchers have shown that pre-sowing treatments can significantly increase seed germination rates (Amoakoh et al., 2017; Azad et al., 2013; TUHETERU et al., 2022) [24 ].
Start of germination day, end of germination day, and seedling vigor index
Table 3presents summary results of germination percentage, germination start day, germination end day, and seedling vigor index (SVI) for Grevillea under nursery conditions. Pretreatment of seeds has a significant impact on days to germination and germination rate of Grevillea robusta seeds. The first germination was recorded at 2 day after sowing from the seeds soaked in cold water under T2 T3, T4, and T9. Grevillea robusta soaked in cold water (T2 T3, T4, and T9) significantly (p < 0.05) increased the germination as compared to the control (seeds sown without any treatment). Compared with other treatments, germination ended later in the control and treatment (T2 T3, T4, and T5) [25].
| Treatments | SDG | EDG | SVI | Germination Rate |
|---|---|---|---|---|
| ΤΙ | 5.000 1.732a | 18.667 ± 3.512a | 38.633 ± 4.023abc | 2.173 ± 0.564c |
| T2 | 2.000 1.000b | 18.000 4.583a | 37.744 ± 2.977bc | 2.980 ± 0.464abc |
| T3 | 2.000 1.000b | 19.667 ± 5.859a | 47.327 ± 1.066b | 3.155 ± 0.636abc |
| T4 | 2.000 1.000b | 19.000 ± 6.083a | 40.622 ± 6.833abc | 3.386 ± 0.478ab |
| T5 | 3.333 ± 0.577ab | 21.000 ± 5.568a | 45.756 ± 3.162ab | 3.287 ± 0.470abc |
| T6 | 4.333 ± 0.577ab | 15.667 ± 2.309a | 38.931 ± 6.647abc | 2.559 ± 0.181bc |
| T7 | 3.000 0.000ab | 14.333 ± 3.512a | 42.717 ± 3.795abc | 2.844 ± 0.608bc |
| T8 | 4.333 3.512ab | 17.333 ± 8.083a | 35.994 ± 14.291c | 2.719 1.436bc |
| T9 | 2.000 1.000b | 17.000 4.359a | 45.079 ± 2.944abc | 3.242 ± 0.363abc |
| T10 | 3.667 ± 0.577ab | 14.333 ± 2.082a | 41.841 ± 10.274abc | 4.042 ± 0.943a |
| CV | 9.24 | 14.87 | 11.06 | 11.05 |
| LSD ( 0.05 ) | 2.5 | 8.7 | 9.5 | 1.2 |
| SDG: Start of germination day; EDG: End of germination day; SVI: seedling vigor index | ||||
Table 3: Effect of seed pre-sowing treatment on mean germination parameters of Grevillea robusta at nursery site.
The results showed that there was no significant difference in the germination cut-off date of each treatment (P > 0.05), but the germination start date, seedling vigor index and germination rate were significantly different (P < 0.05) affected by the pre-sowing method (Table 3). The shortest germination times are T7 (3-14 days) and T10 (4-14 days), and the longest germination times are control (5-19 days), T2 (2-18 days), and T3 (2-20 days). , T4 (2-19 days) and T5 (3-21 days) under nursery conditions. In terms of seedling vigor index (SVI), under nursery conditions, T3 (47.33) was significantly higher than all other pretreatment techniques, while T8 (36) was significantly lower than all other pretreatment techniques. The treated the seeds immersed in cold water in T3 produced seedlings with high vigor flowed by T8 and T9. This means Grevillea robusta might require time to break the dormancy and after that soaking in cold water for 36 hours in second time makes the seed coat softer, which ensures the maximum successful germination of seed (Chapman et al., 2020). Pretreatment under T10 treatment significantly increased the germination rate of Grevillea seeds compared with normal germination (control). The control produced the longest germination days and lower germination rates. Likewise, the results of this study showed that germination percentage and germination rates were improved when Grevillea seeds were subjected to various pre-sowing treatments of cold water soaking. Pre-sowing treatments promote rapid germination (Bonner, 1989; Chapman et al., 2020). Many studies have attempted to break the dormancy of hardcoated seeds to improve germination rates and seedling vigor (Lott et al., 2005; Umarani, 2014) [26].
Effect of seed pre-sowing treatment on average germination parameters of Grevillea robusta in laboratory
Table 4 Summary results of germination rate, germination start day, germination end day and seedling vigor index (SVI) of Grevillea in the laboratory. The results obtained showed that germination rates and duration varied significantly depending on the different pretreatments. The seeds soaked in cold water for 48 hours and soaked in cold water for 24 hours and then dried again and soaked in cold water for 24 hours began to germinate earlier than other treatments. Germination ended in T2, T3 and T4 later in cold water immersion compared to the other treatments. The results showed that there were significant differences (p < 0.05) in germination start and end dates, seedling vigor index, and germination rate between treatments (Table 4). The shortest germination times are T7 (2-9 days), T8 (1-9 days), T9 (2-8 days) and T10 (1-10 days), and the longest germination time is the control under laboratory conditions (6-10 days), T2 (3-13 days), T3 (4-15 days) and T4 (2-12 days), the highest germination rate was observed, T9 (97.33%) followed by T8 (96%), T7 and T10 (92%), control/untreated seeds had the shortest germination rate (14.67%). Untreated seeds (control) had late germination when other treatments had already completed germination. This means that the optimal treatment for Grevillea robusta T9 is considered in terms of germination start and end, germination percentage and germination rate. The main reason why T9 can successfully germinate is that the Grevillea robusta seeds need cold water for the first time to break dormancy, and then soak in cold water for a second time for 36 hours to soften the seed coat and maximize the germination rate of the seeds, which ensures the maximum successful germination of seed [27].
| Treatments | SDG | EDG | GP | GR |
|---|---|---|---|---|
| T1 | 6.000 ± 2.000a | 9.667 0.577cde | 14.667 ± 6.110c | 1.137 ± 0.569c |
| T2 | 3.333 ± 1.528bc | 12.667 ± 1.528ab | 52.000 ± 17.436b | 2.560 ± 0.862bc |
| T3 | 4.000 1.732ab | 14.667 ± 2.082a | 62.667 ± 4.619b | 1.883 ± 0.440bc |
| T4 | 2.333 1.155bcd | 12.333 ± 0.577abc | 64.000 8.000b | 2.557 ± 0.237bc |
| T5 | 1.000 0.000d | 10.667 ± 2.082bcde | 56.000 ± 20.785b | 3.170 ± 1.114b |
| T6 | 2.333 ± 0.577bcd | 11.333 ± 2.309bcd | 66.667 16.166b | 2.880 ± 1.038bc |
| T7 | 1.667 ± 1.155cd | 9.000 1.732ed | 92.000 ± 8.000a | 6.097 ± 1.619a |
| T8 | 1.000 0.000d | 9.000 1.732ed | 96.000 ± 4.000a | 7.877 ± 1.575a |
| T9 | 1.667 ± 1.155cd | 8.000 1.000e | 97.333 ± 4.619a | 6.633 ± 1.761a |
| T10 | 1.333 ± 0.577cd | 10.000 ± 1.732bcde | 92.000 ± 8.000a | 6.263 ± 0.531a |
| CV | 8.35 | 6.12 | 15.22 | 7.35 |
| LSD ( 0.05 ) | 2.1 | 2.8 | 20.11 | 1.92 |
| SDG: Start of germination day; EDG: End of germination day; GP and GR: Germination percentage and Rate | ||||
Table 4: Effect of seed pre-sowing treatment on average germination parameters of Grevillea robusta in laboratory.
Generally speaking, with the extension of soaking time, the germination rate increases significantly. Furthermore, as the cold water soaking time increased, the germination rate increased compared to the control under laboratory conditions. Regardless, these two findings suggest that changes in pre-sowing treatments contribute to the production of viable and viable seeds in the laboratory and to improved survival and growth performance of Grevillea seedlings in the nursery (Kildisheva, 2019; Sagwal, 2020). Furthermore, this plays a key role in producing optimum quality and required quantity of seedlings to achieve the desired development goals of the field plantation. Therefore, in the process of planting and cultivating Grevillea seedlings, we should consider choosing appropriate treatment methods to produce highquality and large quantities of seedlings to maximize the survival rate of seedlings and minimize the mortality of seedlings (Cochrane et al., 2015) [28-36].
Conclusions
The purpose of this study was to explore the effects of pre-sowing treatments on the seed germination and early seedling growth of Grevillea robusta to obtain high-quality seedlings. Studies have shown that pre-sowing treatments can soften the seed coat and have a profound impact on the germination and seedling development of Grevillea, resulting in increased germination rates and better seedling growth. Among different pre-sowing treatments, T9 had the largest plant height (31.05 cm), root neck diameter (0.44 cm) and collar diameter (25.62 mm). Understanding pre-seeding treatments at the early sapling stage is critical to managing seeds and resources. Based on the results obtained, it is clear that Grevillea seeds require pre-sowing treatment to promote seed germination and early seedling growth. This study showed that there were significant differences in the germination rate start and end dates of seed germination, seedling vigor index, and germination rate of Grevillea robusta seeds under different treatments. It can be seen that among the different pre-sowing treatments of silver birch, T9, T7, and T5 performed best in terms of germination rate, germination presentation, plant height, collar diameter, survival rate, root length, and seedling vigor index. In order to establish a tree species nursery and produce high-quality seedlings with minimum labor, time and cost, pre-sowing treatment of seeds is crucial. The results of this study will help large and small nursery operators to increase seed germination rates and seedling sales revenue. Therefore, nursery owners such as government, NGOs and private sector should be aware of the effects of pre-sowing treatments on seed germination and growth so that these treatments can be applied to obtain high quality seedlings for mass production in the shortest possible time. Further research is needed to understand physical and chemical influences under laboratory and nursery conditions and to understand the growth performance of Grevillea [37].
Acknowledgements
The author would like to thank Oromia Agricultural Research Institute for their financial support and the staff of Agroforestry Research Team of Bako Agriculture Research Center for their followup and data collection.
References
- Abdul‐Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria 1. Crop science 13: 630-633.
- Amoakoh OA, Nortey DDN, Sagoe F, Amoako PK, Jallah CK (2017) Effects of pre-sowing treatments on the germination and early growth performance of Pouteria campachiana. Forest science and technology, 13: 83-86.
- Azad MS, Nahar N, Matin MA (2013) Effects of variation in seed sources and pre-sowing treatments on seed germination of Tamarindus indica: a multi-purpose tree species in Bangladesh. Forest Science and Practice 15: 121-129.
- Barton LV (1965) Dormancy in seeds imposed by the seed coat. Differenzierung und Entwicklung/Differentiation and Development pp: 2374-2392.
- Benson EE (2000) Sepecial symposium: In vitro plant recalcitrance in vitro plant recalcitrance: An introduction. In Vitro Cellular & Developmental Biology-Plant 36: 141-148.
- Bezkorowajnyi PG (2001) Developing Fodder Resources on the Forest Grasslands of Tribal Areas in Western India: A Participatory Approach. Bangor University (United Kingdom).
- Bonner FT (1989). Principles and General Methods of Producing and Handling Seeds.
- Bowman DM (2000). Australian rainforests: islands of green in a land of fire. Cambridge University Press.
- Bradbeer JW (2013) Seed dormancy and germination. Springer Science & Business Media.
- Chapman M, Nelson P, Nicholson H, Dunphy M, McAlpin S (2020) Australian Rainforest Seeds: A Guide to Collecting, Processing and Propagation. Csiro publishing.
- Ciesla WM (2001). Forestry Department. Food and Agriculture Organization of the United Nations Forest Plantations Thematic Papers.
- Cochrane A, Yates CJ, Hoyle GL, Nicotra AB (2015) Will among‐population variation in seed traits improve the chance of species persistence under climate change? Global Ecology and Biogeography, 24: 12-24.
- Fischer R, Bohn F, de Paula MD, Dislich C, Groeneveld J, et al (2016) Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecological modelling 326: 124-133.
- Guan Y, Li SG, Fan XF, Su ZH (2016) Application of somatic embryogenesis in woody plants. Frontiers in Plant Science 7: 938.
- Kennedy DN, Swaine MD (1992) Germination and growth of colonizing species in artificial gaps of different sizes in dipterocarp rain forest. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 335: 357-367.
- Kettenring KM, Tarsa EE (2020). Need to seed? Ecological, genetic, and evolutionary keys to seed-based wetland restoration. Frontiers in Environmental Science 8: 109.
- Kheloufi A, Mansouri L, Aziz N, Sahnoune M, Boukemiche S, Ababsa B (2018) Breaking seed coat dormancy of six tree species. Reforesta pp: 4-14.
- Kildisheva OA (2019) Improving the outcomes of seed-based restoration in cold and hot deserts: An investigation into seed dormancy, germination, and seed enhancement.
- Kildisheva OA, Dixon KW, Silveira FA, Chapman T, Di Sacco A, et al (2020) Dormancy and germination: making every seed count in restoration. Restoration Ecology 28: S256-S265.
- Kimaro AA, Liingilie A (2015) Agronomic and Silvicultural Aspects of the Agroforestry System for Biomass Supply for Mbeya Cement Company, Tanzania.
- Krishnan PR, Kalia RK, Tewari JC, Roy MM (2014) Plant nursery management: principles and practices.
- Kuria A, Lamond G, Pagella T, Gebrekirstos A, Hadgu K, Sinclair FL (2014) Local knowledge of farmers on opportunities and constraints to sustainable intensification of crop-livestock-trees mixed systems in Lemo Woreda, SNNPR Region, Ethiopian highlands.
- Kuyah S, Sileshi GW, Luedeling E, Akinnifesi FK, Whitney CW, et al (2020). Potential of agroforestry to enhance livelihood security in Africa. Agroforestry for Degraded Landscapes: Recent Advances and Emerging Challenges 1: 135-167.
- Lal P (2010) Clonal forestry in India. The Indian Forester 136: 17-37.
- Lott R, Sexton G, Novak M, 2005. Seed and seedling supply for farm forestry projects in the tropics and subtropics of eastern Australia. Erskine, PD, Lamb, D. and Bristow, M (eds): 24-48.
- Maass M, Burgos A (2011) Water dynamics at the ecosystem level in seasonally dry tropical forests. Seasonally dry tropical forests: ecology and conservation pp: 141-156.
- Nyambati R, Kioko S (2018) Production and utilization of fruit, fodder and bio-energy trees. Kenya’s water towers protection and climate change mitigation and adaptation (water) programme.
- Othieno H, Awange J, Othieno H, Awange J (2016) Energy Resources in East Africa. Energy Resources in Africa: Distribution, Opportunities and Challenges pp: 33-137.
- Penfield S (2017) Seed dormancy and germination. Current Biology 27: R874-R878.
- Resch TM (1998) Grevillea robusta A. Cunn. Arboles Utiles de la Región Tropical de América Del Norte pp: 229.
- Sagwal SS (2020) Forest Tree Seeds: Handbook. Scientific Publishers.
- Sohel MSI, Islam HN, Ullah MA, Newaz KMN, Khan MFA (2023) Ecological and economic significance of swamp vegetation nursery for successful reforestation program: an insight from Bangladesh. Geology, Ecology, and Landscapes pp: 1-17.
- Tamado T, Schutz W, Milberg P (2002) Germination ecology of the weed Parthenium hysterophorus in eastern Ethiopia. Annals of applied biology 140:263-270.
- Tuheteru FD, Husna H, Albasri A, Arif A, Basrudin B, et al (2022). Effect of pre-sowing treatment on the germination and early growth of Kalappia celebica Kosterm: an endemic and vulnerable tree species of Sulawesi, Indonesia. Biodiversitas Journal of Biological Diversity 23.
- Umarani R (2014) 10 Principles and Practices of Tree Seed Handling Techniques. Industrial Agroforestry Perspectives and Prospectives 131.
- Veiga-Barbosa L, Ruiz C, Correa EC, Pérez-García F (2016). Dormancy imposed by a tough seed coat in Malvella sherardiana (Malvaceae), a highly threatened species of Spain. Botany Letters, 163: 321-327.
- Wang W, Chen Q, Hussain S, Mei J, Dong H, et al (2016) Pre-sowing seed treatments in direct-seeded early rice: consequences for emergence, seedling growth and associated metabolic events under chilling stress. Scientific reports, 6: 19637.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Daba MH (2023) Effects of Pre-Sowing Treatments in Seed Germinationand Early Seedling Growth of Grevillea robusta in Bako Agricultural ResearchCenter Nursery Site, Western Oromia, Ethiopia. J Earth Sci Clim Change, 14: 738.
Copyright: © 2023 Daba MH. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2551
- [From(publication date): 0-2023 - Nov 28, 2025]
- Breakdown by view type
- HTML page views: 2194
- PDF downloads: 357