Research Article Open Access
Effects of Prenatal Fish Oil and Folic Acid Supplementation on Infant Psychomotor and Mental Development: Results from NUHEAL Randomized Controlled Trial
| Cristina Campoy1*, Signe Altmäe1, Rosa Ramos2, Francisco Cruz3, Miguel Perez3, Maria T Salvatierra1, Concepcion Robles1, Milagros Cruz4,Maria T Miranda5, Angel Gil6, Tamas Decsi7 and Berthold V Koletzko8 | |
| 1Department of Paediatrics, School of Medicine University of Granada, Granada, Spain | |
| 2CIBER of Epidemiology and Public Health (CIBERESP), Spain, Laboratory of Medical Investigations, San Cecilio Clinical University Hospital, Granada, Spain | |
| 3Department of Clinical Psychology, Evaluation and Personality, School of Psychology, University of Granada, Granada, Spain | |
| 4Obstetric and Gynaecology Service of the Granada’s Clinical San Cecilio University Hospital, Granada | |
| 5Department of Biostatistics, University of Granada, Granada, Spain | |
| 6Department of Biochemistry and Molecular Biology, School of Pharmacy, University of Granada, Granada, Spain | |
| 7Department of Paediatrics, University of Pécs, Pécs, Hungary | |
| 8Division of Metabolic Diseases and Nutritional Medicine, Dr. von Hauner Children’s Hospital Ludwig-Maximilians-University of Munich, Munich, Germany | |
| Corresponding Author : | Cristina Campoy Department of Paediatrics School of Medicine University of Granada Avda. de Madrid, 11, 18012-Granada, Spain Tel: +34 629308695 E-mail: ccampoy@ugr.es |
| Received September 05, 2014; Accepted January 13, 2015; Published January 15, 2015 | |
| Citation: Campoy C, Altmäe S, Ramos R, Cruz F, Perez M, et al. (2015) Effects of Prenatal Fish Oil and Folic Acid Supplementation on Infant Psychomotor and Mental Development: Results from NUHEAL Randomized Controlled Trial. J Preg Child Health 2:131. doi: 10.4172/2376-127X.1000131 | |
| Copyright: © 2015 Campoy C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Background: Prenatal supply of folic and fatty acids is related to infant’s neurodevelopment; however, the potential beneficial effects on child’s neurologic outcomes remain controversial.
Methods: We analysed 154 Spanish pregnant women in 4 randomized groups of supplementation with fish oil (FO), folic acid (5-MTHF), both, or placebo, and assessed their infant’s mental and psychomotor development at 6 and 20 months of life with Bayley Scales of Infant Development (BSID).
Results: No significant differences in BSID outcomes between the groups were detected. FO+5-MTHF supplementation influenced positively blood phospholipid polyunsaturated fatty acids (PUFAs) and folate levels during pregnancy, at delivery, and in neonates. No effect of FO and/or 5-MTHF supplementation on breast milk PUFA levels was detected. Further, higher maternal DHA and lower n6/n3 ratio at delivery associated positively with offspring’s PDI scores at 20 months. While infants with higher blood folate levels correlated with higher MDI scores at 20 months.
Conclusions: Findings of the current study show no clear effect of FO and folic acid supplementation on child’s neurodevelopment, regardless of the positive effect of supplementation on blood PUFAs and folate levels. However, prenatal PUFAs, especially DHA, and higher folate levels in newborns could have a positive effect on child’s neurodevelopment.
| Keywords |
| Fish oil; Folic acid; LC-PUFA; Pregnancy; Prenatal supplementation; Neurodevelopment |
| Introduction |
| There is growing evidence that early nutrition can affect later cognitive performance. Nevertheless, much of this evidence is from animal studies, and in humans from retrospective and short-term nutritional intervention studies. The current evidence of an association between prenatal nutrition and brain development is more credible for n-3 fatty acids and folate. In humans, considerable amounts of fatty acids accumulate in the foetal brain and retina during the third trimester of pregnancy, and in the infant brain and retina during the first year of postnatal life [1-3]. Human foetuses and young infants have a limited ability to synthesise omega 3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs) de novo, prenatally fatty acids are obtained from maternal stores, and diet, and postnatally from breast milk or infant formulas [4]. Therefore deficiencies in the fatty acids could critically influence neurodevelopment and developmental velocity, and later mental performance in children [5]. Indeed, several studies indicate the beneficial effect of high maternal docosahexaenoic acid (DHA) status on infant’s neurodevelopmental outcomes [6-11]. Also benefits of higher maternal fish consumption (natural source of n-3 LC-PUFAs) during pregnancy on child’s neurodevelopment have been reported [12,13]. Furthermore, maternal supplementation with DHA during pregnancy and lactation has been associated with child’s enhanced psychomotor and cognitive development [14-19]. Nevertheless, the potential beneficial effects of maternal DHA supply on child’s neurologic outcomes, especially the long-term outcomes, remain controversial, as several studies have not detected such effects [20-25]. These inconclusive results could be due to the differences in the dosage and timing of supplementation, source of supplements, methodology used for assessing neurologic condition, and/or limited statistical power. |
| Folates are other group of nutrients that have fundamental importance for brain development. Folates participate in one-carbon biosynthetic and epigenetic processes that facilitate the synthesis and methylation of nucleic acids and proteins [26], and are thus indispensible during periods of rapid cell growth and proliferation, which occur during brain development. Additionally, it has been indicated that folate deficiency can modulate plasma and tissue fatty acid composition [27-30]. Recent cohort studies of folate supplemented mothers have shown that their infants have fewer behavioural and emotional problems [31,32], improved neurodevelopment and reduced hyperactivity and peer problems in children [33,34]. |
| Nevertheless, the recent reviews and meta-analyses of randomised clinical trials conclude that there is no clear long-term benefit of folate and n-3 fatty acids supplementation during pregnancy on child’s neurodevelopment [35-38]. Despite of the growing interest in the topic, the optimal content of micronutrient supplementation and whether there is a long-term impact on child’s neurodevelopment needs to be investigated further. In addition, as folates can influence fatty acid composition in the blood and tissue, the effect of folic acid supplementation together with n-3 fatty acids on child’s neurodevelopment has not been investigated in other clinical trials. |
| In the current study we set out to assess the effects of maternal Fish Oil (FO) and folic acid (5-MTHF) supplementation during the second half of pregnancy on their offspring psychomotor and mental development during the first 20 months of postnatal life. |
| Materials and Methods |
| Study design |
| This study is a part of a European randomized multicentre trial, NUHEAL (Nutraceuticals for a Healthier Life; registration no. NCT01180933). The detailed study design, subject recruitment and characteristics, inclusion criteria, dietary intervention, and collection of data and biological material have been described previously [30,39]. In short, healthy women aged 18 to 40 years with uncomplicated pregnancies were invited to participate in the study before 20 weeks gestation. Babies born within the Spanish cohort (n=154) were assessed for neurodevelopment at 6 and 20 months of postnatal life. Their mothers were randomly allocated in a double-blind fashion to one of four treatment groups during the second half of pregnancy: (a) fish oil (500 mg DHA+150 mg EPA/day) (FO), (b) folic acid (5-methyltetrahydrofolic acid (5-MTHF), 400 μg /day) (5-MTHF), (c) fish oil + folic acid (FO+5-MTHF), and (d) placebo (see [30] for detailed recruitment description). Women were provided with 180 sachets, each containing 15g of a milk-based supplement (Blemil Plus Matter, Ordesa Laboratorios, Barcelona, Spain). Each sachet was to be consumed as one daily dose, providing 500 mg DHA and 150 mg EPA (provided as modified fish oil (Pronova Biocare, Lysaker, Norway)), or 400 μg 5-MTHF (BASF, Ludwigshafen, Germany), both, or placebo together with vitamins and minerals in amounts meeting the recommended intakes during the second half of pregnancy. In more detail, each sachet provided per day: 360 mg calcium, 360 mg phosphorous, 106 mg magnesium, 5.7 mg zinc, 60 μg iodine, 240 μg vitamin A, 0.5 μg vitamin D, 3 mg vitamin E, 0.48 mg vitamin B1, 0.54 mg vitamin B2, 6 mg vitamin B3, 0.63 mg vitamin B6, 0.78 μg vitamin B12 and 60 μg vitamin C. The supplements were taken daily from week 20 of pregnancy until delivery. Subjects were asked not to take any other vitamin or dietary supplements during this time. Compliance was assessed in standardized questionnaires (see [30]) at pregnancy week 30 and at delivery by asking each of the women how many days of supplementation she had missed. In addition, the women were asked to return the non-consumed sachets at each visit, providing thereby adherence to the self-reported supplement intake. Volunteers were excluded if they had consumed fish oil supplements during the first half of the pregnancy or continued to use folate and vitamin B12 containing supplements after 16 weeks gestation. Only singleton births with birth weights appropriate for gestational age were included. |
| Breast-feeding was always recommended and supported after the delivery. As it is assumed that the diet during pregnancy influences the composition of lipid stores, which contribute to milk lipids [40], infants not fully breastfed were provided with or without supplementary DHA according to the nutritional code assigned to the mothers during pregnancy, continuing with the double blind and randomized procedure. Except for fatty acid composition, both formulas were identical and in line with recent European recommendations [41,42]. Children born from the placebo or 5-MTHF group received a formula free of DHA and Arachidonic Acid (AA), and those born to FOsupplemented women received a formula containing 0.5% of total fatty acids as DHA and 0.4% of AA during the first 6 months of postnatal life. Clinical histories of the parents were obtained on enrolment of the mother. Fasting blood samples were drawn from mothers at weeks 20 and 30 of pregnancy and at delivery. Umbilical cord blood was collected at delivery. All blood samples were collected into EDTA tubes. Blood was centrifuged at 3500 rpm for 5 min and plasma collected. Plasma was frozen at -80ºC until analysis. Mature breast milk was collected 8 weeks after the delivery, a random sample manually expressed by the mother. Samples were stored at -80ºC until the milk fatty acids were analysed. |
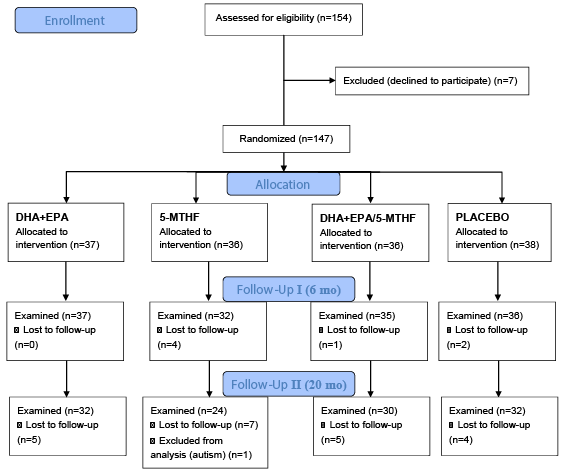
| The local Ethical Committee of San Cecilio University Clinical Hospital of Granada, Spain approved the study protocol. After a careful explanation of the study details, written consent was obtained from all participating women. Neurodevelopment assessment was performed by one psychologist in 147 infants at age of 6 months (n=140) and 20 months (n=118) using the Spanish version of the BSID [43] (Figure 1). These scales are considered valid and reliable for assessing psychometric properties of children from 1 to 30 months of age and are widely used to evaluate the global cognitive development of young children. BSID are intended to measure the level of development in cognitive, motor, and behavioural domains and comprise mental, psychomotor and behaviour rating scales. In this study, the mental and psychomotor scales were considered. Raw BSID scores for the mental and psychomotor scales, Raw Mental Score (RMS) and Raw Psychomotor Score (RPS), based on the number of items successfully completed, were converted to the Mental Development Index (MDI) and Psychomotor Development Index (PDI) standard scores according to the child’s age at the time of testing. The MDI assesses memory, problem solving, discrimination, classification, language and social skills. The PDI measures control of gross and fine muscle groups, including walking, running, jumping, pre-hension and imitation of hand movements. |
| The BSID score differences between 6 and 20 months of infant age were standardised to a mean of 100 corresponding to the mean of the raw scores (range 84-116), and standard deviation of 15 (one standard deviation change) and of 30 (two standard deviation change). Accordingly, three children subgroups were obtained: NC = No Change; OSDC= One Standard Deviation Change; TSDC= Two Standard Deviation Change. For the NC group, MDI mean scores were 104.94 ± 8.61 and 108.39 ± 8.53 points at 6 and 20 month, and PDI scores were respectively 114.43 ± 10.41 and 118.35 ± 9.18 points. For the OSDC group, MDI scores were 99.37 ± 9.21 and 116.80 ± 12.95 points at 6 and 20 month, and PDI scores were respectively 108.32 ± 8.67 and 123.56 ± 8.58 points. Finally, for the TSDC group, MDI scores were 94.73 ± 7.18 and 123.07 ± 11.37 points at 6 and 20 month, and PDI scores were 99.91 ± 10.46 and 131.55 ± 10.66 points, respectively. |
| Fatty acid analyses from blood and breast milk and folate analyses from blood |
| The procedures of these analyses have been extensively described elsewhere [9]. In short, total lipid extraction from maternal and cord blood plasma was performed according to the method of Kolarovic and Fournier [44] and lipids from breast milk were extracted as described before [14]. The analysis results of fatty acids were expressed as percentages by weight (wt %) of total detected fatty acids. Folate analyses in blood and plasma was performed by microbiological assay with the use of a chloramphenicol resistant strain of Lactobacillus casei as described previously [45]. Total homocysteine (tHcy) concentrations in plasma were measured by HPLC with fluorescence detection [46]. For all assays, samples were analysed blind, within 18 months of collection and quality control was provided by repeated analysis of stored batches of pooled samples covering a wide range of values. Intra- and interassay coefficients of variation were <11% for folate and ≤ 2.5% for tHcy. |
| Statistical analysis |
| Power calculation showed that the size of the remained study groups (infants at 6 and 20 months of age) allowed a detection of 1.0 SD points of difference in BSID scores (PDI and MDI) with a Type I error of 0.05 and a statistical power of 85%. Variables were assessed for normality using Kolmogorov-Smirnov or Shapiro-Wilk tests. Descriptive results were expressed as means with standard deviation or, in non-normal data, as median and interquartile range. |
| In order to test whether several confounders differed between the study groups, a set of potential confounders (maternal age, gravidity, parity, delivery mode and maternal smoking in the 20th and 30th week of gestation, and others) were included one by one in the models as dependent variables and study group as exposure (Table 1). Since none of the potential confounders differed among study groups, it is confirmed that main analyses on the effect of the intervention on the study outcomes do not need to adjust for any of these confounders, as expected when the study design and randomization was worked properly. |
| The main analyses of this study relay on testing differences among the different study groups. When comparing two groups, Student t was used for normally distributed data, while when comparing 3 or the main 4 groups in normally distributed data ANOVA test was used. For non-parametric data, the Kruskal-Wallis and Mann-Whitney U test were applied. Effect size of intervention was assessed using Cohen’s d (standardized mean difference) and 95% confidence interval. Taking into account the cut-off values established by Cohen, the effect size (d) can be small (~0.2), medium (~0.5) or large (~0.8). All computations were performed using SPSS statistical program, version 20.0, (SPSS Inc. Chicago, USA); a P value < 0.05 was considered statistically significant. |
| Results |
| Participants’ characteristics and how they relate to study outcomes |
| Out of the 154 infants recruited, 118 completed the whole study up to 20 months of age (Figure 1). There were no differences in the dropout rates between intervention groups. There were no differences concerning baseline characteristics between followed children from the different intervention groups (Table 1). Mean dietary intake of DHA of the participating women was similar in all intervention groups at the 20th and the 30th week of pregnancy. The four groups of mothers did not significantly differ in any anthropometric variables during pregnancy or in peripartal maternal or fetal complications or birth outcomes at delivery, and there were no differences in the confounders analysed (Table 1) (results reported by Krauss-Etschmann et al. [30]. |
| The study population characteristics in association with BSID score difference between infant age at 6 and 20 months are presented in Table 2. This model explains 84% of the variation of the psychomotor development. The MDI at 20 months was related to maternal age, and an improvement in MDI of one or two standard deviations was observed in children whose mothers were ≤ 35 years old at delivery (P=0.006). |
| Primary outcome: Effect of FO and folic acid supplementation during pregnancy on BSID scores at 6 and 20 months of life |
| No significant differences in the psychomotor (PDI) and Mental Development (MDI) scores of infants at 6 and 20 months of life were found as a function of the type of supplementation received by their mother (Table 3). At 6 months of age infants’ MDI scores were similar in all 4 study groups (p = 0.8), with statistically non-significant small effect sizes (Cohen’d < 0.2). PDI scores at 6 months were similar in the 4 supplemented groups (p = 0.4), with the effect sizes of medium to small (Cohen’d= 0.4 – 0.09). When dividing the four study groups into 2: FO and no FO supplementation, there was still no effect of the supplementation on infant’s MDI and PDI scores (p = 0.6 and p = 0.7, respectively), with statistically non-significant small effects (respective Cohen’d= 0.1, C.I. -0.24; 0.43, and Cohen’d= 0.08, C.I. -0.26; 0.41) (Table 3). |
| At 20 months of age the infants’ MDI scores were also similar in all 4 study groups (p = 0.7), with statistically non-significant medium to small effect sizes (Cohen’d= 0.3 – 0.13). PDI scores at 20 months were as well similar in the 4 supplemented groups (p = 0.7), with statistically non-significant small effect sizes (Cohen’d< 0.02). When dividing the four study groups into 2: FO and no FO supplementation, there was still no effect of the supplementation on infant’s MDI and PDI scores (p = 0.4 and p = 0.3, respectively), with statistically non-significant the small effects (respective Cohen’d= 0.14, C.I. -0.22; 0.51, and Cohen’d= 0.18, C.I. -0.18; 0.55) (Table 3). |
| Secondary outcomes: Influence of FO and folic acid supplementation on blood PUFAs and folate levels during pregnancy and at delivery, and on PUFAs in breast milk |
| At the moment of entering the study (pregnancy week 20), all women allocated to different supplementation groups demonstrated similar PUFAs and folates levels in the blood (Table 4, and Supplementary Table 1). Folate levels in the blood were influenced by different FO and/or 5-MTHF supplementations in the second part of the pregnancy (week 30 and at delivery): whole blood folate and plasma folate levels were higher in the 5-MTHF group and highest in the FO+5-MTHF group when compared to solely FO supplemented and to placebo groups at pregnancy week 30 and at delivery (Table 4). The levels of tHcy did not seem to differ between the supplementation groups throughout the pregnancy and at delivery (Table 4). |
| PUFAS, as expected and as we have reported in our previous study [47], were increased during pregnancy, delivery and in cord blood in the study group of FO supplementation; DHA being significantly higher in the two groups where FO was received (groups DHA+EPA; DHA+EPA+5-MTHF), meanwhile AA/DHA and n-6/n-3 ratios being significantly lower during pregnancy week 30 and at delivery (Supplementary Table 1). These associations were even stronger, when all FO supplemented women (groups DHA+EPA & DHA+EPA+5-MTHF) were compared to women with no FO supplementation (5-MTHF & Placebo), where DHA levels were significantly higher and AA, AA/DHA and n-6/n-3 ratios were significantly lower in the FO supplemented group at the second part of pregnancy and at delivery (see Table 5). At delivery, there were close correlations between mothers and their offspring in AA/DHA ratios (r=0.622, P<0.001) and n6/n3 ratios (r=0.30, P<0.005) (Supplementary Figures 1 and 2). As well, plasma folate concentrations were closely correlated to their mothers’ at delivery (r=0.59, P<0.001). PUFAS in the breast milk at week 8 after the delivery did not statistically differ between the 4 differentially supplemented study groups (Table 6). However, marginal significant difference was found in AA/DHA ratios between the 4 study groups (p = 0.06). Post-hoc analyses demonstrated that AA/DHA ratio was borderline significantly lower in FO group when compared to the placebo group (0.8 ± 0.3 vs. 1.2 ± 0.7, p = 0.057). |
| Exploratory outcomes: Comparison of BSID scores at 6 and 20 months as a function of LC-PUFAs and folate levels in mothers and neonates at delivery |
| Although no significant differences in BSID scores at 6 and 20 months were observed among the four/and two supplementation groups, PDI scores at 20 months were higher in infants whose mothers had plasma phospholipid DHA concentrations above the median (11.51 mg/dL) at delivery (>P50): 122.80 (9.20) vs. 126.20 (12.50) (P<0.045), and n6/n3 ratio ≤ median values (≤ P50): 127.40 (10.90) vs. 122.0 (11.10) (P<0.045) (Table 7). Mothers with DHA concentrations in plasma phospholipids above the P50, had an average of DHA intake higher than 44, 20 g/daily, and represented the 39.3% of the whole group of the Spanish pregnant women studied. |
| Furthermore, infants whose mothers had a higher concentrations of DHA in plasma phospholipids at delivery, showed a significant improvements in the standard deviations obtained in the Bayley PDI from 6 to 20 months, which resulted in one or two standard deviations higher than those infants born to mothers with lower concentrations of these fatty acids (Increases in standard deviations of BSDI Psychomotor Development index, between 6 to 20 months of age, as a function of maternal DHA in plasma phospholipids at delivery: 10.37 ± 3.15 mg/dL= no change in PDI; 11.94 ± 3.76 mg/dL= one standard deviation of change in PDI; 13.46 ± 4.44 mg/dL = two standard deviations of change in PDI). |
| Infants with higher folate levels in umbilical cord (>P50) demonstrated higher MDI scores at 20 months of age than infants with folate levels at birth below the median (<P50), 120.26 (9.27) vs. 115.10 (11.71) (P<0.026). Mothers with folic acid levels in plasma above the 50 percentile showed a daily intake of folate ≥ 318 μg/day. 74% to 79% of the pregnant women showed folate deficiency during pregnancy folate levels in plasma <15 μg/L (7 nmol/L). |
| Discussion |
| The data from this current randomized clinical trial does not show any statistically significant effect of maternal FO and/or folic acid supplementation on infant psychomotor and mental development at 6 and 20 months of life. However, as expected, the folate and fatty acid levels during pregnancy and at delivery were positively influenced by the FO and/or folic acid supplementation, which positively associated with child’s neurodevelopmental scores at 20 months of age. |
| The findings of the current study of no effect of prenatal FO and/ or folic acid supplementation on neurodevelopment outcome assessed by Bayley scores at the age of 6 and 20 months does not preclude a beneficial or non-beneficial effect of prenatally acquired FO and/or folic acid on neurodevelopment outcome at a later age. In fact, longer follow-up studies of FO supplementation during pregnancy do provide positive influence of the supplementation on child’s neurodevelopment later in life [14,19,22]. Our study results are in line with previous studies. Tofail et al. demonstrated that FO or soy oil supplementation during the last trimester of pregnancy on psychomotor and mental development of infants at 10 months of age resulted no significant group differences with respect to infant Bayley’s MDI and PDI scores [24]. Another group showed that DHA and AA supplementation during pregnancy and lactation did not influence neurodevelopment at toddler age; however, perinatal DHA and AA status were related to developmental outcomes at 18 months [25]. Also Bouwstra et al. reported significant association between umbilical cord fatty acid composition and neurodevelopmental status at 18 months, and no Bayley test score differences between breast-fed, formula-fed, and LCPUFA formula-fed children [11,48]. Furthermore, the previous papers based in the assessment of the NUHEAL children at 5.5 [23] and 6.5 years old [24], about psychomotor and cognitive performance, have not shown either any statistical difference in the neurological outcomes due to the type of maternal supplementation given during pregnancy. However, even up to 6.5 years later the same positive effect of higher maternal concentrations of DHA at delivery and perinatal folate levels has been shown to be associated to a better neurodevelopmental scoring. Further, a recent study by Mulder et al. [49] demonstrate that language development is robustly constrained risk at different ages and with different tests in infants born to women consuming about 5% energy from linoleic acid, 0.59% energy as α-linolenic acid and 85 mg/d DHA. They conclude that infants of women following typical western diets have risk for DHA insufficiency, which may be reduced by increasing the maternal DHA intake. |
| In the current study, we clearly see the effect of FO and/or folic acid supplementation on the blood LC-PUFAs and folates levels in the second part of the pregnancy and at delivery. Nevertheless, no significant effect of FO/folic acid supplementation during pregnancy on breast milk LC-PUFAs levels 2 months after the delivery was detected. Regarding the positive effects of supplementation on blood folate levels, interestingly the mixed supplementation group (women who received FO+5-MTHF) presented the best plasma folate and whole blood folate levels during pregnancy, delivery and in infants (cord blood), being even better than the pure 5-MTHF group. While plasma PUFAs levels were in the mixed group as good as in women solely receiving FO. The link between folate and plasma and tissue fatty acid composition has been suggested [28,30], which could explain the beneficial effect of mixed supplementation on blood PUFAs and folate levels hypothesized in the NUHEAL study design. |
| The approach obtained in the present study is that not only the suggested beneficial effects of DHA have been shown, but also those seen from folate. The special design of the NUHEAL study has permitted to show that the supplementation of both nutrients seems to be beneficial for brain development, although in different ways: n-3 LC-PUFAs seems to be more related to neuro psychomotor development and folate to mental development in these early stages of life. Furthermore, mothers with higher DHA levels at delivery have babies with higher speed of neuro psychomotor development from 6 to 20 month of age than mothers with lower DHA values. These results permit to suggest that high levels of DHA at delivery has an effect on the Bayley scores, but also on the velocity of the psychomotor development and so, the velocity of brain maturation; this effect is supposed to be related to a better neuropsychological and cognitive outcomes later in life. Nevertheless, further studies on a bigger sample size are needed before any strong conclusions could be drawn. |
| The analysed groups in the current study were rather limited, meaning that the study is not strongly powered in order to provide any conclusive results of the maternal supplementation on infant’s later neurodevelopment. In addition, not including the genotype assessment of enzymes that influence LC-PUFA synthesis and/or folate metabolizing pathway may have reduced the sensitivity and precision of detecting the effects of dietary DHA/folate supply [5]. Further, we cannot rule out the possibility that lifetime nutrient status and maternal stores may be more important for long-term maternal fatty acid and folate statuses than is supplementation during the second half of pregnancy. Future research should consider short-and-long term effects of LC-PUFA and/or folate status prior to and during pregnancy and lactation together with the genotype data in big cohort studies [4]. |
| In conclusion, the results of the current study do not provide direct evidence of a beneficial effect of FO and/or folic acid supplementation during the second half of pregnancy on neurodevelopment in infants at 6 and 20 months of age, regardless of their positive influence on blood PUFAs and folate levels. However, prenatal PUFAs, especially DHA, and neonate folate levels might have a positive effect on child’s neurodevelopment and in the velocity of achieving the improvements between 6 to 20 months of age. |
| Acknowledgements |
| The authors are grateful to the women who participated in the study and to the paediatric and obstetric teams from the Department of Paediatrics at the University of Granada, Spain. Ordesa Laboratories provided the supplementation sachets for women and infant formulas, and participated as partners in the EU FP5 funded NUHEAL randomized control trial. |
| Funding Disclosure |
| This study was partially funded by the Commission of the European Communities within the framework 5 project NUHEAL CLK1- CT-1999-00888 and the University of Granada (Research Strategy Plan), grant from The Spanish Ministry of Education (grant no. SB2010-0025) and Marie Curie post-doctoral fellowship (FP7, n° 329812, NutriOmics). The funding agencies had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data or in the preparation (or approval) of the manuscript. This manuscript does not necessarily reflect the views of the Commission. This trial was registered at clinicaltrials.gov as NCT01180933. |
| Author Contributions |
| Cristina Campoy, Tamas Décsi and Berthold V. Koletzko designed the research and directed its implementation; Rosa Ramos, Francisco Cruz, Maria T. Salvatierra, Concepción Robles, Milagros Cruz, and Angel Gil conducted the research; Cristina Campoy, Signe Altmäe, María T. Miranda and Miguel Pérez analysed the data and/or performed statistical analyses; Cristina Campoy and Signe Altmäe wrote the paper; Cristina Campoy and Berthold V. Koletzko were responsible for administrative support and funding. All authors read and approved the final manuscript. |
References
- Innis SM1 (2007) D ietary (n-3) fatty acids and brain development. J Nutr 137: 855-859.
- Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40: 1-94.
- Uauy R, Mena P, Rojas C (2000) Essential fatty acids in early life: structural and functional role. ProcNutrSoc 59: 3-15.
- Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, Hoesli I, Holzgreve W, Lapillonne A, Putet G, Secher NJ, Symonds M, Szajewska H, Willatts P and Uauy R: The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 2008;36:5-14.
- Anjos T,Altmäe S, Emmett P, Tiemeier H, Closa-Monasterolo R, et al. (2013) Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur J Nutr 52: 1825-1842.
- Hadders-Algra M1 (2008) Prenatal long-chain polyunsaturated fatty acid status: the importance of a balanced intake of docosahexaenoic acid and arachidonic acid. J Perinat Med 36: 101-109.
- Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB and Lammi-Keefe CJ: Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J ClinNutr 2002;76:608-613.
- Colombo J,Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, et al. (2004) Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 75: 1254-1267.
- Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, et al. (2008) Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the inuit of arctic Quebec. J Pediatr 152: 356-364.
- Bouwstra H,Dijck-Brouwer DJ, Decsi T, Boehm G, Boersma ER, et al. (2006) Relationship between umbilical cord essential fatty acid content and the quality of general movements of healthy term infants at 3 months. Pediatr Res 59: 717-722.
- Bouwstra H, Dijck-Brouwer J, Decsi T, Boehm G, Boersma ER, Muskiet FA and Hadders-Algra M: Neurologic condition of healthy term infants at 18 months: positive association with venous umbilical DHA status and negative association with umbilical trans-fatty acids. Pediatr Res 2006;60:334-339.
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, et al. (2005) Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect 113: 1376-1380.
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, et al. (2007) Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet 369: 578-585.
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA (2003) Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 111: e39-44.
- Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, Turcich MR, Llorente AM, Anderson RE and Heird WC: Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J ClinNutr 2005;82:125-132.
- Judge MP, Harel O and Lammi-Keefe CJ: Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J ClinNutr 2007;85:1572-1577.
- Dunstan JA, Simmer K, Dixon G, Prescott SL (2008) Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 93: F45-50.
- van Goor SA,Dijck-Brouwer DA, Doornbos B, Erwich JJ, Schaafsma A, et al. (2010) Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr 103: 235-242.
- Escolano-Margarit MV, Ramos R, Beyer J, Csabi G, Parrilla-Roure M, Cruz F, Perez-Garcia M, Hadders-Algra M, Gil A, Decsi T, Koletzko BV and Campoy C: Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr 2011;141:1216-1223.
- Campoy C, Escolano-Margarit MV, Ramos R, Parrilla-Roure M, Csabi G, Beyer J, Ramirez-Tortosa MC, Molloy AM, Decsi T and Koletzko BV: Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am J ClinNutr 2011.
- Helland IB,Saugstad OD, Smith L, Saarem K, Solvoll K, et al. (2001) Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics 108: E82.
- Helland IB, Smith L, Blomén B, Saarem K, Saugstad OD, et al. (2008) Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 122: e472-479.
- Malcolm CA, McCulloch DL, Montgomery C, Shepherd A and Weaver LT: Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arch Dis Child Fetal Neonatal Ed 2003;88:F383-390.
- Tofail F,Kabir I, Hamadani JD, Chowdhury F, Yesmin S, et al. (2006) Supplementation of fish-oil and soy-oil during pregnancy and psychomotor development of infants. J Health PopulNutr 24: 48-56.
- van Goor SA, Dijck-Brouwer DA, Erwich JJ, Schaafsma A and Hadders-Algra M: The influence of supplemental docosahexaenoic and arachidonic acids during pregnancy and lactation on neurodevelopment at eighteen months. Prostaglandins LeukotEssent Fatty Acids 2011;84:139-146.
- Laanpere M,Altmäe S, Stavreus-Evers A, Nilsson TK, Yngve A, et al. (2010) Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viability. Nutr Rev 68: 99-113.
- Umhau JC,Dauphinais KM, Patel SH, Nahrwold DA, Hibbeln JR, et al. (2006) The relationship between folate and docosahexaenoic acid in men. Eur J ClinNutr 60: 352-357.
- Pita ML, Delgado MJ (2000) Folate administration increases n-3 polyunsaturated fatty acids in rat plasma and tissue lipids. ThrombHaemost 84: 420-423.
- Böhles H, Arndt S, Ohlenschläger U, Beeg T, Gebhardt B, et al. (1999) Maternal plasma homocysteine, placenta status and docosahexaenoic acid concentration in erythrocyte phospholipids of the newborn. Eur J Pediatr 158: 243-246.
- Krauss-Etschmann S, Shadid R, Campoy C, Hoster E, Demmelmair H, Jimenez M, Gil A, Rivero M, Veszpremi B, Decsi T and Koletzko BV: Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J ClinNutr 2007;85:1392-1400.
- Roza SJ, van Batenburg-Eddes T, Steegers EA, Jaddoe VW, Mackenbach JP, Hofman A, Verhulst FC and Tiemeier H: Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br J Nutr 2010;103:445-452.
- Steenweg-de Graaff J, Roza SJ, P. SEA, Hofman A, Verhulst FC, Jaddoe VW and Tiemeier H: Maternal folate status in early pregnancy and child emotional and behavioral problems. The Generation R Study. Am J ClinNutr 2012.
- Julvez J,Fortuny J, Mendez M, Torrent M, Ribas-Fitó N, et al. (2009) Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. PaediatrPerinatEpidemiol 23: 199-206.
- Schlotz W, Jones A, Phillips DI, Gale CR, Robinson SM, et al. (2010) Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry 51: 594-602.
- Skorka A, Gieruszczak-Bialek D, Piescik M and Szajewska H (2012) Effects of prenatal and/or postnatal (maternal and/or child) folic acid supplementation on the mental performance of children. Crit Rev Food SciNutr 52: 959-964.
- Campoy C,Escolano-Margarit MV, Anjos T, Szajewska H, Uauy R (2012) Omega 3 fatty acids on child growth, visual acuity and neurodevelopment. Br J Nutr 107 Suppl 2: S85-106.
- Dziechciarz P, Horvath A and Szajewska H: Effects of n-3 long-chain polyunsaturated fatty acid supplementation during pregnancy and/or lactation on neurodevelopment and visual function in children: a systematic review of randomized controlled trials. J Am CollNutr 2010;29:443-454.
- Gould JF, Smithers LG and Makrides M: The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J ClinNutr 2013;97:531-544.
- Decsi T,Campoy C, Koletzko B (2005) Effect of N-3 polyunsaturated fatty acid supplementation in pregnancy: the Nuheal trial. AdvExp Med Biol 569: 109-113.
- Demmelmair H,Baumheuer M, Koletzko B, Dokoupil K, Kratl G (1998) Metabolism of U13C-labeled linoleic acid in lactating women. J Lipid Res 39: 1389-1396.
- Aggett PJ, Haschke F, Heine W, Hernell O, Koletzko B, et al. (1991) Comment on the content and composition of lipids in infant formulas. ESPGAN Committee on Nutrition. ActaPaediatrScand 80: 887-896.
- Koletzko B,Agostoni C, Carlson SE, Clandinin T, Hornstra G, et al. (2001) Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. ActaPaediatr 90: 460-464.
- Bayley N. (1977) Manual for the Bayley Scales of Infant Development.
- Kolarovic L, Fournier NC (1986) A comparison of extraction methods for the isolation of phospholipids from biological sources. Anal Biochem 156: 244-250.
- Molloy AM and Scott JM: Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol 1997;281:43-53.
- Ubbink JB, Hayward Vermaak WJ, Bissbort S (1991) Rapid high-performance liquid chromatographic assay for total homocysteine levels in human serum. J Chromatogr 565: 441-446.
- Escolano-Margarit MV, Campoy C, Ramirez-Tortosa MC, Demmelmair H, Miranda MT, Gil A, Decsi T and Koletzko BV: Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: a randomised controlled trial. Br J Nutr 2013;109:1647-1656.
- Bouwstra H, Dijck-Brouwer DA, Boehm G, Boersma ER, Muskiet FA and Hadders-Algra M: Long-chain polyunsaturated fatty acids and neurological developmental outcome at 18 months in healthy term infants. ActaPaediatr 2005;94:26-32.
- Mulder KA, King DJ, Innis SM (2014) Omega-3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. PLoS One 9: e83764.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
| Table 5 | Table 6 | Table 7 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15778
- [From(publication date):
February-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11112
- PDF downloads : 4666
