Effects of Plasmalogen on Patients with Moderate-to-Severe Alzheimer's Disease and Blood Plasmalogen Changes: A Multi-Center, Open-Label Study
Received: 29-Jul-2019 / Accepted Date: 13-Aug-2019 / Published Date: 23-Aug-2019 DOI: 10.4172/2161-0460.1000474
Abstract
Objective: Plasmalogens (Pls) are a special class of glycerophospholipids containing a vinyl ether bond at the sn-1 position. Recently, it has become clear that Pls are closely related to Alzheimer’s disease (AD). Pls levels have been found to be decreased in the brain and blood of patients with AD. We previously reported that Pls could improve cognitive function in AD animal models and in a randomized controlled trial of patients with mild cognitive impairment and mild AD. This study aimed to investigate the effect of Pls on patients with moderate-to-severe AD in an open-label study.
Methods: Eligible patients were Japanese patients aged 60-85 years who had <20 points of the Mini-Mental State Examination (MMSE) score. They received 1.0 mg or 0.5 mg of scallop-derived Pls per day for 12 weeks. The primary outcome was the MMSE score, and the secondary one was blood concentration of phosphatidylethanolamine Pls (PlsPE).
Results: A total of 157 patients were enrolled, and 142 participants completed the study. The patients showed a statistically significant improvement in the MMSE score after the treatment, and the increase did not differ by treatment dose. Erythrocyte and plasma PlsPE, which were prominently lower than those of normal subjects at baseline, increased significantly after the treatment in the whole patients. While the increase in erythrocyte PlsPE did not significantly differ in the 1.0 mg and 0.5 mg groups, plasma PlsPE increased more markedly in the 0.5 mg group
than in the 1.0 mg group (P=0.001). The change in erythrocyte PlsPE, but not the change in plasma PlsPE, showed a modest degree of correlation with the change in MMSE score (Pearson’s r=0.20, P=0.01).
Conclusion: These findings suggest that orally administered scallop-derived Pls improve cognitive function and that the measurement of blood Pls is valuable to assess the severity and treatment progress in patients with moderate-to-severe AD.
Keywords: Plasmalogen; Scallop; Alzheimer’s disease; Moderate-tosevere AD; Cognitive function; Biomarker of severity; Mini-mental state examination
Introduction
A marked global increase in the number of patients with cognitive impairment, especially Alzheimer’s disease (AD), has been identified as the highest priority to tackle among problems associated with the ageing population [1]. However, the measures thus far taken are quite insufficient to control AD in communities and individuals, and effective therapeutic and preventive measures against AD remain to be developed urgently. A well accepted hypothesis is that the pathological process of AD leading to cognitive decline is initiated by the accumulation of β-amyloid plaques and tau protein tangles in the brain, and pharmaceutical agents targeting the amyloid formation and clearance have been tested in the past decade [2]. However, clinical trials have been unsuccessful in demonstrating efficacy of these novel candidates [2,3].
Recent research has suggested a potential efficacy of plasmalogen (Pls), a special class of phospholipids, in the treatment of AD, as noted in recent reviews [4-7]. Pls levels have been found to be decreased in the postmortem AD brain and in the blood of AD patients [8- 15]. Pls are found in the cell membrane of many mammalian tissues, especially of brain, heart, skeletal muscle, leukocytes, and sperm. Phosphatidylethanolamine Pls (PlsPE) are abundantly found in the brain while Phosphatidylcholine Pls (PlsPC) are abundant in the heart. Among the various functions of Pls, special attention is focused on the antioxidant and anti-neuroinflammatory properties that are linked to the chemical structure of Pls characterized by the vinyl ether bond at the sn-1 position of glycerol backbone. Other well-known properties include ion transport, membrane fusion, cholesterol efflux, and precursor of biologically active substances. These properties are all vital to maintain life [4-7].
We developed a simple method to extract large amounts of Pls from animals, and have accelerated research on Pls treatment and AD [16]. Our studies in animal models demonstrated that Pls reduced β-amyloid accumulation and improved cognitive and memory functions by suppressing neuroinflammation [17-19]. We further conducted a placebo-controlled trial, in which Pls were orally administered to patients with Mild Cognitive Impairment (MCI) or mild AD for a period of 24 weeks [20,21]. Pls treatment improved memory in AD patients and also improved spatial orientation in MCI patients [20,21]. In the present open-label study, we investigated the effect of a 12-week treatment with Pls (1.0 mg or 0.5 mg per day) on cognitive function in patients with moderate-to-severe AD.
Methods
Study design and participants
The present study was a multi-center open-label study to examine the effect of scallop-derived Pls (0·5 mg or 1·0 mg per day) on cognitive function in patients with moderate-to-severe AD. The study period lasted for 12 weeks. The severity of cognitive impairment was determined by study physicians in accordance with the Fifth Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V).
Eligible patients were male or female Japanese patients aged 60- 85 years with moderate-to-severe AD; they had a score of less than 20 on the Mini-Mental State Examination (MMSE); they did not have vascular dementia or other type of dementia as confirmed by a CT scan or MRI taken within the previous six months; and they were on a stable dosage for at least three months prior to enrollment if they were taking anti-dementia drugs. All caregivers were required to accompany participants at all visits throughout the period of study and to offer information about participants’ daily life and health status. Participants were excluded if they had an allergy to scallops, raw materials of test substance. Written informed consent was obtained from either participants or their caregivers. The study was approved by the Institutional Review Boards of BOOCS Clinic Fukuoka (Fukuoka, Japan), and was performed in compliance with the Declaration of Helsinki.
Procedures
Choice of Pls dose (1·0 or 0·5 mg per day) was arbitrarily decided by study physicians, but they were requested to adjust the dose selection so as to equalize the number of patients in the two groups as much as possible at each study site.
Enrolled participants were instructed to ingest the test substance (0.5 mg of Pls per one portion) once or twice per day for 12 weeks, and received an amount of the test substance required until the next visit. They were also provided with an extra amount of the test substance for one-month use in case that they should miss the next scheduled visit. Unconsumed test substance was returned at each visit for evaluation of adherence to the treatment. Any complications and adverse events were recorded at study sites on the basis of participants’ report at each visit. Each study physician also judged the causal relationship of an adverse event with treatment by indicating one of the three categories (unrelated, possibly related, and related). Adverse events were classified in accordance with the CTCAE version 5.0 (U.S. Department of Health and Human Services, 2017).
Outcomes
The primary outcome was change in the MMSE score, which ranges from 0 to 30 with lower scores representing poorer cognitive function. The secondary outcomes were the levels of PlsPE in erythrocyte and plasma. Cognitive function was evaluated at baseline and weeks 4, 8, and 12. Participants had fasting blood drawn at baseline and week 12 for the measurement of erythrocyte PlsPE and plasma PlsPE. Erythrocyte PlsPE levels were expressed as weight percentages of total phospholipids. Measurement of PlsPE was carried out with the use of the method previously reported [16,22]. Safety was assessed by reported adverse events, physical examination, and, if needed, biochemical blood tests including liver function, renal function, blood sugar, and lipid levels.
Statistical analysis
Post-treatment change in the MMSE score and Pls levels was statistically assessed by paired t-test. Data were presented as mean change from baseline and 95% Confidence Interval (CI). The betweendose difference in means was assessed by unpaired t-test. Chi-square test was used for assessment of the between-group difference in proportions. Post-treatment change in the MMSE score was also evaluated categorically. The degree of improvement in the MMSE score was classified into four categories: remarkable improvement (ΔMMSE ≥ 4 points), improvement (ΔMMSE 2 to 3 points), no change (ΔMMSE ± 1 point), and worsening (ΔMMSE ≤−2 points). The relation between post-treatment change in blood Pls and change in MMSE at 12 weeks was assessed by Pearson’s correlation with graphical inspection. Statistical significance was declared if two-sided P was <0.05. The study is registered at the University Hospital Medical Information Network as ID UMIN000016008.
Results
Study participants
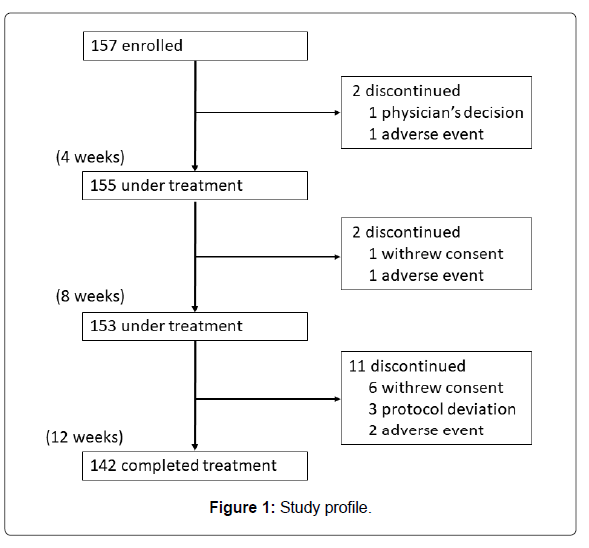
A total of 157 participants were registered at 23 hospitals or clinics located in Kanto and Kyushu regions in Japan from February 19, 2015 to December 25, 2015. Those who completed the 12-week treatment numbered 142. The number of patients ingesting Pls 1.0 mg/day was almost equal to that of those ingesting 0.5 mg/day (74 versus 68). They ingested 80% or more of the provided test substance. Figure 1 shows the study profile. Baseline characteristics are summarized in Table 1.
| Variable | All | Dose per day | Pvalue* | |
|---|---|---|---|---|
| 1.0 mg | 0.5 mg | |||
| Number of patients | n = 142 | n = 74 | n = 68 | |
| Male, n (%) | 54 (38.0) | 32 (43.2%) | 22 (32.4%) | 0.18 |
| Age in year | 77.6 (5.2) | 76.6 (4.9) | 78.5 (5.3) | 0.03 |
| MMSE | 13.2 (5.1) | 13.4 (5.0) | 13.0 (5.2) | 0.66 |
| Erythrocyte PlsPE (%) | 7.88 (0.89) | 7.90 (0.96) | 7.86 (0.80) | 0.78 |
| Plasma PlsPE (mg/dl)† | 2.96 (1.23) | 3.10 (1.20) | 2.83 (1.25) | 0.22 |
Values are mean (SD) unless otherwise specified.
MMSE= Mini-Mental State Examination.
PlsPE= phosphatidylethanolamine plasmalogen.
*†Unpaired t-test for mean and chi-square test for proportions in the between-dose comparison. †Number of patients: n=62 in the 1.0 mg group and n=63 in the 0.5 mg group.
Table 1: Baseline characteristics of participants.
Post-treatment changes in MMSE score and blood PlsPE
Table 2 shows changes from baseline in MMSE score and PlsPE levels by treatment dose as well as in the whole patients. Overall, the MMSE score improved by 1·82 points, and the increase was highly statistically significant (P<10−10). The MMSE score was also highly significantly increased in the 1.0-mg group (P<10−4) and 0.5-mg group (P<10−6). The increase seemed to be greater in the 0.5-mg group, but the between-group difference was far from the statistical significance (P=0.12). The item-specific analysis showed a significant improvement with respect to orientation to time, orientation to place, three-word registration, attention and calculation, and three-word recall in the whole subjects. None of these changes did not significantly differ by treatment dose.
| Variable | All | (n = 142) | 1.0 mg | (n=74) | 0.5 mg | (n=68) | Pvalue* |
|---|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | ||
| MMSE (total score) | 1.82 | (1.32; 2.33) | 1.45 | (0.77; 2.12) | 2.24 | (1.48; 2.99) | 0.12 |
| MMSE (item-specific) | |||||||
| 1. Orientation to time | 0.42 | (0.22; 0.62) | 0.38 | (0.10; 0.66) | 0.47 | (0.18; 0.76) | 0.65 |
| 2. Orientation to place | 0.44 | (0.24; 0.64) | 0.35 | (0.08; 0.62) | 0.54 | (0.24; 0.84) | 0.34 |
| 3. Three-word registration | 0.1 | (0.00; 0.20) | 0.07 | (-0.09; 0.22) | 0.13 | (0.01; 0.25) | 0.51 |
| 4. Attention and calculation | 0.31 | (0.11; 0.51) | 0.22 | (-0.07; 0.50) | 0.41 | (0.12; 0.71) | 0.34 |
| 5. Three-word recall | 0.36 | (0.18; 0.54) | 0.28 | (0.01; 0.56) | 0.44 | (0.21; 0.67) | 0.38 |
| 6. Language (naming) | 0.06 | (-0.04; 0.15) | 0 | (-0.13; 0.13) | 0.12 | (-0.01; 0.25) | 0.21 |
| 7. Language (repeating) | 0.04 | (-0.04; 0.11) | 0.07 | (-0.02; 0.16) | 0 | (-0.12; 0.12) | 0.36 |
| 8. Language (3-step command) | 0 | (-0.11; 0.11) | 0.03 | (-0.14; 0.20) | -0.03 | (-0.17; 0.11) | 0.61 |
| 9. Language (reading) | 0.05 | (-0.00; 0.10) | 0.03 | (-0.03; 0.08) | 0.07 | (-0.02; 0.17) | 0.39 |
| 10. Language (writing) | 0.01 | (-0.05; 0.07) | -0.01 | (-0.10; 0.07) | 0.04 | (-0.04; 0.13) | 0.34 |
| 11. Visual construction | 0.04 | (-0.03; 0.11) | 0.04 | (-0.05; 0.14) | 0.03 | (-0.08; 0.14) | 0.87 |
| Erythrocyte PlsPE (%)† | 0.22 | (0.09; 0.35) | 0.15 | (-0.02; 0.33) | 0.29 | (0.11; 0.47) | 0.28 |
| Plasma PlsPE (mg/dl)‡ | 0.7 | (0.48; 0.92) | 0.32 | (0.06; 0.59) | 1.07 | (0.74; 1.40) | 0.001 |
MMSE= Mini-Mental State Examination.
PlsPE= phosphatidylethanolamine plasmalogen.
*Unpaired t-test for difference between 1.0 mg and 0.5 mg groups.
†Number of patients: n= 67 in the 0.5 mg group.
‡Number of patients: n= 62 in the 1.0 mg group and n= 63 in the 0.5 mg group.
Table 2: Mean changes at 12 weeks from baseline in MMSE score and blood plasmalogen levels by treatment
CI = confidence interval.
Both erythrocyte and plasma levels of PlsPE increased significantly in the whole patients (P=0.001 for erythrocyte PlsPE and P<10−8 for plasma PlsPE). While the increase in erythrocyte PlsPE did not significantly differ in the 1.0-mg and 0.5-mg groups, plasma PlsPE increased more markedly in the 0.5-mg group than in the 1.0-mg group (P=0.001).
Categorical assessment of post-treatment change in MMSE
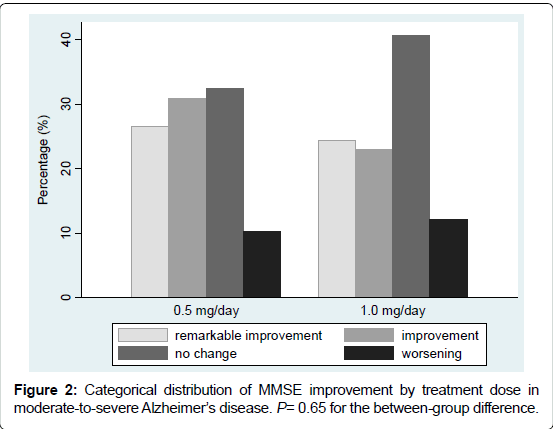
The post-treatment MMSE score improved in more than half of the patients. Numbers of the patients according to improvement categories were: remarkable improvement 36 (25.4%), improvement 38 (26.8%), no change 52 (36.6%), and worsening 16 (11.3%). The distribution of improvement categories did not differ by dose group (P=0.65), as illustrated in Figure 2.
Relation of changes in blood PlsPE to change in MMSE
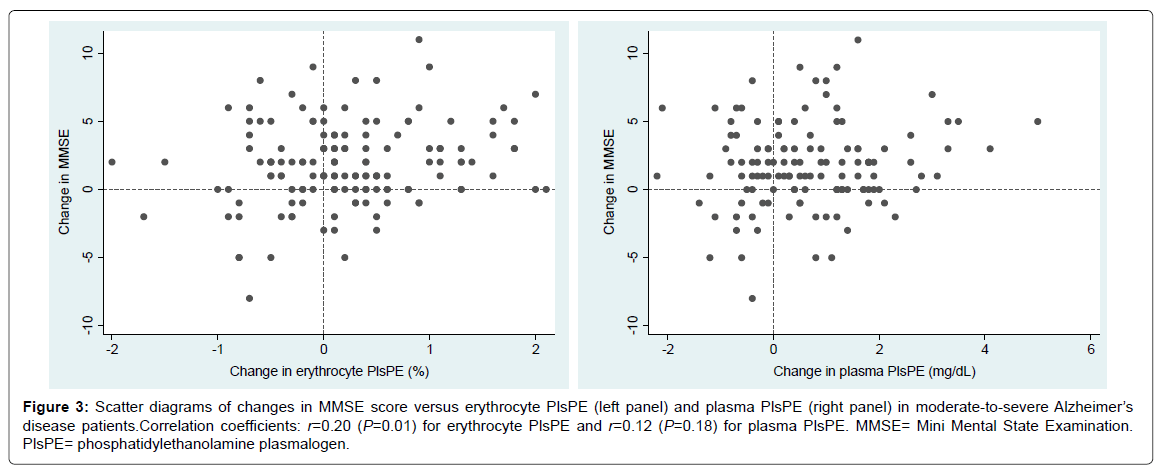
The change in erythrocyte PlsPE, but not the change in plasma PlsPE, showed a modest degree of correlation with the change in MMSE score (Figure 3). Pearson’s correlation coefficients were 0.20 (P = 0.01) for erythrocyte PlsPE and 0.12 (P = 0.18) for plasma PlsPE.
Figure 3:Scatter diagrams of changes in MMSE score versus erythrocyte PlsPE (left panel) and plasma PlsPE (right panel) in moderate-to-severe Alzheimer’s disease patients.Correlation coefficients: r=0.20 (P=0.01) for erythrocyte PlsPE and r=0.12 (P=0.18) for plasma PlsPE. MMSE= Mini Mental State Examination. PlsPE= phosphatidylethanolamine plasmalogen.
Clinical safety
Observed adverse events are listed in Table 3. Out of 157 participants enrolled in the study, 22 patients reported 23 adverse events. None of the reported adverse events was unrelated to the Pls treatment except for one episode, in which manic state was reported to be possibly related to Pls ingestion.
| Adverse event | Number of episode |
|---|---|
| Blood and lymphatic system disorders | |
| Anemia | 1 |
| Eye disorders | |
| Cataract | 1 |
| Vision decreased* | 1 |
| Gastrointestinal disorders | |
| Nausea | 1 |
| General disorders and administration site conditions | |
| Edema limbs | 1 |
| Malaise | 1 |
| Infections and infestations | |
| Upper respiratory infection | 2 |
| Injury, poisoning and procedural complications | |
| Bruising | 1 |
| Fracture | 3 |
| Metabolism and nutrition disorders | |
| Anorexia | 2 |
| Musculoskeletal and connective tissue disorders | |
| Muscle cramp | 1 |
| Nervous system disorders | |
| Intracranial hemorrhage* | 1 |
| Psychiatric disorders | |
| Agitation† | 1 |
| Sundown syndrome†‡ | 1 |
| Manic state* | 1 |
| Renal and urinary disorders | |
| Urinary frequency | 1 |
| Respiratory, thoracic and mediastinal disorders | |
| Pneumonitis* | 1 |
| Skin and subcutaneous tissue disorders | |
| Eczema | 1 |
| Pruritus | 1 |
*Treatment was discontinued after the adverse event
†Two episodes were recorded on an identical patient at the same time
‡Not listed in the CTCAE version 5.0
Table 3: Summary of adverse events.
Discussion and Conclusion
The present study showed that Pls ingestion resulted in an evident improvement in cognitive function as measured by MMSE in moderateto- severe AD patients regardless of ingested dose (1.0 mg or 0.5 mg per day). The findings add to evidence that Pls are beneficial in AD patients [20,21].
The present study did not set a control (placebo) group, thereby causing a concern that the observed beneficial effect may have been ascribed to a placebo effect. It is, however, conjectured that placebo effect is unlikely to appear in moderate-to-severe AD in the same way as observed in general physical illnesses and symptoms. Patients with moderate-to-severe AD are considered to be less likely to develop the concepts of expectation and hope due to impaired cognitive function under the premise that placebo effect is produced by patients’ expectation and hope [23,24].
To address the magnitude of placebo effect, we retrieved randomizedcontrolled trials of Japanese patients with moderate and severe AD by using PubMed and CiNii database. Studies of Japanese patients only were selected so as to eliminate ethnicity-related bias [25-27]. The placebo group in each study consistently exhibited a deterioration in cognitive function as measured by the Severe Impairment Battery and Alzheimer’s Disease Assessment Scale-cognitive subscale in the 12-week period of treatment [25-27]. One of these studies measured MMSE and reported a decrease of 0.3 points at 24 weeks of placebo treatment [26]. While there is no direct evidence that placebo does not improve MMSE score at 12 weeks of treatment, placebo effect, if any, is unlikely to convert the natural course of deterioration in the MMSE score in AD patients [28,29]. It is notable that a quarter of the patients exhibited a remarkable improvement in cognitive function (increase of ≥ 4 points in MMSE). Furthermore, the positive correlation between the post-treatment change in erythrocyte PlsPE and the change in MMSE was an important finding to support the effect of Pls improving cognitive function.
A clear improvement in MMSE was observed in both of the 1.0- mg and 0.5-mg groups, and no measurable difference was observed between the two groups. It is very interestingly that very small amounts of Pls had a favorable effect on cognitive function. Recent studies have identified the counterpart of Pls among orphan G protein-coupled receptors on neuronal membrane, suggesting that Pls are a hormonelike substance [19,30,31]. This functional property may be a possible reason for Pls of small amounts such as 1.0 mg or 0.5 mg per day exerting an overt effect of improving cognitive function.
It is also interesting that improvement was more remarkable in moderate-to-severe AD than in mild AD and MCI with the same dosage of Pls. In a previously reported study, patients with mild AD and MCI showed a small increase of 0.40 points in the MMSE score (95% CI 0.04–0.80 points) after a 12-week of treatment with Pls of 1.0 mg/day [20]. It could be possible that patients with moderate-to-severe AD have relatively less normal brain tissue and more damaged tissue due to brain atrophy than those with mild AD and MCI, and therefore the former may demand and consume less hormone-like substance, Pls, than the latter. In addition, the brain activity of mild AD and MCI is almost as high as that of healthy individuals, and thus consumes as much Pls. On the other hand, the brain activity of moderate-to-severe AD is lower and consumes less Pls than that of healthy individuals. This hypothetical reasoning leads to a conclusion that even a very small amount of Pls is effective for moderate-to-severe AD. Conversely, if a larger amount of Pls (i.e., 2–3 mg per day) is administered to mild AD and MCI, the same effect as moderate-to-severe AD might be attained. Further studies concerning the dose-response effect are needed to determine the influence of severity.
Post-treatment Pls levels increased by 0.22% in erythrocytes and 0.70 mg/dL in plasma. It may be of interest how low the Pls levels at baseline were in comparison with the levels of normal subjects and to what extent the post-treatment Pls approached to the normal levels. In our unpublished data of 39 elderly aged 65-86 years with normal cognitive function (MMSE 29-30), the mean values of erythrocyte and plasma Pls were 8.56% (SD 0.94%) and 4.27 mg/dL (SD 1.07 mg/ dL), respectively. At baseline, erythrocyte Pls were relatively 8% lower (P<10−4) and plasma Pls were 31% lower (P<10−7) as compared with the respective values of the normal elderly. The post-treatment erythrocyte and plasma Pls still remained to be 5% lower (P=0.006) and 14% lower (P=0.03), respectively. These findings suggest that the measurement of blood Pls is valuable to assess the severity and treatment progress in AD. It remains to be confirmed whether higher dosages of Pls can result in further increases in blood Pls levels nearer to the normal levels.
Plasma PlsPE increased more markedly after treatment in the 0.5 mg group than in the 1.0 mg group while erythrocyte PlsPE did not show such a between-dose difference. We have no clear explanation for this rather puzzling finding. The finding might be a chance because plasma PlsPE at baseline were slightly lower in the 0.5 mg group. Alternatively, there might be an unknown reason related to the interplay between erythrocyte and plasma Pls. Erythrocyte and plasma Pls were correlated with each other to some extent: Pearson’s correlation coefficients were 0.33 for baseline values and 0.31 for post-treatment change. Further research is needed to clarify the effects of Pls dose on erythrocyte and plasma levels of Pls.
This study has some problems and limitations including a short duration of Pls administration in addition to being open-labelled study. A randomized controlled trial of a longer period in patients with moderate-to-severe AD is highly warranted in the near future.
Funding Sources
The study was funded by The Japanese Plasmalogen Society (Pls2014-02) (Fukuoka, Japan) as an investigator-initiated trial.
Conflict of Interest
All authors declare that they have no conflicts of interest. All authors have approved the final article.
Acknowledgments
The authors thank all patients and caregivers who took part in the study. The following contributed to implementation of the study as study physicians: A Shirakawa (Shirakawa Hospital, Usuki), E Nishikawa (Nishikawa-naika Clinic, Shimonoseki), F Yoshida (Yoshida Clinic, Usuki), H Fujino (Fujino Clinic, Yanagawa), H Kondo (Tohwa Hospital, Kitakyushu), J Miyagi (Miyagi Neurosurgery Clinic, Fukuoka), K Nishiura (Hakata Gion Mental Clinic, Fukuoka), K Saito (BOOCS Clinic Fukuoka, Fukuoka), K Yasumatsu (Fukuoka Megumi Hospital, Koga), M Ichimaru (BOOCS Holistic Clinic Tokyo, Tokyo), M Kureshima (Kitakyushu General Hospital, Kitakyushu), M Munaka (Hayama Clinic, Munakata), N Takehara (Atagohama Family Clinic, Fukuoka), S Hosokawa (Hosokawa Naika-Shinkeinaika Clinic, Kitakyushu), S Miyoshi (Asagi Hospital, Onga), S Mori (Mori Clinic of Internal Medicine & Neurology, Itoshima), S Nakamura (Sangenjaya Nakamura Mental Clinic, Tokyo), T Fujino (Fujino Cardiovascular & Internal Medicine Clinic, Usuki), T Kaneko (Kaneko Hospital, Yanagawa), T Nomura (Nomura Naika-Shinkeinaika Clinic, Fukuoka), T Yoshimatsu (Mito Hospital, Kasuya), Y Ikushima (Ikushima Clinic, Yanagawa), Y Sekine (Sekine Clinic, Hirakata). The authors are grateful to Ms. Chizuko Kanemaru for her support in preparing the manuscript.
References
- World Alzheimer Report 2018. The state of the art of dementia research: New frontiers. Alzheimer’s Disease International, London.
- Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8: 595-608.
- Anderson RM, Hadjichrysanthou C, Evans S, Wong MM (2017) Why do so many clinical trials of therapies for Alzheimer's disease fail? Lancet 390: 2327-2329.
- Farooqui AA, Horrocks LA (2001) Plasmalogens: workhorse lipids of membranes in normal and injured neurons and glia. Neuroscientist 7: 232-245.
- Su XQ, Wang J, Sinclair AJ (2019) Plasmalogens and Alzheimer's disease: a review. Lipids Health Dis 18: 100.
- Paul S, Lancaster GI, Meikle PJ (2019) Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog Lipid Res 74: 186-195.
- Braverman NE, Moser AB (2012) Functions and biosynthesis of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822: 1442-1452.
- Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL (1995) Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer's disease brain. Brain Res 698: 223–226.
- Guan Z, Wang Y, Cairns NJ, Lantos PL, Dallner G, et al. (1999) Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J Neuropathol Exp Neurol 58: 740–747.
- Han X, Holtzman DM, McKeel DW Jr (2001) Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem 77: 1168–1180.
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, et al. (2007) Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res 48: 2485–2498.
- Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, et al. (2010) Circulating plasmalogen levels and Alzheimer disease assessment scale-cognitive scores in Alzheimer patients. J Psychiat Neurosci 35: 59–62.
- Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL (2015) Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects. Acta Neuropsychiatr 7: 270–278.
- Oma S, Mawatari S, Saito K, Wakana C, Tsuboi Y, et al. (2012) Changes in phospholipid composition of erythrocyte membrane in Alzheimer's disease. Dement Geriatr Cogn Disord Extra 2: 298–303.
- Yamashita S, Kiko T, Fujiwara H, Hashimoto M, Nakagawa K, et al. (2015) Alterations in the levels of amyloid-ß, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer's disease: possible interactions between amyloid-ß and these lipids. J Alzheimers Dis 50: 527–537.
- Mawatari S, Okuma Y, Fujino T (2007) Separation of intact plasmalogens and all other phospholipids by a single run of high-performance liquid chromatography. Anal Biochem 370: 54–59.
- Katafuchi T, Ifuku M, Mawatari S, Noda M, Miake K, et al. (2012) Effects of plasmalogens on systemic lipopolysaccharide-induced glial activation and ß-amyloid accumulation in adult mice. Ann NY Acad Sci 1262: 85–92.
- Hossain MS, Ifuku M, Take S, Kawamura J, Miake K, et al. (2013) Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS One 8: e83508.
- Hossain MS, Mineno K, Katafuchi T (2016) Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling. PLoS One 11: e0150846.
- Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, et al. (2017) Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer's Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. E Bio Medicine 17: 199-205.
- Fujino T, Yamada T, Asada T, Ichimaru M, Tsuboi Y, et al. (2018) Effects of Plasmalogen on Patients with Mild Cognitive Impairment: A Randomized, Placebo-Controlled Trial in Japan. J Alzheimers Dis Parkinsonism 8: 1-5.
- Mawatari S, Hazeyama S, Fujino T (2016) Measurement of ether phospholipids in human plasma with HPLC–ELSD and LC/ESI–MS after hydrolysis of plasma with phospholipase A1. Lipids 51: 997–1006.
- Benedetti F (2013) Placebo and the new physiology of the doctor-patient relationship. Physiol Rev 93: 1207–1246.
- Kirsch I (1985) Response expectancy as determinant of experience and behavior. Am Psychologist 40: 1189–1202.
- Homma A, Imai Y, Tago H, Asada T, Shigeta M, et al. (2008) Donepezil Treatment of Patients with Severe Alzheimer’s Disease in a Japanese Population:Results from a 24-Week, Double-Blind, Placebo-Controlled, Randomized Trial. Dement Geriatr Cogn Disord 25: 399–407.
- Nakamura Y, Imai Y, Shigeta M, Graf A, Shirahase T, et al. (2011) A 24-Week, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of the Rivastigmine Patch in Japanese Patients with Alzheimer’s Disease. Dement Geriatr Cogn Dis Extra 1: 163–179.
- Nakamura Y, Kitamura S, Homma A, Shiosakai K, Matsui D (2014) Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer’s disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan. Expert Opin Pharmacother 15: 913–925.
- Mendiondo MS, Ashford JW, Kryscio RJ, Schmitt FA (2000) Modelling mini mental state examination changes in Alzheimer's disease. Stat Med 19: 1607–1616.
- Kavanagh S, Van Baelen B, Schäuble B (2011) Long-term effects of galantamine on cognitive function in Alzheimer's disease: a large-scale international retrospective study. J Alzheimers Dis 27: 521–530.
- New DC, Wong YH (2007) Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J Mol Signal 2: 2.
- Pike LJ, Han X, Chung KN, Gross RW (2002) Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088.
Citation: Fujino T, Yamada T, Mawatari S, Shinfuku N, Tsuboi Y, et al. (2019) Effects of Plasmalogen on Patients with Moderate to Severe Alzheimer’s disease and Blood Plasmalogen Changes: A Multi-Center, Open-Label Study. J Alzheimers Dis Parkinsonism 9: 474. DOI: 10.4172/2161-0460.1000474
Copyright: © 2019 Fujino T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7932
- [From(publication date): 0-2019 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 6918
- PDF downloads: 1014