Research Article Open Access
Effects of Perinatal 2,3,7,8-Tetrachlorodibenzo-P-Dioxin Exposure on Development of Taste Preference in Rat Offspring
Nishijo M1*, Tran NN1, Nakagawa H1, Hori E2, Torii K3, Takashi K3 and Nishijo H21Department of Public Health, Kanazawa Medical University, Uchinada, 920-0293, Japan
2System Emotional Science, Graduate School of Medicine, University of Toyama, Toyama, 930-0194, Japan
3Frontier Research Laboratories, Institute for innovation, Ajinomoto Co., Inc., Kawasaki, 210-8681, Japan
- *Corresponding Author:
-
Nishijo M
Department of Public Health, Kanazawa Medical University
1-1, Daigaku, Uchinada, Ishikawa 920-0293, Japan
Tel: +81-76-286-2211
Fax: +81-76-286-3728
E-mail: ni-koei@kanazawa-med.ac.jp
Received date: December 30, 2013; Accepted date: February 07, 2014; Published date: February 14, 2014
Citation: Nishijo M, Tran NN, Nakagawa H, Hori E, Torii K, et al. (2014) Effects of Perinatal 2,3,7,8-Tetrachlorodibenzo-P-Dioxin Exposure on Development of Taste Preference in Rat Offspring. J Addict Res Ther 5:173. doi:10.4172/2155-6105.1000173
Copyright: © 2014 Nishijo M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Perinatal 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) exposure affects various motivated behaviors controlled by limbic system of the brain in offspring. In this study, effects of perinatal TCDD exposure on taste preference were investigated in the rat offspring whose dams were orally exposed to TCDD on the 15 gestational days. After weaning, offspring were given free access to six amino acid solutions with different tastes (sweet, umami, or bitter), saline (salty taste), and distilled water in a choice paradigm. In the TCDD exposed males, daily food intake was significantly decreased on Postnatal Day (P.D.) 33, whereas no differences of total fluid and any kind of solution intake were found between TCDD exposure and control groups in males. In the exposed females, daily intake of MSG was significantly lower on P.D. 32-34 respectively. Moreover, mean MSG intake rates during P.D. 29-35 was significantly decreased in TCDD exposure females. These results suggest that the TCDD-exposed females need less protein than the control females because of poorer growth than the control females, because MSG has “umami” taste, which reflects preference protein intake. In contrast, daily histidine intake on P.D. 34 was significantly increased in TCDD exposed females, and mean lysine intake rate during PND 29-35 was significantly higher than that of control group. These results suggest that rats preferred bitter taste of histidine and lysine solutions under the chemical stress of TCDD exposure. In conclusion, perinatal TCDD exposure affected development of taste preference of offspring female rats, and more study to clarify neural mechanisms is necessary in future.
Keywords
Dioxin; Offspring; Rat; Taste; Amino acids; Preference; Perinatal exposure
Introduction
2,3,7,8-Tetrachlorodibenzo-P-Dioxin (TCDD) is a highly toxic man-made chemical found ubiquitously in the environment. Maternal exposure to TCDD during pregnancy has been reported to induce deficits in higher brain functions of off-spring, such as working memory in a radial arm maze, operant responding in running wheels or two-lever chamber, and discrimination reversal learning [1-4]. We also reported that perinatal TCDD exposure influenced emotional learning, such as active avoidance learning, in young offspring rats [5]. Recently, Nguyen et al. investigated the effects of prenatal TCDD exposure on socio-emotional behaviors in offspring rats and found TCDD exposure induced social-interaction deficits in the male rats, and hyperactivity in female rats [6].
Male reproductive behavior is organized during sexual differentiation of the brain in the perinatal period, and it is suspected that TCDD exposure in utero may affect brain development, because of its endocrine effects. Other sexually demographic behavior such as play preference of sweet solution is also organized via hormonal influence. Amin et al. reported that perinatal TCDD and dioxin-like PCBs exposure altered expression of saccharin preference behavior in adult female rat offspring [7]. Decreased sweet preference was also observed in female rat offspring exposed to non-dioxin-like PCBs during perinatal period [8].
Amino acids preference can be altered by amino acid composition and quantity of protein in diet to maintain adequate protein intake for life. However, previous studies have suggested that not only dietary change but also central nervous system, especially Lateral Hypothalamic Area (LHA) could regulate amino acid intake [9,10]. Kondoh et al. reported that histidine preference was increased during specific Alteration of Rhythm of Environmental Temperature (SART) stress, in which spontaneous neuron activity was higher in the LHA, a feeding center [11]. However, effects of prenatal TCDD exposure on taste preference for sapid stimuli such as amino acids have not studied.
In the present study, we compared the ingestive behaviors for amino acid solutions between TCDD exposed and control offspring to investigate the influence of prenatal TCDD exposure on taste preference in rat offspring.
Materials and Methods
Dams and TCDD exposure during pregnancy
Seven virgin Wistar female rats aged 10 weeks old (body weight: 190-224 g) were mated with male rats of same species. The following day, their conception was confirmed by detecting vaginal plugs or sperm in their smears, and that day was denoted Gestational Day (GD) 0. On the GD 15, pregnant rats were assigned to the TCDD exposed group (n=4, mean ± standard deviation of body weight (BW)=279.4 ± 16.3 g) and control (n=3, 280.2 ± 5.0 g) group. The appropriate volume (1.3-1.5 ml) based on body weight of TCDD solution dissolved in corn-oil to be 1.0 μg/ml, was administrated to the dams of the TCDD exposed (1.0 μg/kg BW) group, by single intragastric injection using an oral cannula. The control group received only corn-oil in same way. This protocol of TCDD exposure, a single oral dose of 1.0 μg/kg b.w. on GD 15, has been confirmed in previous studies to increase level of pup’s brain tissue at low level and to cause adverse developmental effect on endocrinal function in the brain[12]. We also have applied this protocol for Wister rats and reported adverse neurodevelopmental effects in our previous studies [5,6].
All experimental procedures and experimental design were approved by Ethical Review Board at Kanazawa Medical University.
Offspring and Procedure of preference test
Both the exposed and control off-springs were fed with ad libitum access of maternal breast milk after birth, and weaned at the 21 day after birth (P.D. 21). After weaning, each offspring rats of TCDD exposed group (16 males and 14 females) and control group (17 males and 12 females) was housed individually in a plastic cage and free access to food as well as seven chemical solutions (0.15 M NaCl, 0.4 M threonine, 0.5 M glycine, 0.15 M monosodium glutamate (MSG), 0.2 M lysine HCl, 0.05 M histidine, 0.05 M arginine) and distilled water in a choice paradigm for 2 weeks from P.D. 22 to P.D. 35. These six amino acids were used, since these chemical structures are similar, but their tastes are different; NaCl for salty taste, threonine and glycine for sweet taste, MSG for umami taste, and lysine, histidine, and arginine for bitter taste. NaCl was used for control solution of MSG, because of MSG has salty taste, too. This set of these solutions were showed their usefulness to test taste preference in previous reports, and the concentration of solutions used in the present study were reported to be most preferred when rats were fed a 5% purified whole-egg protein diet [10,11,13]. Offspring rats were housed in a room where temperature (24 ± 1°C), relative humidity (55 ± 10%) and light (8 a.m-8 p.m.) were controlled. Daily intake (ml) of each solution and food intake and body weight was measured at 5-6 p.m. every day. After recording daily intakes, the location of each solution cylinder was randomly changed every day to avoid the animal’s choice of place.
Statistical analysis
The statistical package SPSS (ver.11.0) was used for statistical analysis. The differences in the daily intakes of food, total fluid, and each solution were examined between dioxin exposure and control groups by gender using student t test. Means of weekly fluid intake rate (each solution intake/total fluid intake) for each solution in each gender were compared by 2-way ANOVA with 2 fixed factors; TCDD exposure groups and early and later childhood, P.D. 22-28 and P.D. 29-35. Posthoc test was performed by tests of simple main effect. The statistical criterion was P<0.05.
Results
Body weight and food/fluid intakes
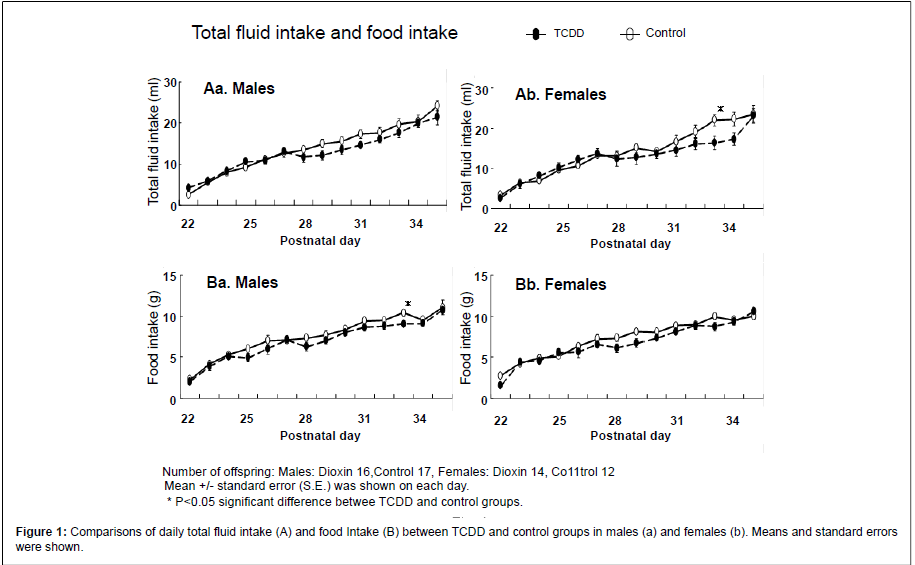
Daily total fluid and food intakes (mean ± standard error; SE) for 2 weeks from P.D. 22 to P.D. 35 were compared between the TCDD-exposed and control groups in each gender (Figure 1). In the males, there was no significant difference in total fluid intake between TCDD exposure and control groups (Aa). However, in the females, total fluid intake was lower in the TCDD-exposed group during P.D. 32-34, with significant decrease on P.D. 33 compared with the control group (Ab). Daily food intake tended to be lower after P.D.28 in the male TCDD-exposed group, and food intake on P.D. 33 was significantly lower than that in the controls (Ba). However, no significant difference in daily food intake was found in the females (Bb). In addition, there was also no significant difference in body weight measured everyday between the TCDD-exposed and control groups in both genders (data not shown).
Daily amino acid solution intakes
Generally, daily consumption of glycine and MSG solutions, around 30-60% of total fluid intake, was higher than that of other solutions. In the males, glycine solution intake was constantly maintained at the highest value in both the TCDD-exposed and control groups without difference between these two groups. In the females, glycine solution intake was decreased after P.D. 28 in both the TCDD-exposed and control groups, but no difference in glycine solution intake was found between these two groups (data not shown).
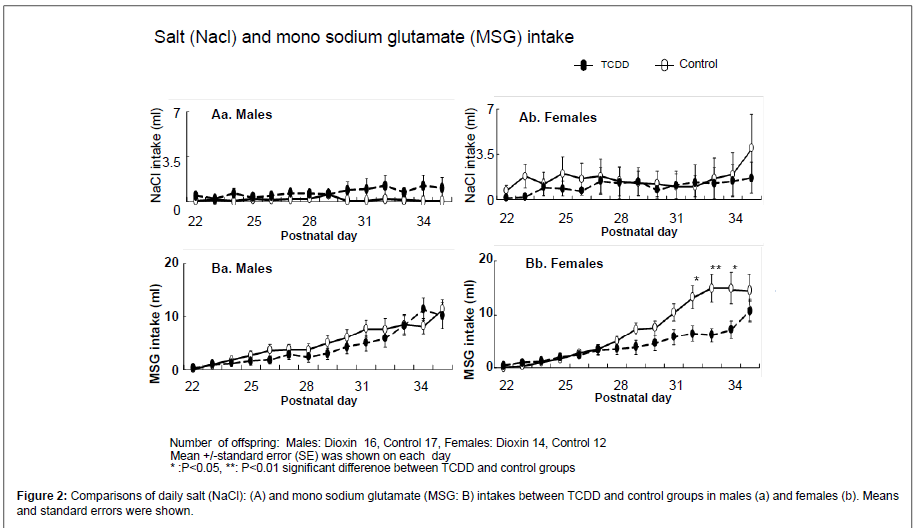
Figure 2 shows daily intakes (mean ± SE) of NaCl and MSG solutions in both genders. In both genders, NaCl solution intakes were relatively constant, and no difference was found between the TCDDexposed and control groups (Aa,b). Although, in the males, daily MSG intake was gradually increased during experimental period with no difference between the TCDD-exposed and control groups (Ba), MSG intake was increased in the control group compared with the TCDDexposed group after P.D. 29 in the females, with significant differences between the two groups on P.D. 32-34 (Bb). On 35th, MSG intake of control females was still high, but no significant difference was observed, because of a little bit increased MSG intake of the TCDDexposed females.
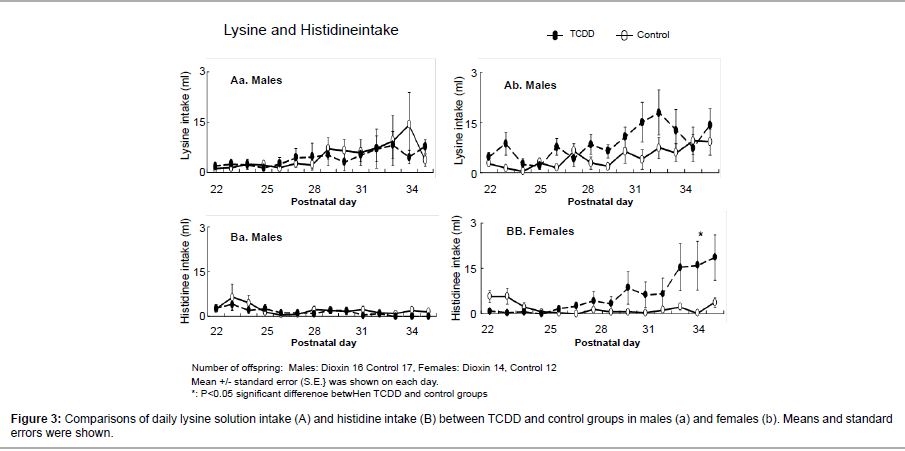
Daily lysine intakes (mean ± SE) tended to increase in the TCDDexposed group more than control group in females after P.D. 28-33 (Figure 3Ab), but there was no significant difference between the TCDD-exposed and control groups. Histidine intake in the males were always little during the experiment, and there was no significant difference between the TDCC-exposed and control groups (Figure 3Aa). However, in females, the histidine intake on P.D. 34 was significantly higher in TCDD-exposed offspring rats than the controls (Figure 3Bb).
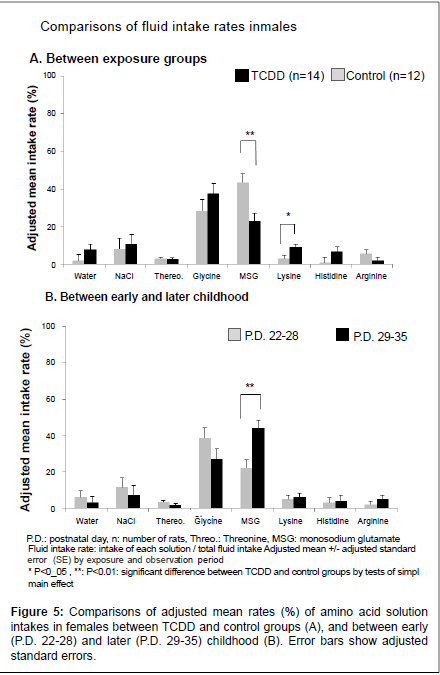
Average amino acid solution intake rates of TCDD and control groups in early and later childhood
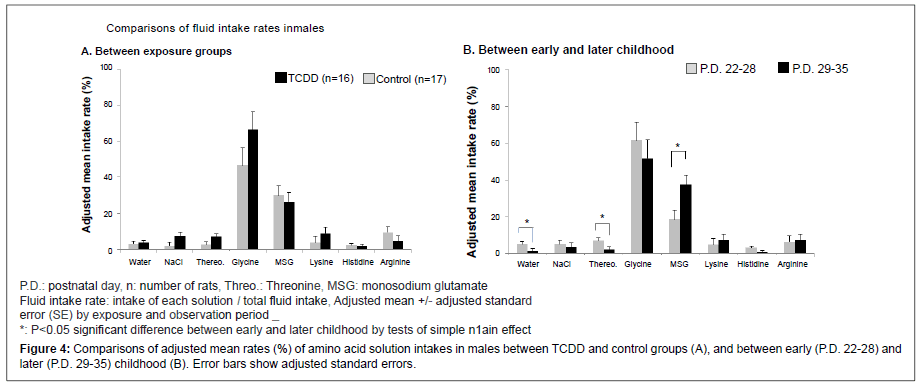
Adjusted mean weekly fluid intake rates of each solution with adjusted SE between TCDD exposed offspring rats and controls by observation periods, early (P.D. 22-28) and later period (P.D. 29-35), are shown in Figure 4A for the males and Figure 5A for the females. Also, adjusted mean weekly intake rates between early and later periods by TCDD exposure groups are shown in Figure 4B for the males and Figure 5B for the females. In the males (Figure 4), there was no difference in any amino acid solution intake rates between the TCDD-exposed and control groups after adjusting two periods (A), but MSG intake rate was significantly increased in later period (B) compared with early period. In the females (Figure 5), MSG intake rate was significantly decreased in the TCDD-exposed group compared with the control group (A). In contrast, lysine intake rate was significantly increased in the TCDDexposed female group (Figure 5A). Histidine intake rate also tended to increase in the TCDD-exposed females, but the difference between the TCDD-exposed and control groups was not statistically significant (Figure 5A). In later childhood MSG, intake rate was significantly increased compared with early period in females (Figure 5B), as well as male offspring rats.
Discussion
Effects of TCDD on taste preference
In the present study, we examined effects of the perinatal TCDD exposure on taste preference in the rat offspring. Effects of perinatal TCDD exposure on taste preference were more evident in the females than the males. Particularly, MSG intake, which increased in later childhood in controls, was not increased, but lysine intake was increased in the TCDD-exposed females.
Intake of highly palatable fluids or foods have been shown to increase during stress in rats, but intake of “salty” or “sweet” fluids or foods has been reported to be unchanged in any forms of stress [14-17]. Consistent with these reports, preference of NaCl (salty taste) and glycine (sweet taste) were not changed in the present study. MSG has not only salty taste but also “umami” taste, which reflects preference protein intake [18]. In the present survey, offspring rats preferred to take MSG in later childhood (P.D. 29-35) compared with that in early childhood (P.D. 22- 28) in both sexes, suggesting that they develop taste preference for their growth and maturity. Particularly, the control female rats preferred to MSG most of all solutions in later childhood; more than glycine which was the most preferring solution in early childhood. However, TCDD-exposed female rats took significantly less amount of MSG solution, although daily MSG intake was gradually increased in later childhood. These results suggest that change of taste preference in the TCDD-exposed females is late than the control females because of late maturity. However, there was no significant difference in body weight measured everyday between the TCDD-exposed and control groups in both genders. These findings suggest that changes in preference of MSG solution in the TCDD-exposed females were not ascribed to changes in body size between the TCDD-exposed and control females. Moreover, maturity starts generally later in males compared with females. This fact might be one reason for gender difference of TCDD exposure effect on taste preference of offspring rats. Because, glycine solution was the most preferred solution for male offspring rats in both TCDD exposed and control groups in all observation periods, albeit their MSG intake was increasing in the later childhood.
Kondoh et al. reported that rats preferred histidine solution (bitter taste) under the SART stress (changes in environmental temperature). Lysine has also bitter taste, and intake of lysine solution tended to increase in the SART stressed rats, consistent with the present study. It has been reported that spontaneous neuronal activity was higher in the LHA (a feeding center) in the SART stressed rats [11]. These findings suggest that the changes in preference might be ascribed to activity changes in the brain areas such as the LHA. However, further neurological studies are necessary to clarify the changes induced TCDD exposure in the brain areas including limbic system.
Decreased sweet preference indicating by increased saccharin solution intake has been reported in female adult rat offspring exposed to TCDD or dioxin-like PCBs or non-dioxin-like PCBs during perinatal period [7,8]. Sweet preference is known as a sexually dimorphic behavior, and these alterations in female offspring were suspected to be caused by anti-estrogenic or anti-androgenic effects of TCDD or PCBs [7,8]. However, in the present survey, no preference difference was found in intakes of glycine and threonine with sweet taste, although alteration of taste preference was dominant in the females compared with the males. Consistent with the present results, allelic variation of Tas1r3 gene, which encodes the T1R3 receptor protein, affected taste responses to saccharin and sucrose, but did not affect responses to threonine and glycine [19]. These results suggest that neural coding of sweet tastes might be heterogeneous, and that TCDD does not generally affect all sweet tastes.
Possible biological mechanisms of dioxin effects
In our previous study using the same protocol of TCDD exposure, perinatal TCDD exposure induced increased Ca2+/calmodulindependent protein kinase IIα (CaMKIIα) activity in the limbic system involved in taste preference and motivation in the female rat offspring [6]. Furthermore, the levels of total(t)-N-methyl-D-aspartate (NMDA) type glutamate receptor subunit 1 (NR1) and phosphorylated(p)- α-amino 3-hydroxy 5-methyl 4-isoxazole propionate (AMPA) type glutamate receptor subunit 1 (GluR1) in the amygdala, and p-GluR1 and p-synapsin 1 (Syn 1) in the hippocampus were significantly increased in the TCDD-exposed females. These results suggest that activity in the pre- and post-synaptic membrane was increased in the limbic system of the TCDD-exposed females. These results are consistent with the idea that synaptic activity as well as CaMKIIα activity was increased in the limbic system of female rat brains in response to TCDD exposure. Furthermore, over activation of CaMKIIα disturbed synaptic plasticity in the limbic system of the rat brain [20]. In addition, it is reported that activity of CaMKII in the brain is modulated by estradiol, and that development of brain regions including the amygdala, hippocampus, and cortical regions with estrogen/androgen receptors and aromatase are medicated by sexual steroid hormones [21-24]. Therefore, alterations in sexual hormones in later childhood (adolescence), which occur earlier in females than males due to perinatal TCDD exposure, might alter CaMKII and synaptic activity in the limbic system, leading to change in taste preference in TCDD exposed offspring.
Conclusions
Perinatal TCDD exposure affected development of taste preference of offspring female rats. More studies using animal offspring are necessary to confirm effects of TCDD exposure on taste preference.
Acknowledgements
Acknowledge any contributions not in the nature of authorship here. The authors thank Dr. Jun-ichiKuriwaki for his valuable advices to experimental methods.
Funding Sources
This work was supported partly by Project research from the Japan Society for the promotion of science (Grant-in-Aid for Scientific Research (B) (25305024) and (25290005), and JSPS (Japan Society for the Promotion of Science) Asian Core Program.
The founders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
References
- Seo BW, Sparks AJ, Medora K, Amin S, Schantz SL (1999) Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). NeurotoxicolTeratol 21: 231-239.
- Markowski VP, Zareba G, Stern S, Cox C, Weiss B (2001) Altered operant responding to motor reinforcement and the determination of benchmark dosed following perinatal exposure to low-level 2,3,7,8-Tetrachlorodibenzo-p-dioxin. Environ Health Perspect 109: 621-627.
- Markowski VP, Cox C, Preston R, Weiss B (2002) Impaired cued delayed alternation behavior in adult rat offspring following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on gestation day 15. NeurotoxicolTeratol 24: 209-218.
- Schantz SL, Bowman RE (1989) Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). NeurotoxicolTeratol 11: 13-19.
- Nishijo M, Kuriwaki J, Hori E, Tawara K, Nakagawa H, et al. (2007) Effects of maternal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on fetal brain growth and motor and behavioral development in offspring rats. ToxicolLett 173: 41-47.
- Nguyen AT, Nishijo M, Hori E, Nguyen NM, Pham TT, et al. (2013) Influence of Maternal Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin on Socioemotional Behaviors in Offspring Rats. Environ Health Insights 7: 1-14.
- Amin S, Moore RW, Peterson RE, Schantz SL (2000) Gestational and lactational exposure to TCDD or coplanar PCBs alters adult expression of saccharin preference behavior in female rats. NeurotoxicolTeratol 22: 675-682.
- Lilienthal H, Heikkinen P, Andersson PL, Viluksela M (2013) Sexually dimorphic behavior after developmental exposure to characterize endocrine-medicated effects of different non-dioxin-like PCBs in rats. Toxicology 311: 52-60.
- Rogers QR, Leung PM (1973) The influence of amino acids on the neuroregulation of food intake. Fed Proc 32: 1709-1719.
- Tabuchi E, Ono T, Nishijo H, Torii K (1991) Amino acid and NaCl appetite, and LHA neuron responses of lysine-deficient rat. PhysiolBehav 49: 951-964.
- Kondoh T, Nishijo H, Takamura Y, Torii K, Ono T (1996) Increased Histidine preference during specific alteration of rhythm of environment temperature stress in rats. BehavNeurosci 110: 1187-1192.
- Kakeyama M, Tohyama C (2003) Developmental neurotoxicity of dioxin and its related compounds. Ind Health 41: 215-230.
- Torii K, Mimura T, Yugari Y (1987) Biochemical mechanism of umami taste perception and effect of dietary protein on the taste preference for amino acids and sodium chloride in rats. In: Kawamura Y, Kare MR, New York, Marcel Dekker, Umami: A basic taste 513-563.
- Antelman SM, Rowland NE, Fisher AE (1976) Stimulation bound ingestive behavior: a view from the tail. PhysiolBehav 17: 743-748.
- Vaswani K, Tejwani GA, Mousa S (1983) Stress induced differential intake of various diets and water by rat: the role of the opiate system. Life Sci 32: 1983-1996.
- Yoshida T, Okuno T, Kawabata T, Morimoto T (1994) Salt consumption and body fluid balance during cold exposure in rats. PhysiolBehav 55: 163-167.
- Kant GJ, Bauman RA (1993) Effects of chronic stress and time of day on preference for sucrose. PhysiolBehav 54: 499-502.
- Kondoh T, Mori M, Ono T, Torii K (2000) Mechanisms of umami taste preference and aversion in rats. J Nutr 130: 966S-70S.
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, et al. (2007) Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 32: 82-94.
- Kaitsuka T, Fukunaga K, Soeda F, Shirasaki T, Miyamoto E, et al. (2007) Changes in Ca(2+)/calmodulin-dependent protein kinase II activity and its relation to performance in passive avoidance response and long-term potentiation formation in mice prenatally exposed to diethylstilbestrol. Neurosci 144: 1415-24.
- Sawai T, Bernier F, Fukushima T, Hashimoto T, Ogura H, et al. (2002) Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res 950: 308-311.
- Wagner C, Morrell JI (1995) Distribution and steroid hormone regulation of aromatase MRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurology 370: 71-84.
- Wagner CK, Morrell JI (1997) Neuroanatomical distribution of aromatase MRNA in the rat brain: indications of regional regulation. J Steroid BiochemMolBiol 61: 307-314.
- Handa RJ, Price Jr RH, Wilson ME (1997) Steroid hormone receptors in the developing brain. Biomed Rev 7: 51-66.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14376
- [From(publication date):
February-2014 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 9807
- PDF downloads : 4569