Research Article Open Access
Effects of Naphthalene Acetic Acid (NAA) and Indole -3- Butyric Acid (IBA) on In Vitro Rooting of Sugarcane (Saccharum officinarum L.) Micro- Shoots
Belay Tolera*Ethiopian Sugar Corporation, Research and Training Division, Biotechnology Research Team, Wonji Research Center, P.O. Box 15, Wonji, Ethiopia
- Corresponding Author:
- Belay Toler
Ethiopian Sugar Corporation
Research and Training Division
Variety Development Directorate
Biotechnology Research Team
Wonji Research Center
P.O. Box 15, Wonji, Ethiopia
Tel: +25191081644
E-mail: belaytolera@yahoo.com
Received date: September 17, 2015; Accepted date: December 28, 2015; Published date: January 04, 2016
Citation: Tolera B (2016) Effects of Naphthalene Acetic Acid (NAA) and Indole -3- Butyric Acid (IBA) on In Vitro Rooting of Sugarcane (Saccharum officinarum L.) Micro-Shoots. J Biotechnol Biomater 6:215. doi:10.4172/2155-952X.1000215
Copyright: © 2016 Tolera B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
In Vitro rooting of micropropagated micro-shoots of two commercial sugarcane varieties was carried out with the aim of evaluating the root induction responses of the sugarcane varieties (B41-227 and N14) to alpha naphthalene acetic acid (NAA) and Indole -3- butyric acid (IBA). Accordingly, four levels of NAA (0, 1, 2, and 3 mg L-1) and IBA (0, 1, 2 and 3 mg L-1) in a completely randomized design with 4 × 4 × 2 factorial treatment combination arrangements were tested. Data on the number of roots per shoot and average root length (cm) were collected after 30 days of culture on ½ MS root induction medium. Analysis of variance revealed that the interaction effects of NAA, IBA and the sugarcane genotypes on number of roots per shoot and average root length of both sugarcane varieties was very highly significant (P<0.0001). Culture medium containing 2 mg L-1 NAA and 1 mg L-1 IBA for B41-227 and 1 mg L-1 NAA alone for N14 was found to be optimum. On these medium, B41-227 gave 33 ± 0.15 roots per shoot with 2.92 ± 0.18 cm root length and N14 produced 35± 0.20 roots per shoot with 3.2 ± 0.25 cm root length. The rooted plantlets were survived 100% after four weeks of acclimatization in greenhouse on Farmyard manure and soil at 2:8 ratios. The optimized protocol can be used to develop healthy and profuse root system in the sugarcane micro-shoots, an essential stage in sugarcane micropropagation.
Keywords
In Vitro rooting; Micropropagation; Sugarcane; NAA; IBA
Introduction
Sugarcane (Saccharum officinarum L.) is one of the major cash crops grown extensively all over the world [1] ranking among the ten most planted crops [2,3] encompassing approximately half of the globe [4] and is an economically important multipurpose crop in tropical and subtropicalregions of several countries [4-7]. In Ethiopia, sugarcane is cultivated on more than 60,000 ha and the four sugar mills produce about 300,000 tonnes of sugar per annum which only covers about 60% of the annual demand for domestic consumption. In spite of this fact, Ethiopia is endowed with favorable climate, vast fertile land and water resources for large scale irrigated sugarcane agriculture [8,9]. Besides, sugarcane has now emerged as a multiproductcrop used for food (sugar), energy and raw material for a number of by-products [10-12]. Accordingly, the Ethiopian Government is implementing a large scale expansion and new green field sugar development programs with the objective of boosting the country’s annual sugar production both to satisfy the domestic sugar demand and exploit the international sugar market. Following the completion of this development program, the total cane development area will reach 342,000 ha. As a result, the existing annual sugar production of 300,000 tonnes shall be boosted to 5 million with 181,604 m3 of ethanol and 101 megawatt electric power generation. However, availability of adequate quantity quality disease free planting materials of sugarcane within a short time is the major limiting factor to attain the intended plan. In addition, the yield of the existing few and old commercial cane varieties is declining sharply and some productive varieties were obsolete due to lack of alternative technologies for disease cleansing and rejuvenation. Moreover, commercialization of improved introduced and adapted sugarcane varieties took several years through the conventional propagation method. The spectacular findings in micropropagation have generated outstanding interests, enthusiasm and optimism over the world [13].
Micropropagation using shoot tip or apical meristem culture has been widely used to produce virus-free plants [14,15] and rapid multiplication of new variety [16,17] and for obtaining rapid rejuvenation and mass production of true to type and uniform planting materials of sugarcane [18,19]. Moreover, tissue culture raised sugarcane plants were reported to give superior cane and sugar yield [20-25]. However, the wide spread use of this technology is restricted by the often high percentage of plantlets loss when transferred to ex vitro condition [26]. Besides correction of plantlets physiology, anatomy and the other morphological characteristics of micropropagated plantlets, the status of root development determines the survival rate and hence the profitability of micropropagation technology. Profuse and healthy root induction of micro-shoots is a crucial stage in micropropagation for successful establishment and survival of the plantlets to soil transfer. Naphthalene acetic acid (NAA) is reported to be the strongest auxin followed by indole-3-butyric acid (IBA) and indole-3- acetic acid (IAA) in the order of decreasing strength for In Vitro rooting and plantlet growth in sugarcane [27]. Different researchers found optimum rooting responses of sugarcane micro-shoots on ½ MS medium fortified with different concentrations and combinations of NAA and IBA, indicating specificity of sugarcane genotypes to different concentration and combinations of auxins [28-35]. Therefore, this study was carried out with the objective to find out the optimum concentration and combination of NAA and IBA on In Vitro rooting of micropropagated micro-shoots of two selected sugarcane (B4-227 and N14) varieties widely grown in the Ethiopian Sugar Estates.
Materials and Methods
The same batches of micro-shoots of sugarcane varieties N14 and B41-227 derived from shoot tip explants and having similar sizes (3 to 4 cm shoot length with 4 to 5 leaves) were used to study the root induction responses of the two sugarcane varieties under similar culture growth conditions In Vitro. These varieties were selected as they are well adapted and have high cane and sugar yield and are among a few very productive ones widely used in the Ethiopian Sugar Estates. Half strength Murashige and Skoog (1962), (MS) medium, supplemented with different concentrations and combinations of naphthalene acetic acid (NAA) and indole-3-acetic acid (IBA) was used for the rooting experiment. The MS medium contained 60 g/l sucrose (table sugar) as a carbon source, the pH of the medium adjusted to 5.8 before gelling with 8 g/l agar and autoclaved at 121ºC and 15 psi for 20 minutes. Then, molten medium of 40 ml was dispensed per each culture jar. The experiment was carried out at a temperature of 25 ± 2ºC under 16-hours light and eight hours dark photoperiod regimes maintained under fluorescent light having 2500 μmol m-2 S-1 light intensity and 75 to 80% relative humidity of the growth room. The experiment was laid out in a three factor (sugarcane varieties with NAA and IBA) factorial treatment combinations arrangements with a completely randomized design. After four weeks of culture transfer to rooting medium, data on the number of roots per shoot and average root length was collected from five randomly selected plantlets among 15 plantlets per treatment combinations and the collected data were subjected to analysis of variance (ANOVA) using SAS statistical software (version 9.2). Treatments’ means were separated using the procedure of REGWQ (Ryan-Einot-Gabriel-Welsch).
Results and Discussion
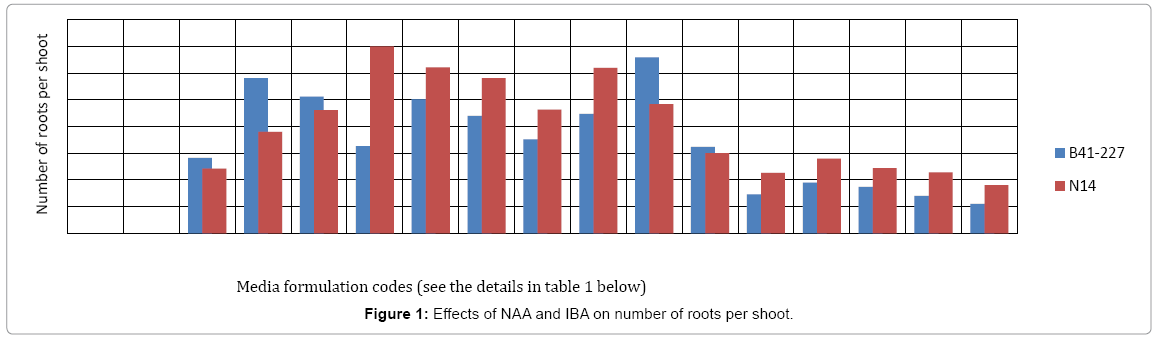
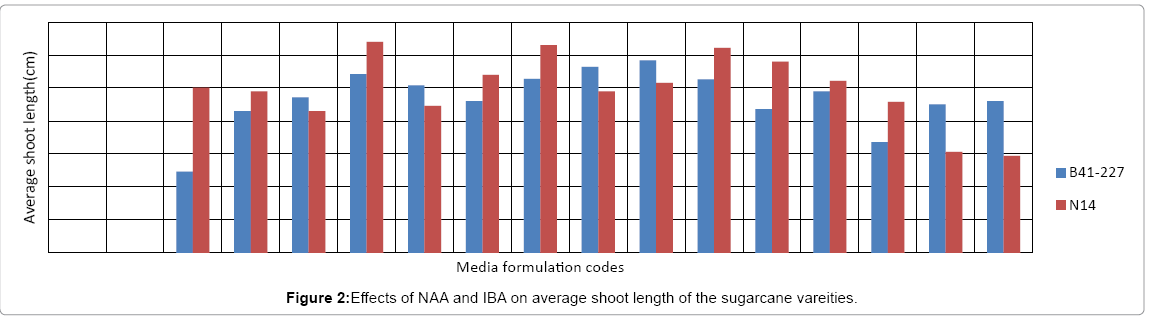
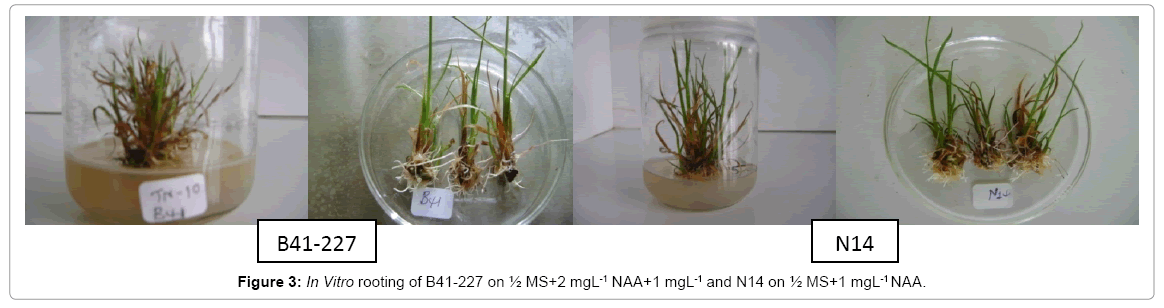
Analysis of variance (ANOVA) revealed that the interaction effects of sugarcane genotypes, naphthalene acetic acid (NAA) and indole- 3-butyric acid (IBA) was very highly significant (p<0.0001) on the number of roots per shoot and average root length of the two sugarcane varieties tasted. The two varieties also showed significant variation both in the number of roots per shoot and average root length. However, on MS medium lacking auxins (NAA and/or IBA), no root induction occurred in the micro-shoots of both sugarcane varieties (Figures 1 and 2). This is due to the well-established fact that auxins are integral to root induction and development as they control basic processes in cell division and elongation [36-38]. Interaction of IBA and NAA promoted starch hydrolysis during root development and subsequently reduced the C:N ratio and increased the protein-nitrogen activity during the development of root primordial [39]. Auxins trigger enzyme activities which regulate different biochemical pathways like protein, carbohydrates, nitrogen, and phenolics, during root induction process [39-41]. Sugarcane variety B41-227 gave the largest (33 ± 0.15) number of roots per shoot with 2.92 ± 0.18 cm average root length on ½ MS medium supplemented with 2 mg L-1 NAA with 1 mg L-l IBA (Figures 1-3) while only 24.17 ± 0.00 roots per shoot with 2.58 ± 0.00 cm average root length were observed in N14 on the same medium composition. The relative degree of activity of auxins is very variable and differs not only from plant to plant, but also from organ to organ, tissue to tissue, cell to cell [36]. In addition, the difference in the concentration of receptor molecules (proteins) for the auxins and the metabolic capabilities of the genotypes may result in the difference in the rooting response between the two genotypes (Table 1).
| PGRs (mg/l) | Details of Media formulation codes for Figures 1 and 2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | T14 | T15 | T16 | |
| NAA | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 |
| IBA | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
Table 1: Description of media formulation codes.
In sugarcane vareity N14, the highest number (35 ± 0.20) of roots per shoot with 3.20 ± 0.25 cm average root length was obtained on ½ MS medium supplemented with 1 mgL-1 NAA without IBA (Figures 1-3) while the same treatment combination resulted in only 16.25 ± 0.57 roots per shoot with 2.71 ± 0.00 cm average root length in B41-227 (Figures 1 and 2). Increasing the concentration of IBA from 0 to 1 mg L-l at 0 mg L-l NAA significantly increased the number of roots per shoot (from 0 ± 0.00 to 14.05 ± 0.35) and average root length (from 0 ± 0.00 to 1.23 ± 0.44 cm) in B41-227. Similarly, N14 showed a significant increase in the number of roots per shoot (from 0 ± 0.00 to 12.1 ± 0.57) and average root length (from 0 ± 0.00 to 2.5 ± 0.06 cm) because of the increase in the concentration of IBA from 0 to 1 mg L-l at 0 mg L-l NAA. However, keeping the concentration of IBA at 0 mg L-l and increasing NAA beyond the supra-optimal concentration resulted in significant reduction both in the number of roots per shoot (from 35 ± 0.20 to 31 ± 0.69) and average root length (from 3.20 ± 0.25 cm to 2.45 ± 0.01 cm) in N14. This might be related to the fact that higher concentrations of NAA is inhibitory both to root induction and elongation in sugarcane [30]. Increased concentrations of auxins stimulate natural ethylene production which is inhibitory to rooting. However, despite many reports on the physiological action of both phytohormones, the molecular mechanisms of their effect(s) on cell expansion, cell division, differentiation, organogenesis, and the mechanisms of their interactions have not yet been elucidated [36]. Nevertheless, the main steps in auxin signaling are initial perception of the hormone signal by the receptor protein, the signal transductioncascade, and the final physiological response (root induction). Auxin signal perception by analogy to animal systems each target plant cell is presumed to possess receptors which are able to detect hormonal signals and then to initiate the chain of molecular events leading to the final physiological response [42,43].
In case of B41-227, maintaining the concentration of NAA at 2 mg L-l and increasing IBA from 0 mg L-l to 1 mg L-l showed a significant increase in the number of roots per shoot (from 22.42 ± 0.17 to 33 ± 0.15) and average root length (from 2.82 ± 0.00 to 2.92 ± 0.18 cm). However, further increase in the concentration of IBA from 1 to 2 mg L-l in B41-227 showed a significant reduction in the number of roots per shoot (Figure 1) and average shoot length to 16.18 ± 0.23 and 2.63 ± 1.20 cm (Figure 2), respectively. This inhibition of root induction and elongation at higher concentrations of IBA may be due to deposition of ethylene in the culture jars as auxins of all types stimulate plant cell to produce ethylene which retards root induction and elongation [37]. The present results in N14 are in contrast with the results obtained in sugarcane vareity HSF-240 on ½ MS medium containing 1 mg L-l NAA [38]. One 1 mg L-l NAA was best for rooting response in sugarcane [39].
The results obtained in B41-227 are different from the root induction results obtained in sugarcane variety CP-77- 400 and BL- 4 on medium containing 2 mg L-l NAA and 1 mg L-l IBA [28]. PhytohormonesNAA and IBA are the most widely used auxins in commercial vegetative propagation practices in sugarcane. They are chosen because of their rhizogenic efficacy, which results from their high stability in plant tissues [41]. This, in turn, also makes them the preferred choice for varieties that are difficult to root and do not respond well to IAA application. However, the most potent auxin may not necessarily be the most suitable one in terms of root development and quality [44,45]. Thus, IAA (Indole-3-acetic acid) either alone or in combination with NAA and/or IBA shall be investigated to further improve of the optimized protocol.
Acclimatization
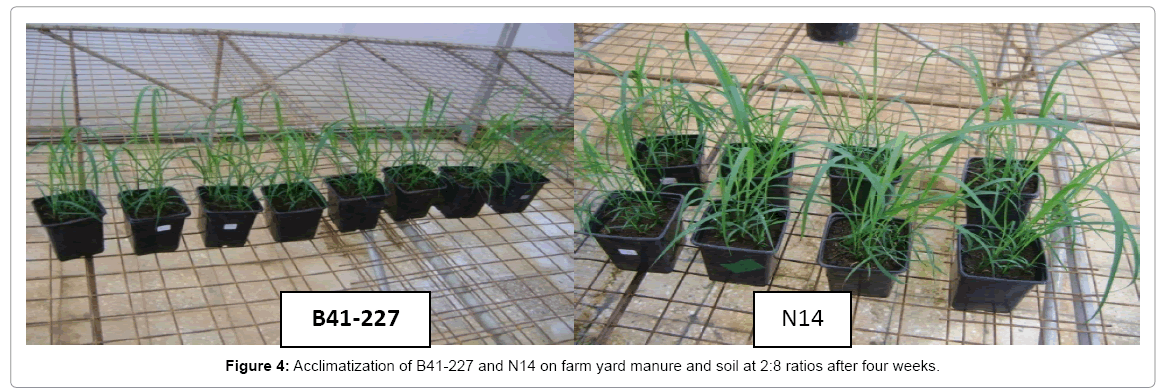
The rooted plantlets were taken carefully out of the culture jar and the roots washed with running tap water gently to remove traces of culture medium particularly sucrose. Since the plantlets were from high sucrose concentration medium and the chance of fungal attack could potentially be minimized through washing, the collected plantlets were transferred to plastic pots filled with autoclaved farmyard manure and soil in the ratio of 2:8 as a potting mixture. Fifty plantlets of each variety were used for acclimatization. After four weeks of transfer to greenhouse, 100% of the plantlets of each of the varieties were successfully acclimatized and survived (Figure 4).
Conclusion
Lack of steady supply of adequate quantity quality and disease free sugarcane planting materials is one of the most challenging issues for attaining the intended production plans of the Ethiopian Sugar Industry using the conventional method of propagation. In Vitro mass propagation of sugarcane through shoot tip or apical meristem explant ensures rapid multiplication of true to type and disease free planting materials of sugarcane within a short period of time. Rooting of In Vitro propagated micro-shoots is an important stage in micropropagation technology that affects the ex-vitro survival rate. In the present study, an efficient protocol for In Vitro rooting was optimized with subsequent acclimatization of micropropagated sugarcane micro-shoots of shoot tip explant derived sugarcane varieties: B41-227 and N14. Accordingly, ½ MS medium containing 2 mg L-1 NAA and 1 mgL-1 IBA for B41-227 and 1 mgL-1 NAA without IBA for N14 were found to be optimum. On these medium, B41-227 could produce 33 ± 0.15 roots per shoot with 2.92 ± 0.18 cm average root length and N14 produced 35± 0.20 roots per shoot with 3.2 ± 0.25cm average root length after 30 days of culture transfer to root induction medium. The plantlets showed 100% survival on planting medium containing farm yard manure and soil in 2:8 ratios after four weeks of planting in greenhouse conditions. Thus, the optimized protocol can be used to develop healthy and profuse root system for the sugarcane varieties and can play a key role in the stage of rapid supply of quality planting material of the sugarcane varieties and minimizes the current challenge of the Sugar Industry. As a subsequent activity, effects of IAA either alone or in combination with NAA and/ or IBA on In Vitro rooting and ex-vitro rooting of the sugarcane micro-shoots to merge rooting and acclimatization stage as a low cost alternative to the usual In Vitro propagation shall be investigated.
Acknowledgement
We would like to express our thanks to Ethiopian Sugar Corporation for funding the research and Jimma University College of Agriculture and Veterinary Medicine for providing Plant Tissue culture laboratory and facilities.
References
- Singh N, Kumar A, Garg GK (2006) Genotype influence of phytohormone combination and sub culturing on Micropropagation of sugarcane varieties. Indian Journal of biotechnology 5: 99-106.
- FAO (2013) Food and Agriculture Organization of the United States of America. World sugarcane production statistics.
- Suprasana P (2010) Biotechnological Interventions in sugarcane improvement: Strategies, methods and progress. Nuclear Agriculture and Biotechnology Division, Technology Development Article.
- Katia CS, SilvanaCreste, TercilioCalsa Jr., Mauro AX, Marcos GAL, et al. (2012) Challenges, Opportunities and Recent Advances in Sugarcane Breeding.
- Chatenet M, DelageC, RipollesM (2001) Detection of sugarcane yellow leaf curl virus in quarantine and production of virus-free sugarcane by apical meristem culture. Plant disease 85: 1177-1180.
- Guimarces CT, Sobral WS (1998) The Saccharum complex. Relation to other anderopogneae.Plant breed Rev 16: 269-288.
- Sawant RA, Tawar PN (2011) Use of Sodium Hypochlorite as Media Steriliant in Sugarcane Micropropagation at Commercial Scale. Sugar Tech 13: 27-35.
- ESISC (2008) Investment opportunity profile for sugarcane plantation and processing in Ethiopia. Ethiopian Investment agency, Addis Ababa, Ethiopia 18-22.
- Rezene F (2009) The status of bio-fuels in Ethiopia. IUCN regional workshop on bio-fuels, Nairobi Kenya.
- Gallo-Meagher, English RG, AbouzidA(2000)Thidiazuron stimulates shoot regeneration of sugarcane embryonic callus. In Vitro cellular and developmental biology plant 36:37-40.
- Jalaja NC, Neelamathi D, Sreenivasan TV (2008) Micropropagation for quality seed Production in sugarcane in Asia and the Pacific. Sugarcane pub 13-60.
- Naturland EV (2000) Organic farming in the tropics and Sub tropics. Kleinhaderner, Germany 10-15.
- Ali A, Naz S, Siddiqui FA,Iqbal J (2008) An efficient protocol for large-scale production of sugarcane through micropropagation. Pak J Bot 40:139-149.
- Hendre RR, Iyer RS, Kotwal M (1983) Rapid multiplication of sugarcane by tissue culture. Sugarcane 1:58.
- Parmessur y, Aljanabi A, Saumtally S,Dookun-SaumtallyA (2002) Sugarcane yellow leaf virus and sugarcane yellows phytoplasma: Elimination by tissue culture. Plant pathology 51:561-566.
- Anita PJ, Sehrawat RK, PuniaA (2000) Efficient and cost effective micropropagation of two early maturing varieties of sugarcane (Saccharum Spp.). India sugar 50:611-618.
- Sandhu SK, Gossal SS, Thind KS, Uppal SK, Sharma B, et al. (2009) Field performance of micrpropagated plants and potential of seed cane for stock yield and quality in sugarcane. Sugar tech research article 11: 34-38.
- Heinz DJ, Mee GW (1969) Plant differentiation from callus tissue of Saccharum species. Crop sci 9:346-348.
- Lakshmanan (2012) Sugarcane tissue culture. Sugarcane for the future. Information sheet IS13034.
- Anonymous (2002) Micropropagation: Tissue culture techniques in sugarcane. Indian Institute of sugarcane Research, Directorate of sugarcane Development 1-2.
- Comstock JC, Miller JD (2004) Yield comparison: Disease free tissue cultures versus bud propagated planted sugarcane plants and healthy versus yellow leaf virus infected plants. Journal American Society Sugar Cane Technologists 24:31-40.
- Geetha S,Padmanabhan D (2001) Effect of hormones on direct somatic embryogenesis in sugarcane. Sugar Tech 3:120-121.
- Nand L, Ram K (1997) Yield comparison in sugarcane crop rose from conventional and mericlone derived seedcane. Ind Sugar47:617-621.
- Soodi N, Gupta PK, Srivastava RK, Gosal SS (2006) Comparative studies on field performance of micrpropagated and conventionally propagated sugarcane plants. Plant tissue cult and Biotech 16:25-29.
- Ramanand M Lal, Singh SB (2005) Comparative performance of micropropagated and conventionally raised crops of sugarcane. Sugar tech 7:93-95.
- Pospisilova J, Ticha I, Kadlecek P, HaiselD, PlzakovaS (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biologiaplantarum 42: 481-497.
- Lal J, PandeHP, Awasthi SK (1996) A general micropropagation protocol for sugarcane varieties. New Bot 23:13-19.
- Ali A, Naz S, Siddiqui FA,Iqbal J (2008)An efficient protocol for large-scale production of sugarcane through micropropagation. Pak J Bot 40:139-149.
- Bahera KK, Sahoo S (2009) Rapid In Vitro micropropagation of sugarcane (SaccharumofficinarumL.cv-Nayana) through callus culture. Nature and science 7: 1545-0740.
- Biradar S, Biradar BP, Patil VC, Patil VC, Kambar NS (2009) In Vitro plant regeneration using shoot tip culture in commercial cultivars of sugarcane. Karnataka Journal of Agricultural Science 22: 21-24.
- Eldessoky DS, Ismail RM, Abdel-Hadi AH, Abdallah NA (2011) Establishment of regeneration and transformation system of sugarcane cultivar GT54-9 (C9). GM Crops 2: 126-134.
- Gopitha K, Bhavani AL, Sentilmanickam J (2010) Effect of the different auxins and cytokinins on callus induction, shoot, root regeneration in sugarcane. International journal of pharma and biosciences 1: 4-5.
- Khan IA, Dahot MU, Yasmin S, Kahtri A, Seema N,et al. (2006) Effect of sucrose and growth regulators on the micropropagation of sugarcane clones. Pakistan Pak J Bot 389:961-967.
- Pawar SV, Patil SC, Jambhale VM, Naik RM, Mehetre SS ( 2002) Rapid multiplication of commercial sugarcane varieties through tissue culture. Indian sugar 11:183-186.
- Sahoo DP, Samantrai D, Rout GR (2011) Rapid clonal propagation of Saccharumofficinarum L. vars.CO-6907 and CO-86249 and to assesse the genetic uniformity through molecular markers. Plant biosystems 145: 445-451.
- George EF, Machakova I,Zazimalova E (2008) Plant propagation by tissue culture. Springer, Netherlands.
- Hartmann HT, Kester DE, Davies FT, Geneve RL (1997) Plant Propagation: Principles and Practices. Prentice Hall International, London, UK.
- Muhammad Nakhooda, Maria Paula WATT, David MYCOCK. (2014) The choice of auxin analogue for In Vitro root induction influences post-induction root development in Eucalyptus grandis. Turk J Agric For 37: 258-266.
- Das P, Basak UC, Das AB (1997) Metabolic changes during rooting in pre-grilled stem cuttings and air layers of Heritiera. Bot Bull Acad Sin 38: 1-4.
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95: 707-735.
- Gaspar T, Kevers C,HausmanJF (1997) Indissociable chief factors in the inductive phase of adventitious rooting. In: A. Altman and Y. Waisel (eds.), Biology of Root Formation and Development. Plenum Press, New York.
- Druege U, Zerche KR, Ernst M (2000) Relationship between nitrogen status, carbohydrate distribution and subsequent Rooting of Chrysanthemum Cuttings as Affected by Pre-harvest Nitrogen Supply and Cold-storage. Ann Bot 85: 687-701.
- Friedman R, Altman A, Bachrach U (1985) Polyamines and Root Formation in Mung Bean Hypocotyl Cuttings: II. Incorporation of Precursors into Polyamines. Plant Physiol 79: 80-83.
- Weiler EW (1984) Immunoassay of plant growth regulators. Annual review of plant physiology 35:85-95.
- Mato MC, Rua ML, Ferro E (1988) Changes in levels of peroxidase and phenolics during root formation in Vitis cultured In Vitro. P hysiol Plant 72: 84-88.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 27207
- [From(publication date):
March-2016 - Jul 04, 2025] - Breakdown by view type
- HTML page views : 25658
- PDF downloads : 1549