Research Article Open Access
Effects of Mesenchymal Stromal Cells on the Neuropathic Pain Induced by Chronic Constriction Injury in Rats
Deniz Genc1*, Noushin Zibandeh1, Yasin Yildiz2, Sezer Aslan2, Faruk Demirtas2, Erdal Karaoz3,4 and Tunc Akkoc1,5
1Department of Pediatric Allergy and Immunology, School of Medicine, Marmara University, Istanbul, Turkey
2School of Medicine, Marmara University, Istanbul, Turkey
3Center for Regenerative Medicine and Stem Cell Research & Manufacturing, Liv Hospital, Istanbul, Turkey
4Department of Histology and Embryology, Istinye University, Istanbul, Turkey
5Department of Sports Health Sciences, Marmara University, ├?┬░stanbul, Turkey
- *Corresponding Author:
- Deniz Genc
Department of Pediatric Allergy and Immunology, School of Medicine
Marmara University, Istanbul, Turkey
Tel: +905337495534
E-mail: denizdogan2000@gmail.com
Received date: August 28, 2017; Accepted date: September 19, 2017; Published date: September 25, 2017
Citation: Genc D, Zibandeh N, Yildiz Y, Aslan S, Demirtas F, et al. (2017) Effects of Mesenchymal Stromal Cells on the Neuropathic Pain Induced by Chronic Constriction Injury in Rats. J Pain Relief 6:302. doi:10.4172/2167-0846.1000302
Copyright: © 2017 Genc D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Background: Neuropathic pain remains a persistent clinical problem and characterized by mechanical allodynia and heat hyperalgesia. Chronic pain conditions are among the major health problems which are difficult to treat. Bone marrow-derived mesenchymal stromal cells (BMSCs) have generated great interest as an option for cell-based therapy. BMSCs are easy to isolate and expand ex vivo. Clinical studies show that direct injection of BMSCs does not produce side effects as rejection and is well tolerated by the immune system.
Methods: Neuropathic pain model in rats was developed with the ligation of the sciatic nerve. BMSCs were isolated from femur and tibia aspirates of rats and kept in culture media. rBMSCs were injected locally into injured sciatic nerve area of rats and efficiency of the therapy was observed with thermal sensitivity and motor functions for 4 weeks after injection of rBMSCs.
Results: After injection into injured area rBMSCs were located in sciatic nerve tissue. In the present study, we showed that a single systemic local injection (into the lesion site) of rBMSCs reversed pain hypersensitivity in rats after injury and decreased the pain symptoms for 2 weeks and these effects got back in 4 weeks.
Discussion: Our results revealed that single injection of rBMSCs showed relief of pain in short-term follow-up and further booster injection needed for long term prolonged therapeutic approach.
Keywords
Rat; Bone marrow; Mesenchymal stromal cells; Cellular therapy; Neuropathic pain
Abbreviations
CCI: Chronic Constriction Injury; MSC: Mesenchymal Stromal Cell; NP: Neuropathic Pain
Introduction
Neuropathic Pain (NP) was defined as the pain arising as a direct consequence of a lesion or disease affecting the somatosensory system according to Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP) [1]. The lesion or disease affect nervous system either centrally or periphery. The prevalence of chronic pain reported as 8-9% in Europe with the characteristic of neuropathy [2]. 10% of community adults have shown symptoms of neuropathic pain in the United States [3].
NP is associated with low levels of health utility that were generally lower than some chronic conditions such as cancer, heart failure, chronic obstructive pulmonary disease, motor neuron disease, type 2 diabetes [4]. The pain is characterized by burning, stabbing or like an electric shock. Stimulus-response function can be damaged in NP and results with allodynia (evoked pain to non-painful stimulus) and hyperalgesia (exaggerated pain perception as result of damaged peripheral pain fiber). The four most common types of NP are postherpetic neuralgia, trigeminal neuralgia, phantom limb pain and painful diabetic neuropathy [5]. First line treatment of neuropathic pain is oral amitriptyline or pregabalin and oral duloxetine for painful diabetic neuropathy according to the guidance of National Institute for Health and Clinical Excellence (NICE) [6]. Insufficient pain relief and intolerable side effects of drugs were experienced in NP patients [7]. Some interventional methods are considered for treatment of NP patients that included in neurosurgical interventions, neural blockade and spinal cord stimulation but the effectiveness of interventional management of NP is also limited [8]. Therefore, it is thought that the mechanism-based treatment is needed.
Some neurobiological mechanisms were caused to induce neuropathic hypersensitivity. Decrease in the threshold of sodium channels, ectopic excitability of sensory neurons, central sensitization is one of the important reason for neuropathic pain [9]. Inflammatory mediators, calcitonin gene-related peptide and substance P which are released form nociceptive terminals and prostaglandins, bradykinin, growth factors and cytokines which are released from injured area contribute to peripheral sensitization. These substances can decrease firing thresholds and establish ectopic discharges in nociceptors [10].
Recently, evidence for a role of the immune cells in neuropathic pain is increasing. The factors that are released by injured neurons and their associated glial cells activate resident immune cells and recruit more immune cells from the blood circulation. These immune cells release many cytokines and chemokines that change the transduction and transmission of nociceptive signals by sensory neurons at the level of their target fields, the nerve trunk, cell bodies in the dorsal root ganglion and synaptic terminations within the dorsal horn of the spinal cord [11]. T cell infiltration to the injured sciatic nerve after chronic constriction injury was showed by researchers. When congenitally athymic nude rats and their heterozygous littermates were comparing after the injury, nude rats have developed a reduced mechanical allodynia and thermal hyperalgesia. Moreover passive transfer of Th1 (T helper) cells into nude rats produced proinflammatory cytokines, but Th2 cells produced anti-inflammatory cytokines [12]. Proinflammatory cytokines such as TNF-α, IL-1, and IL-6 released by inflammatory cells promote neuropathic pain development and progression [13]. The inflammatory response in the injured sciatic nerve suppressed by daily injections of dexamethasone led to blocking the development of thermal hyperalgesia [14].
Chronic constriction injury (CCI) in rat sciatic nerve is widely used the model to produce peripheral mononeuropathy. In this model, the sciatic nerve is encircled by four ligatures of the chronic gut which constricts nerve, produced ischemia, initiates inflammatory reactions, and damage axons. After sciatic nerve injury in rats, allodynia and hyperalgesia were appeared similar to human neuropathic pain [15].
Mesenchymal stromal cells (MSCs) are multipotent cells that are a heterogeneous population of plastic adherent fibroblast-like cells. These cells are able to self-renewing and have the potential to differentiate into numerous cell types as osteoblast, adipocyte, chondrocytes in the culture [16]. The differentiation feature of these cells contributes to promise in regenerative medicine, such as myocardial infarction and peripheral nerve injury [17,18]. MSCs have the ability on immunosuppression and modulate the immune function of the major cell populations, such as antigen presenting cells, T cells and NK cells [19]. Thus, the treatment of graft vs. host disease, rheumatoid arthritis, multiple sclerosis and other autoimmune disorders with transplantation of MSCs have been applied in mice models [20]. MSCs also mediate neuroprotection in a variety of neurodegeneration and nervous system injury models, as Parkinson disease and neuropathic pain. Recently, stromal cell therapy provides cellular therapy for neurodegenerative diseases including Parkinsons’ Disease (PD), Huntington's disease (HD), Alzheimer's and amyotrophic lateral sclerosis (ALS). The key mechanism underlying in neurodegenerative diseases is the loss of structure, function or number of neurons [21]. Today, researchers suggest that human bone marrowderived cells SB623 cells which secrete glial cell line-derived neurotrophic factor can regenerate host strial dopaminergic fibers and dopaminergic-dependent behavioral recovery in the rat model of Parkinson’s disease [22]. Lateral cerebral ventricle microinjection of human MSCs decreased pain-like behaviors, such as mechanical allodynia and thermal hyperalgesia, in neuropathic mice injured sciatic nerve [23]. Transplantation of rat BMSCs into hind limb skeletal muscles of rats alleviated algesia in rats with streptozotocin-induced diabetes [24]. The fact that the MSCs have the neuro-protection effect, stromal cell therapy seems one of the new therapeutical approaches for the treatment of NP. Taken together, MSCs may be uncourageous cell source for the treatment of cold allodynia and neuropathic pain, because of their secretion of anti-inflammatory mediators such as TGF-β, PGE2 and IDO.
In the present study, we aimed to research the effect of local administration of rBMSCs to sciatic nerves of chronic constructive injury rat model on cold allodynia and sciatic functions in rats.
Materials and Methods
Animals and study design
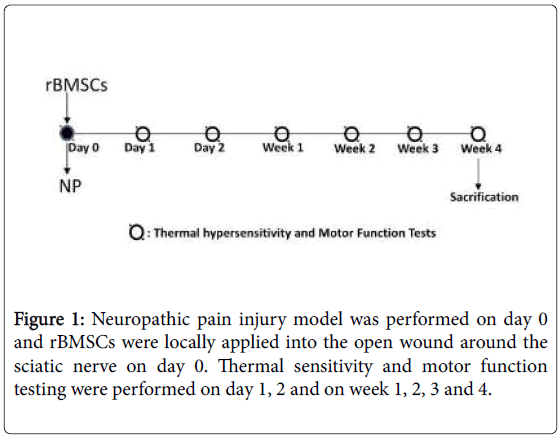
Male Wistar albino (WA) rats were obtained from TUBITAK (The Scientific and Technological Research Council of Turkey) at 6 weeks of age. All rats were housed in separate cages under the stable temperature at 22°C ± 2°C and on a 12 h light/dark cycle, and were supplied standard laboratory rat chow with water ad libitum. Rats weighing 160 and 220 g were used for a model for neuropathic pain. All experimental protocols were carried according to the Regulations for Animal Experiments in Marmara University and were approved by Laboratory Animals Ethics Committee of Marmara University. Rats were divided into three groups as CCI group, CCI+MSC group, and Sham-operated group (n=6 per group) (Figure 1).
Isolation and characterization of rBMSCs
Experiments were performed as the protocol described [25]. Briefly, rats were anesthetized with ketamin 10% and rompun 10% (4:1 v/v, total volume: 1 ml) and sacrificed. Both ends of femurs and tibiae were cut off from epiphysis by scissors and marrow was excised in order to obtain MSCs. Bone marrow was flushed with complete culture media containing DMEM supplemented with 10% FBS, 1% Penicillin/ Streptomycin (Gibco Invitrogen, Life Technologies, Paisley, UK). The supernatant containing thrombocytes and erythrocytes were discarded, and the cell pellet was resuspended in complete culture media. The cells were seeded in plastic tissue culture flasks and incubated for 3 days at 37 °C in a humidified atmosphere containing 5% CO2. After the incubation period, non-adherent cells were removed by changing medium. The culture medium was changed three times a week until cells reached 80% confluency. MSCs were washed with Ca2+-Mg2+ free phosphate buffered solution (PBS; Gibco Invitrogen) and were passaged using 0.25% trypsin-EDTA solution (Gibco, Invitrogen) incubation period for 3 minutes at 37 °C and the enzyme activity was ended by adding 5 ml of MEM. The cell numbers were calculated by the hemocytometer and subjected to flow cytometry analysis. Surface markers of rBMSCs were analyzed with antibodies against the following rat antigens: positive markers CD29 (APC), CD105 (FITC), CD73 (PE) and CD90 (PE), and negative markers CD20 (APC), CD14 (PE) and CD34 (FITC). All of the antibodies were supplied by Becton Dickinson (BD Biosciences, San Diego, USA). Flow cytometry was performed by using FACS Calibur (BD Biosciences). The data were analyzed with Cell Quest software (BD Biosciences).
In vitro differentiation of rBMSCs
In order to determine the multipotency of isolated bone marrow mesenchymal stem cells, rBMSCs were harvested in the third passage and divided into three groups: adipogenic, osteogenic and neurogenic differentiation protocols were performed as described previously [26]. DMEM supplemented with 10% FBS, 1% Penicillin/Streptomycin were added to each group and incubated for 2 days for confluency. After the incubation period, the cells were washed three times with phosphate buffer solution, and the medium was changed separately for each group. For adipogenic differentiation of MSCs were induced by adding standard culture media with supplementations (0.5 mM isobutylmethylxanthine, 10-6M dexamethasone, 10 μg/ml insulin, 200 μM indomethacin) for two weeks. For osteogenic differentiation, the cells were incubated in osteogenic differentiation medium (standard culture media supplemented with 100 nM dexamethasone, 0.05 mΜ ascorbate-2-phosphate, 10 mm β-glycerophosphate) for two weeks. The medium was changed every 3 days. Neurogenic differentiation was performed by incubating cells in culture medium supplemented with 10 ng/ml basic fibroblast growth factor, 10 ng/ml epidermal growth factor, 10 ng/ml Brain-derived neurotrophic factor, 0.5 mM isobutylmethylxanthine for 24-72 h. After incubation period oil red staining protocol was performed for adipogenic differentiation, alizarin red staining protocol for osteogenic differentiation and Nissle staining for evaluation of neurogenic differentiation potential.
Labeling mesenchymal stromal cells with GFP vector
rBMSCs labeled with pGFP vector prior to application to visualize the migration of these cells in the tissue. pGFP (Clontech, Palo Alto, CA, USA), was transfected by electroporation (Neon Transfection System, Invitrogen, Carlsbad, CA, USA) with respect to the instructions provided by the manufacturer. The transformed cells were cultured in 2 ml DMEM-medium with 10% FBS, and without antibiotics. After 48 h of incubation, the cells were continued culturing with fresh medium MEM, 10% FBS and 1% Ampicillin. Culture medium was changed three times a week until cells reach 70-80% confluency.
Immunohystochemistry
Two rats were randomly selected from CCI+MSC group on the second week for the observation of rBMSCs homing in the sciatic nerve tissue. Rats were sacrified and injured area of the sciatic nerve was removed for paraffin embedding. Paraffin sections were stained with anti-GFP antibody. Staining protocol was performed as described previously [27]. Briefly, to detect the localization of rBMSCs two weeks after stem cells application, the left sciatic nerve area was removed for staining with anti-GFP antibody. Tissue samples were fixed in formalin (10%, pH 7.0-7.6) for 24 h, and embedded in paraffin. Transversal sections in 4 μm thickness were taken. Slides were deparaffinized with xylene for 5 min and rehydrated in a series of graded alcohol solutions. Sections were antigen retrieved using a steamer-citrate buffer antigen retrieval method. The sections were incubated in the mixture of primary antibody against GFP (SC-9996 or SC-5385) and were mounted with mounting medium containing DAPI (Santa Cruz).
Neuropathic pain injury model and transplantation of rBMSCs
In the present study, peripheral mononeuropathy was developed as the protocol described [28]. Procedures were carried out under pentobarbital anesthesia (50 mg/kg body weight in intraperitoneally). Skin incision extended from the left sciatic notch to the distal thigh and the sciatic nerve was exposed in left hind limb through a musclesplitting incision. A chronic constrictive injury (CCI) was created by four ligations done with 4 chromatic gut sutures (4-0) that were tied loosely with square knots around the left sciatic nerve. A brief twitch in the muscle surrounding the exposure served as an indicator of the desired degree of constriction. The right sciatic nerve was mobilized. CCI group received 500 μl of PBS into the tissue in the open wound around the sciatic nerve. Sham surgical controls received 500 μl of PBS surrounding tissue without ligations. In CCI+MSC group, rBMSCs (2 × 105 cells/500 μl PBS) in each rat was locally applied into the tissue in the open wound around the sciatic nerve. Incisions were closed layerto- layer with 3-0 silk sutures. The rats were allowed to recover from anesthesia. After surgery, the animals were individually maintained in disinfected plastic cages with solid floors covered with sawdust (Figure 2).
Behavioral testing
Thermal sensitivity of the sciatic nerve was assessed under forcing conditions. On day 1 and 2, and 1st, 2nd, 3rd and 4th weeks after injury, we analyzed the degree of and thermal allodynia. Baseline behavioral tests were carried out before the injury. Animals were habituated to the test environment before any measurements were taken. Cold allodynia was evaluated by using a cold plate. The animals were placed in a glass cage (18 × 28 × 13 cm) with a metal floor that was either chilled by ice bucket. For 15 minutes session, the time was recorded during which the animal held either hind paw above the floor while animal sitting or standing (cumulative time) and the numbers of their paw withdrawal were counted.
Functional assessment
WA rats were evaluated by walking track analysis preoperatively and on day 1 and 2, and 1st, 2nd, 3rd and 4th weeks after injury. Rats were tested in a glass walking pathway (50 cm × 10 cm × 8 cm) with a mirror (50 cm × 10 cm) placed underneath the apparatus at an angle of 45°. When the animals stopped, at the end of the corridor, three photos were acquired with Sony camera that was placed 1 meter away from apparatus. All images were obtained in the same conditions [28]. Several measurements were taken from footprints: (1) the distance between the third toe and the heel (PL-The Print Length), (2) the first and the fifth toe (TS-The Toe Spread), and the second and the fourth toe (ITS-The Intermediary Toe Spread). All three measurements were taken from injured (E-Experimental) and uninjured (N-Normal) side. The SFI was calculated as follows: SFI=-38.3 × (EPL-NPL)/NPL+109.5 × (ETS-NTS)/NTS+13.3 × (EITS-NITS)/NITS-8.8. The SFI oscillates around 0 for normal nerve function and around -100 represents total dysfunction.
Statistical analyses
All experiments have executed a minimum of three times. All data presented as a mean ± standard error. All statistical analyses were performed using SPSS 17. Differences between groups were regarded as statistically significant when p<0.05.
Results
Isolation, characterization, and differentiation of rBMSCs
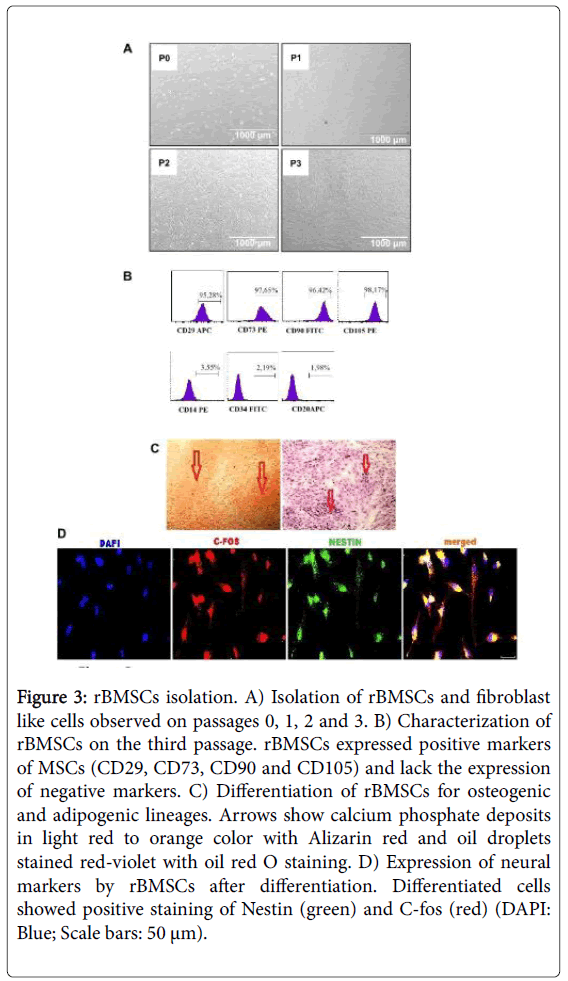
WA rat bone marrow was flushed out from femur and tibiae with culture medium. The suspension was cultured in T25 culture plates and on the third passage cells were analyzed for the positive and negative markers via flow cytometry. The cultured cells were all spindle-shaped and large round nucleus and corresponding with the morphology of bone marrow stromal cells (Figure 3A). Flow cytometer analyses confirmed that the cell populations were consistent with bone marrow MSCs (Figure 3B). The cell surface expression of bone marrow MSCs were positive for CD29, CD73, CD90, CD105 and negative for CD14, CD34, and CD20. MSCs were incubated separately with osteogenic, adipogenic and neurogenic differentiation media in order to observe the multipotency. MSCs were differentiated into adipogenic, osteogenic (Figure 3C) and neuronal cell lineages as observed with positively stained specific cell types (Figure 3D).
Figure 3: rBMSCs isolation. A) Isolation of rBMSCs and fibroblast like cells observed on passages 0, 1, 2 and 3. B) Characterization of rBMSCs on the third passage. rBMSCs expressed positive markers of MSCs (CD29, CD73, CD90 and CD105) and lack the expression of negative markers. C) Differentiation of rBMSCs for osteogenic and adipogenic lineages. Arrows show calcium phosphate deposits in light red to orange color with Alizarin red and oil droplets stained red-violet with oil red O staining. D) Expression of neural markers by rBMSCs after differentiation. Differentiated cells showed positive staining of Nestin (green) and C-fos (red) (DAPI: Blue; Scale bars: 50 μm).
rBMSCs located in sciatic nerve tissue after injury
rBMSCs were labeled with pGFP vector to follow-up whether MSC was located in the damaged area of the sciatic nerve. Rats were sacrificed at week 2 and GFP-labeled rBMSCs illustrated with immunohistochemistry studies that MSCs transferred to the injured area of sciatic nerve were permanent (Figures 4A and 4B). This data suggest that MSCs are located at damaged nerve area and these cells may contribute the improvement of cold allodynia and motor function.
Local administration of rBMSCs reduced number of paw withdrawal
Thermal sensitivity was evaluated during 28 days after sham and NP surgery. Paw withdrawal of rats in all groups were observed in cold plate test. The number of paw withdrawal was increased in CCI group which shows one of the symptoms of sciatic nerve injury. The number of paw withdrawal was significantly reduced in CCI+MSC group compared to CCI group at week 2 (p<0.05). There was no difference between CCI+MSC group and sham group on the paw withdrawal number statistically (p>0.05).
The number of paw withdrawal progressively increased from week 2 in CCI+MSC group. There was no statistical significance on the number of paw withdrawal between groups CCI+MSC and CCI on week 4 (p>0.05). And also our results show that at the end of week 4 number of paw withdrawal in sham group was lower compared to CCI +MSC and CCI groups (Figure 5).
Local administration of rBMSCs improved the motor function of sciatic nerve in CCI model
To assess the efficacy of rBMSCs therapy on functional improvement after sciatic nerve injury, rats were evaluated by walking tract analysis. After the injury, the mean motor function index (MFI) was decreased to -82 in CCI+MSC group and -90.3 in CCI group on day 1 due to loss of sciatic nerve function. There was statistically significant difference on MFI in MSC applied CCI group compared to only CCI group on week 2 (p<0.05). There was a decrease in MFI in MSC applied to group and no significant difference between CCI group on week 4 (p>0.05) (Figure 6).
Discussion
Neuropathic pain is caused by damage or disease affecting the somatosensory nervous system. Neuropathic pain may be associated with abnormal sensations or pain from normally nonpainful stimuli (allodynia). It may have continuous or paroxysmal components. Common suffering in patients includes burning or coldness, sensations, and itching [29].
Neuropathic pain affects 7-8% of the European population and 5% of these cases are severe [30]. Neuropathic pain may be classified as peripheral neuropathic pain, central neuropathic pain or mixed (peripheral and central) neuropathic pain and can be very difficult to treat with only some 40-60% of people achieving partial relief [31].
Current treatments for neuropathic pain include antidepressants, anticonvulsants, topical lidocaine and opioid analgesics [32]. In this study, we focused on the therapeutic efficacy of bone marrow MSCs on neuropathic pain as a new approach.
The use of mesenchymal stromal cells as a therapeutic strategy is a current approach due to the reported safety in transplantation and efficacy in a range of conditions [33]. There are various MSC sources which were used to treat numerous diseases. The adipose tissuederived MSCs have a range of benefits for the treatment of autoimmune diseases [34]. In our previous study, it was shown that dental derived MSCs have immunosuppressive effects on the inflammatory response of immune system cells [35]. The safety of autologous MSC administration as a cellular therapy is wellestablished and accepted in human studies [33,36]. Local transplantation of rBMSCs to an experimental model of neuropathic pain showed a selective migration from the lesion to dorsal root ganglions [37]. In another study, dental derived MSC treatment showed beneficial effects with the suppression of innate immune system cells in an inflammatory disease myasthenia gravis in mice [38].
Therapeutical benefits of MSCs administration on neuropathic pain models depends on numerous factors, such as cell sources, the number of cells and administration way (local or systemic) [39]. The efficacy of the treatment changes with the administration time and repeats of application of MSCs during an injury [40].
In the present study, we focused on bone marrow-derived MSCs for the ability to migrate into the injured area. Flow cytometry analyses indicated that rBMSCs of this study were positive for CD29, CD73, CD90 and CD105 and negative for CD14, CD34 and CD20 cell surface markers associated with lymphohematopoietic cells. Therefore, it was proved that there were no hematopoietic precursors in the cultures. Additionally, the expression of surface markers of cultured cells was indicating that the cells used in this study had the characteristics of MSCs. Additionally, rBMSCs were differentiated to adipogenic, osteogenic and neurogenic lineages. The present study demonstrated that the GFP-labeled cells survived in the sciatic nerve-injured area. rBMSCs were observed around the injured area and had selective migration. rBMSCs were located and serve for sciatic nerve reconstruction. At the same time, immunohistochemistry staining results supported consistently localization of MSCs applied after injury. These results sustained that there is a correlation between cell viability and migration of MSCs towards the injured area and serve for functional improvement. Besides, there was a significant reduction in the injured area in the rats receiving rBMSCs when compared with CCI group and sham group, after 2 weeks. Our results revealed that CCI group that received rBMSCs showed improvement in recovery of injury compared with only CCI group. The results of walking tract analysis and cold plate test support the efficacy of rBMSCs therapy on functional improvement after sciatic nerve injury. Results showed that rats with CCI model had increasing pain after injury, rBMSC received group had a reduction of pain at week 1 and 2. However, pain was progressively increased from week 2 to 4. This result showed that single MSC administration controlled and decreased pain symptoms in short term follow-up. We thought that local administration of rBMSCs should be repeated at the end of week 2 to get optimum improvement.
Conclusion
The present study showed that transplantation of rBMSCs into the injured sciatic nerve enhances the damaged neural tissue and improves locomotor recovery. Transplantation of BMSCs might be an effective strategy to improve functional outcomes following traumatic injuries to the sciatic nerve. We conclude that stromal cell transplantation could be a useful therapeutic tool in the future of regenerative medicine. Further studies might be performed with different types of MSCs that have the potential to differentiate into neuronal cells. Clinical trials with BMSCs in traumatic sciatic nerve injury might be started since the prosperous results of applications of rBMSCs in experimental models reveal effective for its therapeutic use in injuries of the sciatic nerve.
Acknowledgements
The authors would like to thank Feyza Ar├?┬▒c├?┬▒o├?┬?lu for her skillful assistance. This study was supported by Marmara University research project BAPKO No: SAG-A-200611-0193.
References
- Treede RD, Jensen T, Campbell JN, Cruccu G, Dostrovsky JO, et al. (2008) Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 70: 1630-1635.
- Torrance N, Ferguson JA, Afolabi E, Bennett MI, Serpell MG, et al. (2013) Neuropathic pain in the community: More under-treated than refractory? Pain 154: 690-699.
- Yawn BP, Wollan PC, Weingarten TN, Watson JC, Hooten WM, et al. (2009) The prevalence of neuropathic pain: Clinical evaluation compared with screening tools in a community population. Pain Med 10: 586-593.
- Doth AH, Hansson PT, Jensen MP, Taylor RS (2010) The burden of neuropathic pain: A systematic review and meta-analysis of health utilities. Pain 149: 338-344.
- Hall GC, Carroll D, Parry D, McQuay HJ (2006) Epidemiology and treatment of neuropathic pain: The UK primary care perspective. Pain 122: 156-162.
- Tan T, Barry P, Reken S, Baker M (2010) Pharmacological management of neuropathic pain in non-specialist settings: Summary of NICE guidance. BMJ 340: c1079.
- Cornelius VR, Sauzet O, Williams JE, Ayis S, Farquhar-Smith P, et al. (2013) Adverse event reporting in randomised controlled trials of neuropathic pain: Considerations for future practice. Pain 154: 213-220.
- Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, et al. (2013) Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 154: 2249-2261.
- Woolf CJ (2011) Central sensitization: Implications for the diagnosis and treatment of pain. Pain 152: S2-15
- Cohen SP, Mao J (2014) Neuropathic pain: Mechanisms and their clinical implications. BMJ 348: f7656.
- Calvo M, Dawes JM, Bennett DL (2012) The role of the immune system in the generation of neuropathic pain. Lancet Neurol 11: 629-642.
- Moalem G, Xu K, Yu L (2004) T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 129: 767-777.
- Sommer C, Kress M (2004) Recent finding on how inflammatory cytokines cause pain: Peripheral mechanisms in inflammmatory and neuropathic hyperalgesia. NeurosciLett 361: 184-187.
- Feng X, Yuan W (2015) Dexamethasone Enhanced Functional Recovery after Sciatic Nerve Crush Injury in Rats. BioMed Res Int 2015: 627923.
- Austin PJ, Wu A, Taylor GM (2012) Chronic Constriction of the Sciatic Nerve and Pain Hypersensitivity Testing in Rats. J Vis Exp.
- Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA (2015) Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J Tissue Eng 6: 2041731415592356.
- Tong J, Ding J, Shen X, Chen L, Bian Y, et al. (2013) Mesenchymal stem cell transplantation enhancement in myocardial infarction rat model under ultrasound combined with nitric oxide microbubbles. PLoSOne 8: e80186.
- Ladak A, Olson J, Tredget EE, Gordon T (2011) Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol 228: 242-252.
- Rasmusson I (2006) Immune modulation by mesenchymal stem cells. Exp Cell Res 312: 2169-2179.
- Yi T, Song SU (2012) Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. ArchPharmRes 35: 213-221.
- Sakthiswary R, Raymond AA (2012) Stem cell therapy in neurodegenerative diseases. Neural Regen Res 7: 1822-1831.
- Glavaski JA, Virag T, Mangatu TA, McGrogan M, Wang XS, et al. (2010) Glial cell line-derived neurotrophic factor secreting genetically modified human bone marrow derived mesenchymal stem cells promote recovery in a rat model of Parkinson's disease. J NeurosciRes 88: 2669-2681.
- Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, et al. (2010) Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci 67: 655-669.
- Shibata T, Naruse K, Kamiya H, Kozakae M, Kondo M, et al. (2008) Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 57: 3099-3107.
- Karaoz E, Aksoy A, Ayhan S, Sariboyaci AE, Kaymaz F, et al. (2009) Characterization of mesenchymal stem cells from rat bone marrow: Ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol 132: 533-546.
- Karaoz E, Kabatas S, Duruksu G, Okcu A, Subasi C, et al. (2012) Reduction of Lesion in Injured Rat Spinal Cordand Partial Functional Recovery of Motility afterBone Marrow Derived Mesenchymal Stem CellTransplantation. Turk Neurosurg 22: 207-217.
- Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, et al. (2013) Recovery of Fertility in Azoospermia Rats after Injection of Adipose-Tissue-Derived Mesenchymal Stem Cells: The Sperm Generation. Biomed Res Int 2013: 529589.
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB (2004) Detection of Neuropathic Pain in a Rat Model of Peripheral Nerve Injury. Anesthesiology 101: 476-487.
- Tetik C, Erol B, Cabukoglu C, Unsal M (2000) Comparison of the functional evaluation methods in rat sciatic nerve model by a new system, ActaOrthopTraumatolTurc 34: 523-527.
- Torrance N, Smith BH, Bennett MI, Lee AJ (2006) The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 7: 281-289.
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C (2008) Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136: 380-387.
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, et al. (2007) Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 132: 237-251.
- de Girolamo L, Lucarelli E, Alessandri G, Avanzini MA, Bernardo ME, et al. (2013) Mesenchymal Stem/Stromal Cells: A New "Cells as Drugs" Paradigm. Efficacy and Critical Aspects in Cell Therapy. Curr Pharm Des 19: 2459-2473.
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, et al. (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277-2286.
- Yildirim S, Zibandeh N, Genc D, Ozcan EM, Goker K (2016)The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int 2016: 4682875.
- Waterman RS, Betancourt AM (2011) Treating Chronic Pain with Mesenchymal Stem Cells: A Therapeutic Approach Worthy of Continued Investigation. J Stem Cell Res Ther.
- Coronel MF, Musolino PL and Villar MJ (2006) Selective migration and engraftment of bone marrow mesenchymal stem cells in rat lumbar dorsal root ganglia after sciatic nerve constriction, Neurosci. Lett. 405: 5-9.
- Ulusoy C, Zibandeh N, Yildirim S, Trakas N, Zisimopoulou P, et al. (2015) Dental follicle mesenchymal stem cell administration ameliorates muscle weakness in MuSK-immunized mice. J Neuroinflammation 12: 231.
- Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, et al. (2012) Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: A long-term safety study. Cytotherapy 14: 56-60.
- Yousefifard M, Nasirinezhad F, Manaheji HS, Janzadeh A, Hosseini M, et al. (2016) Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Res Ther 7: 36.
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 4427
- [From(publication date):
September-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 3595
- PDF downloads : 832